STANDARD GRADE CHEMISTRY CALCULATIONS Calculations involving the mole

- Slides: 7

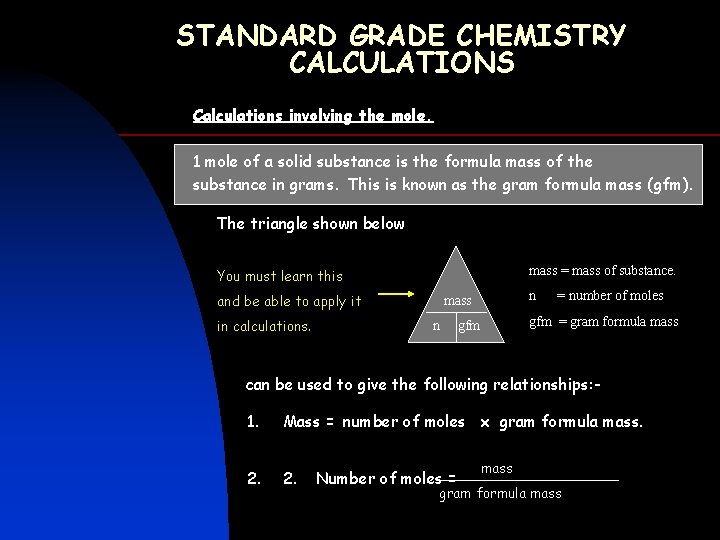
STANDARD GRADE CHEMISTRY CALCULATIONS Calculations involving the mole. 1 mole of a solid substance is the formula mass of the substance in grams. This is known as the gram formula mass (gfm). The triangle shown below mass = mass of substance. You must learn this and be able to apply it in calculations. n mass n = number of moles gfm = gram formula mass gfm can be used to give the following relationships: - 1. Mass = number of moles x gram formula mass. 2. Number of moles = mass gram formula mass

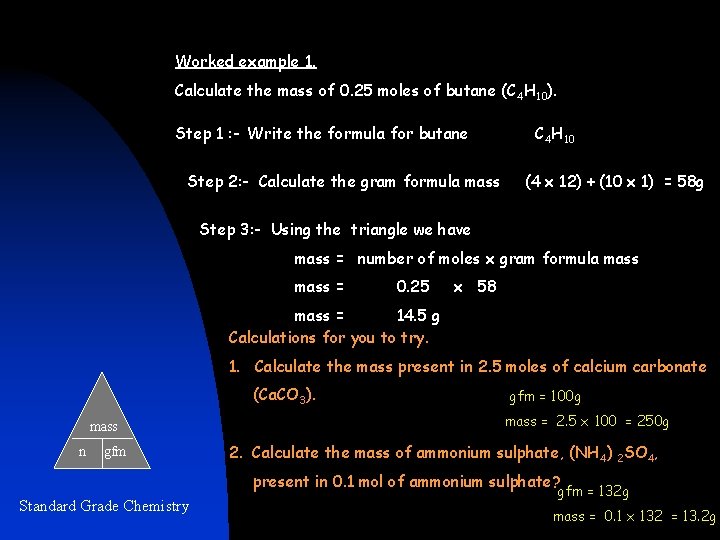
Worked example 1. Calculate the mass of 0. 25 moles of butane (C 4 H 10). Step 1 : - Write the formula for butane Step 2: - Calculate the gram formula mass C 4 H 10 (4 x 12) + (10 x 1) = 58 g Step 3: - Using the triangle we have mass = number of moles x gram formula mass = 0. 25 x 58 mass = 14. 5 g Calculations for you to try. 1. Calculate the mass present in 2. 5 moles of calcium carbonate (Ca. CO 3). mass n gfm = 100 g mass = 2. 5 x 100 = 250 g 2. Calculate the mass of ammonium sulphate, (NH 4) 2 SO 4, present in 0. 1 mol of ammonium sulphate? Standard Grade Chemistry gfm = 132 g mass = 0. 1 x 132 = 13. 2 g

