The Quantum Mechanical Model of the Atom Electron




- Slides: 20

The Quantum Mechanical Model of the Atom Electron Cloud


Bohr's Model of the Atom (cont. ) • Bohr’s model explained hydrogen’s spectral lines, but failed to explain any other element’s lines. • The behavior of electrons is still not fully understood, but it is known they do not move around the nucleus in circular orbits.

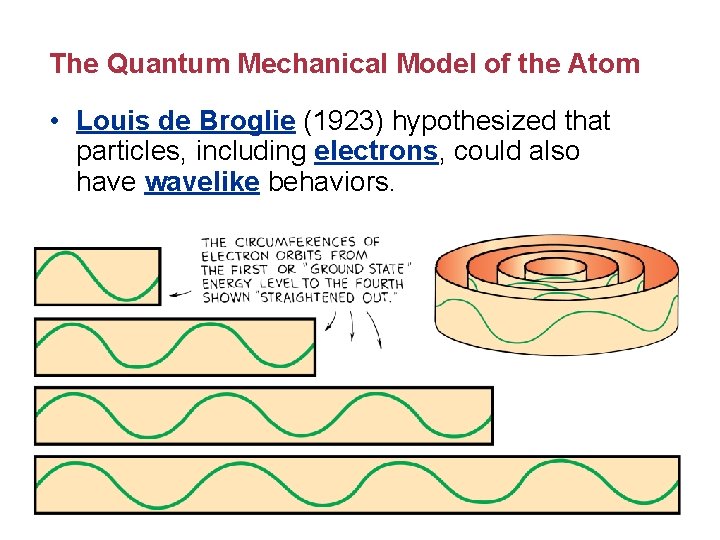
The Quantum Mechanical Model of the Atom • Louis de Broglie (1923) hypothesized that particles, including electrons, could also have wavelike behaviors.

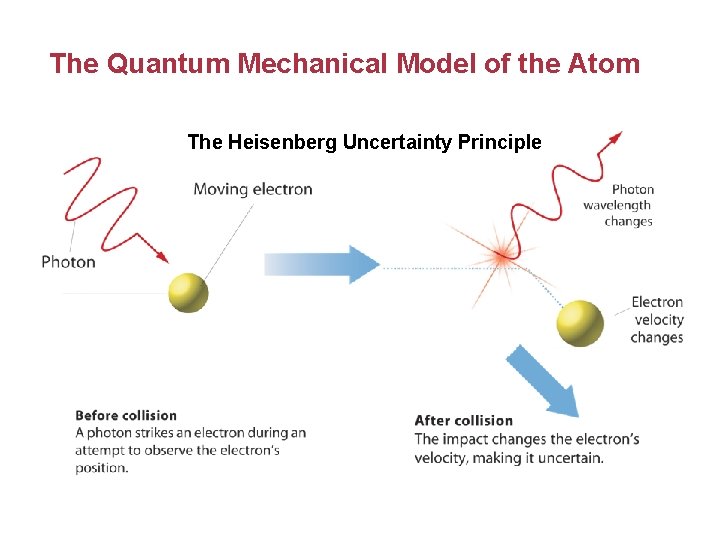
The Quantum Mechanical Model of the Atom • Heisenberg showed it is impossible to take any measurement of an object without disturbing it. • The Heisenberg uncertainty principle states that it is fundamentally impossible to know precisely both the velocity and position of a particle at the same time. • The only quantity that can be known is the probability for an electron to occupy a certain region around the nucleus.