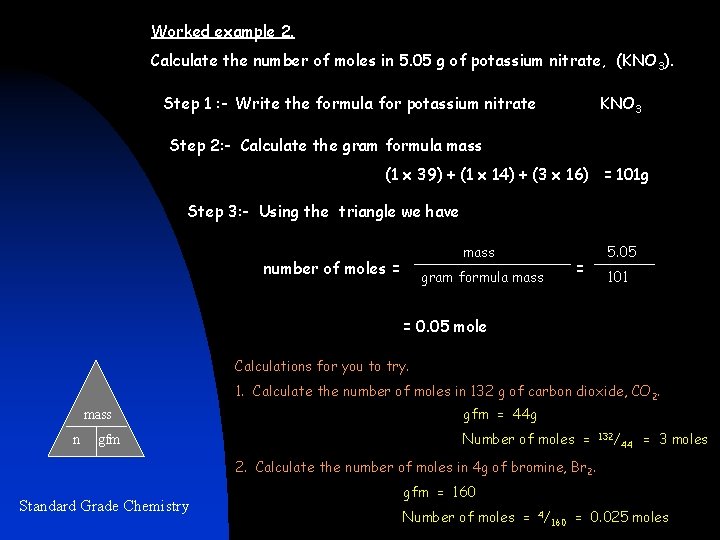
Worked example 2. Calculate the number of moles in 5. 05 g of potassium nitrate, (KNO 3). Step 1 : - Write the formula for potassium nitrate KNO 3 Step 2: - Calculate the gram formula mass (1 x 39) + (1 x 14) + (3 x 16) = 101 g Step 3: - Using the triangle we have mass number of moles = = gram formula mass 5. 05 101 = 0. 05 mole Calculations for you to try. 1. Calculate the number of moles in 132 g of carbon dioxide, CO 2. mass n gfm = 44 g Number of moles = 132/ 44 = 3 moles 2. Calculate the number of moles in 4 g of bromine, Br 2. Standard Grade Chemistry gfm = 160 Number of moles = 4/ 160 = 0. 025 moles

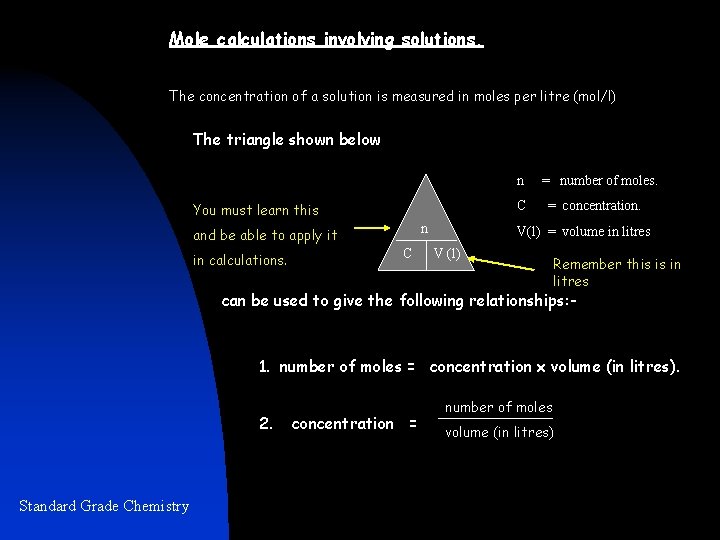
Mole calculations involving solutions. The concentration of a solution is measured in moles per litre (mol/l) The triangle shown below n C You must learn this n and be able to apply it in calculations. C = number of moles. = concentration. V(l) = volume in litres V (l) Remember this is in litres can be used to give the following relationships: - 1. number of moles = concentration x volume (in litres). 2. Standard Grade Chemistry concentration = number of moles volume (in litres)

Worked example 1. Calculate the number of moles in 200 cm 3 of 0. 5 mol/l sodium hydroxide solution. Step 1 : - Change the volume into litres. 0. 2 litres Step 2 : - Using the triangle gives number of moles = concentration x volume (in litres). = 0. 5 x 0. 2 = 0. 1 moles Calculations for you to try. 1. Calculate the number of moles in 50 cm 3 of 0. 1 mol/l zinc sulphate solution. n C Volume = Number of moles 50/ 1000 = 0. 05 litres = 0. 1 x 0. 05 = 0. 0005 moles V (l) 2. Calculate the number of moles in 0. 2 litres of 2 mol/l sodium hydroxide solution Standard Grade Chemistry Volume = 0. 2 litres Number of moles = 0. 2 x 2 = 0. 4 moles