The Quantum Mechanical Model of the Atom The Heisenberg Uncertainty Principle

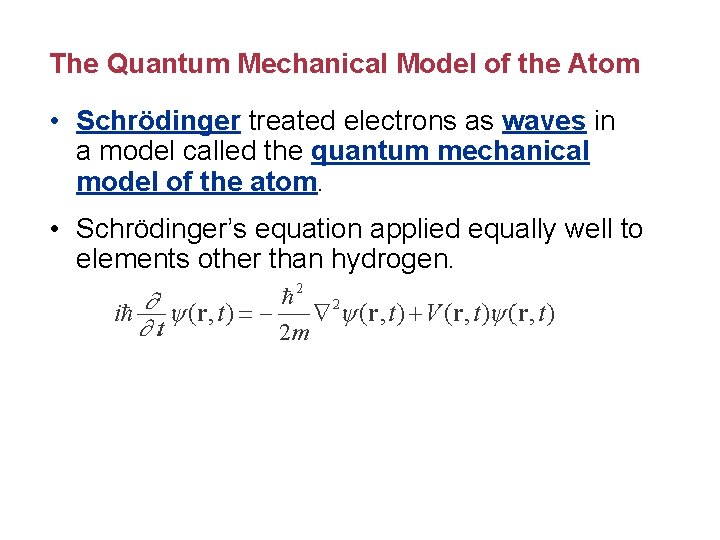
The Quantum Mechanical Model of the Atom • Schrödinger treated electrons as waves in a model called the quantum mechanical model of the atom. • Schrödinger’s equation applied equally well to elements other than hydrogen.

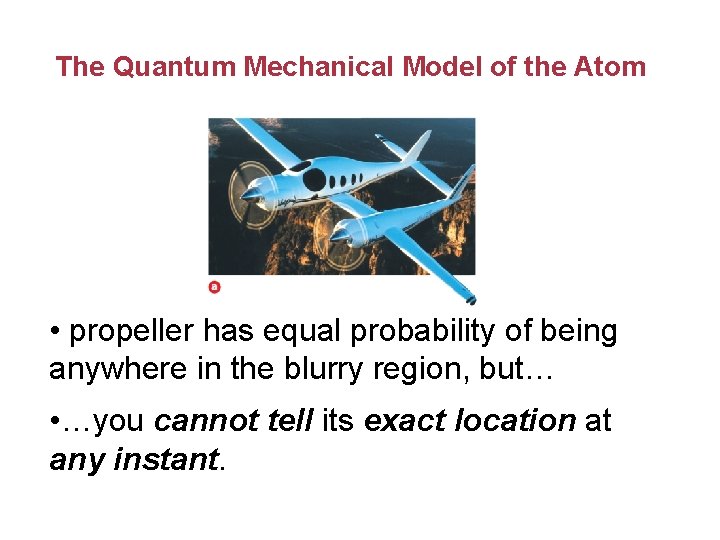
The Quantum Mechanical Model of the Atom • propeller has equal probability of being anywhere in the blurry region, but… • …you cannot tell its exact location at any instant.

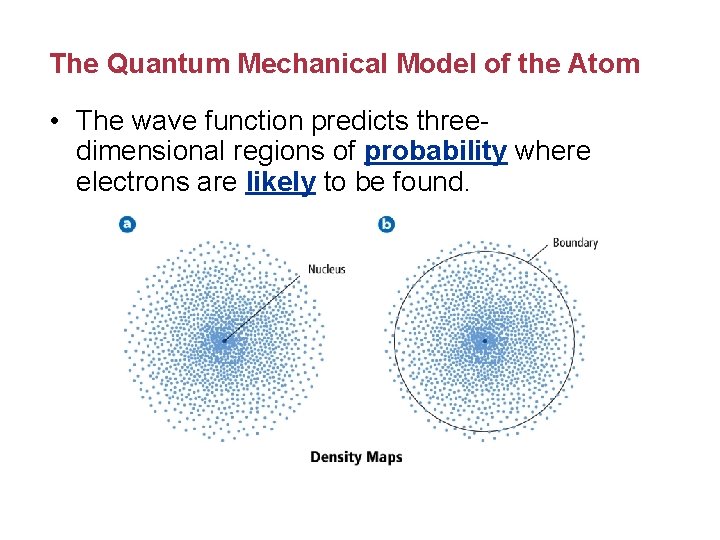
The Quantum Mechanical Model of the Atom • The wave function predicts threedimensional regions of probability where electrons are likely to be found.