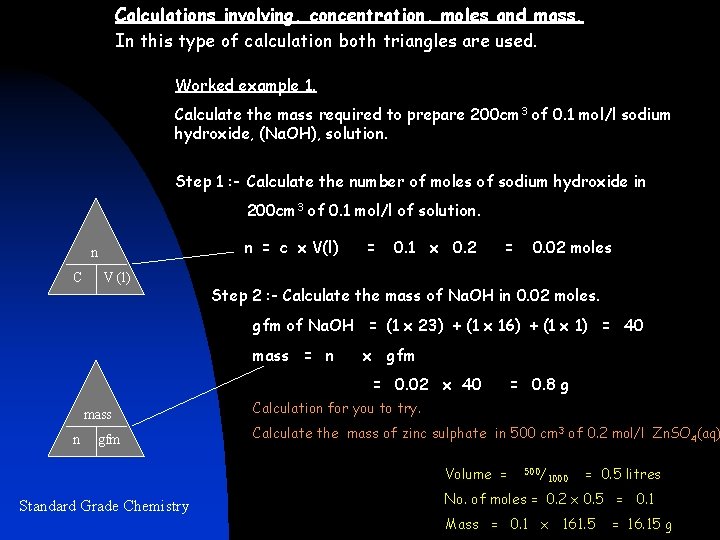
Calculations involving, concentration, moles and mass. In this type of calculation both triangles are used. Worked example 1. Calculate the mass required to prepare 200 cm 3 of 0. 1 mol/l sodium hydroxide, (Na. OH), solution. Step 1 : - Calculate the number of moles of sodium hydroxide in 200 cm 3 of 0. 1 mol/l of solution. n = c x V(l) n C V (l) = 0. 1 x 0. 2 = 0. 02 moles Step 2 : - Calculate the mass of Na. OH in 0. 02 moles. gfm of Na. OH = (1 x 23) + (1 x 16) + (1 x 1) = 40 mass = n x gfm = 0. 02 x 40 mass n gfm Calculation for you to try. Calculate the mass of zinc sulphate in 500 cm 3 of 0. 2 mol/l Zn. SO 4(aq) Volume = Standard Grade Chemistry = 0. 8 g 500/ 1000 = 0. 5 litres No. of moles = 0. 2 x 0. 5 = 0. 1 Mass = 0. 1 x 161. 5 = 16. 15 g

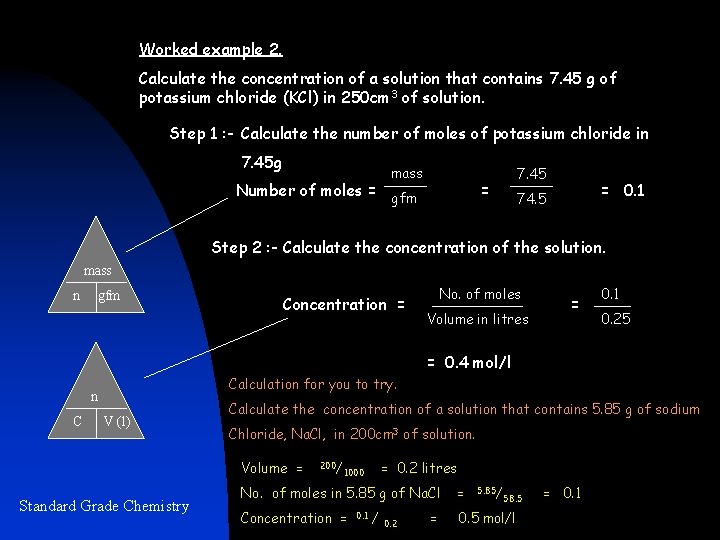
Worked example 2. Calculate the concentration of a solution that contains 7. 45 g of potassium chloride (KCl) in 250 cm 3 of solution. Step 1 : - Calculate the number of moles of potassium chloride in 7. 45 g mass Number of moles = gfm 7. 45 = = 0. 1 74. 5 Step 2 : - Calculate the concentration of the solution. mass n gfm Concentration = No. of moles Volume in litres = 0. 1 0. 25 = 0. 4 mol/l Calculation for you to try. n C V (l) Calculate the concentration of a solution that contains 5. 85 g of sodium Chloride, Na. Cl, in 200 cm 3 of solution. Volume = Standard Grade Chemistry 200/ = 0. 2 litres 1000 No. of moles in 5. 85 g of Na. Cl = Concentration = 0. 5 mol/l 0. 1 / 0. 2 = 5. 85/ 58. 5 = 0. 1