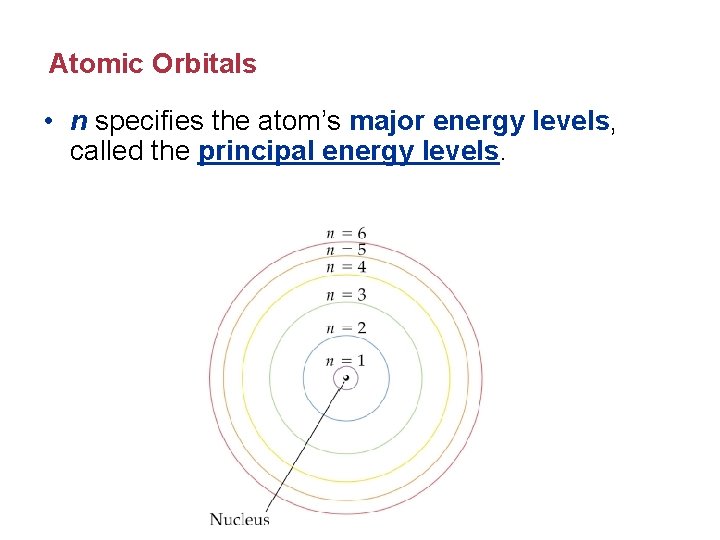
Atomic Orbitals • n specifies the atom’s major energy levels, called the principal energy levels.

Atomic Orbitals • Energy sublevels are contained within the principal energy levels.

Atomic Orbitals • Each energy sublevel relates to atomic orbitals of different shape. Atomic Orbitals are 3 -dimensional regions with a high probability of electrons

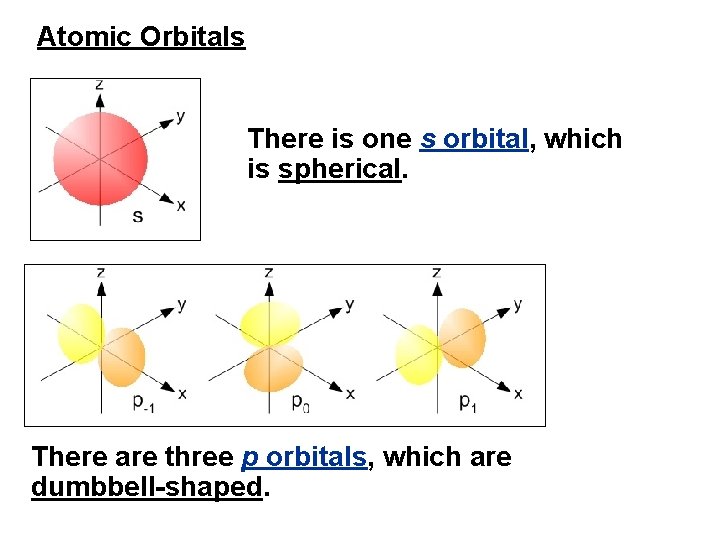
Atomic Orbitals There is one s orbital, which is spherical. There are three p orbitals, which are dumbbell-shaped.

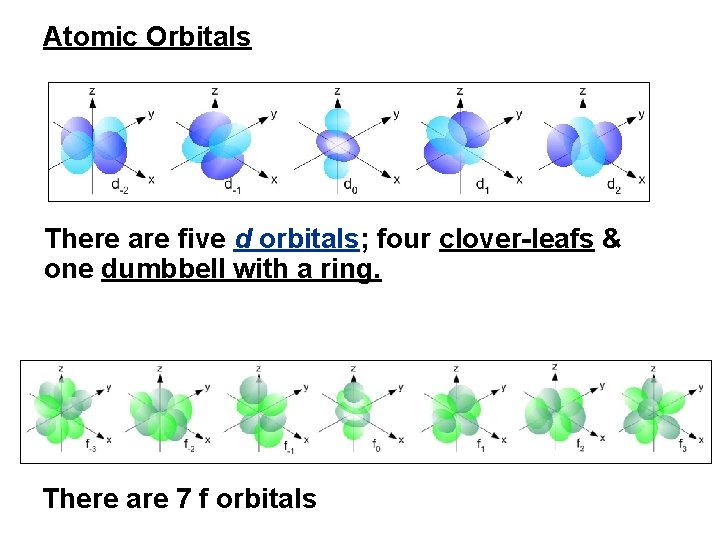
Atomic Orbitals There are five d orbitals; four clover-leafs & one dumbbell with a ring. There are 7 f orbitals

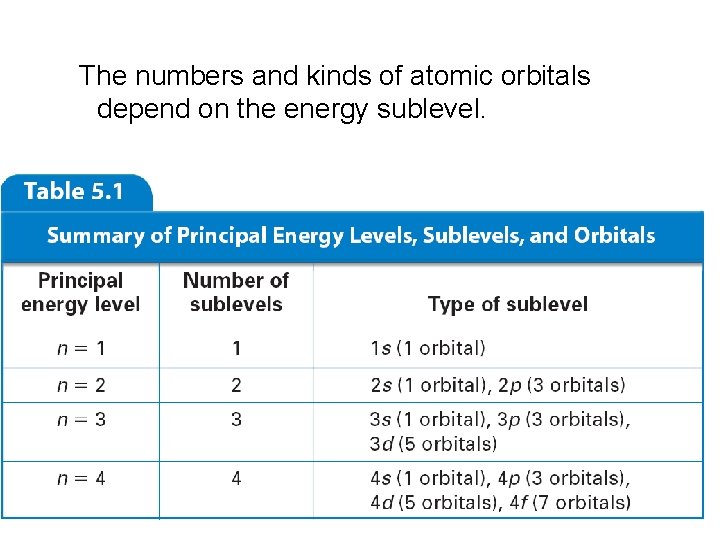
The numbers and kinds of atomic orbitals depend on the energy sublevel.

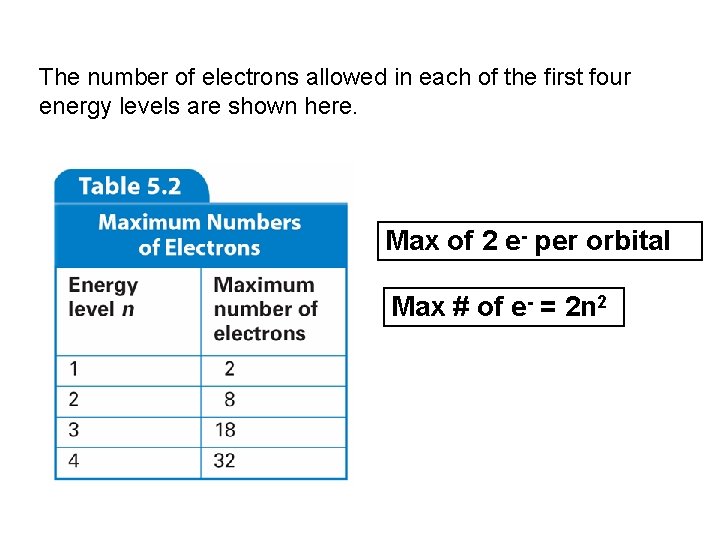
The number of electrons allowed in each of the first four energy levels are shown here. Max of 2 e- per orbital Max # of e- = 2 n 2

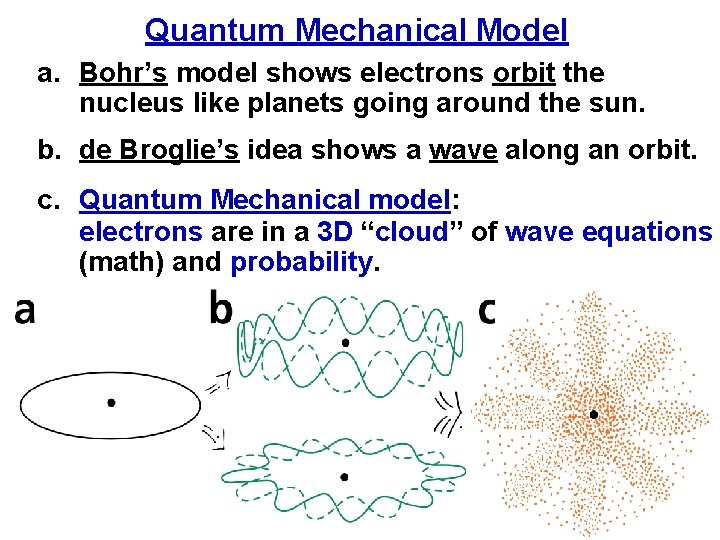
Quantum Mechanical Model a. Bohr’s model shows electrons orbit the nucleus like planets going around the sun. b. de Broglie’s idea shows a wave along an orbit. c. Quantum Mechanical model: electrons are in a 3 D “cloud” of wave equations (math) and probability.

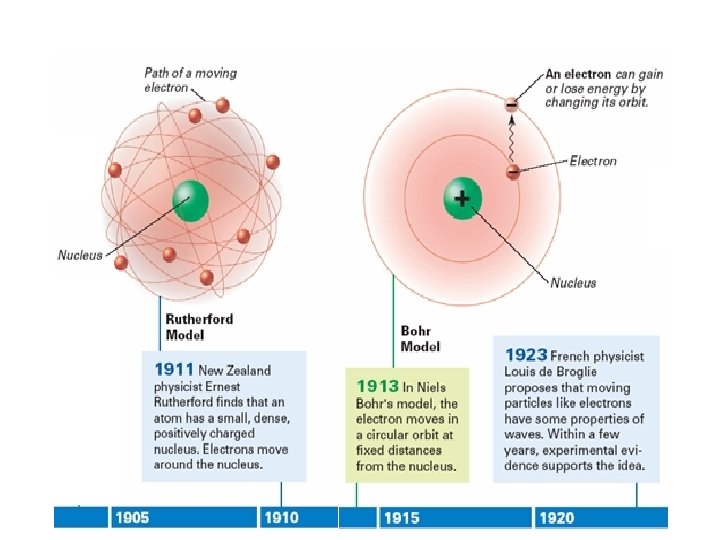
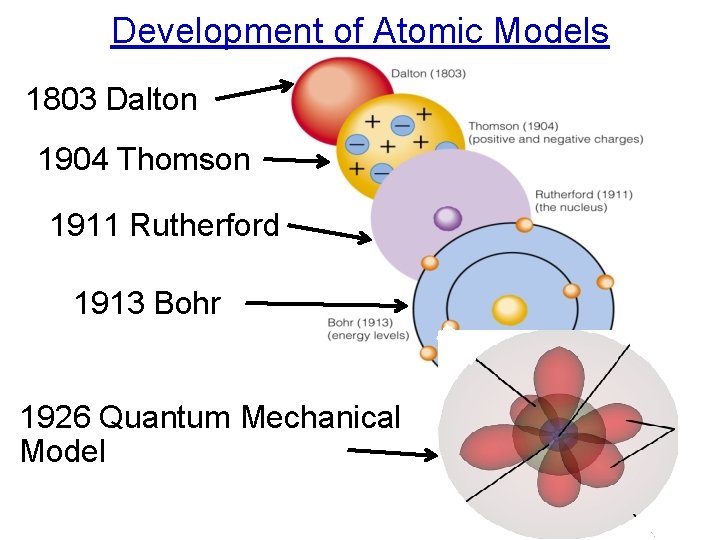
Development of Atomic Models 1803 Dalton 1904 Thomson 1911 Rutherford 1913 Bohr 1926 Quantum Mechanical Model

Section 5. 2 Assessment Which atomic suborbitals have a “dumbbell” shape? A. s B. f C. p D. d A. B. C. D. A B C D

Section 5. 2 Assessment Who proposed that particles could also exhibit wavelike behaviors? A. Bohr B. Einstein C. Rutherford D. de Broglie A. B. C. D. A B C D