The Mole Chemistry 11 The Mole The Mole






























- Slides: 57

The Mole Chemistry 11

The Mole

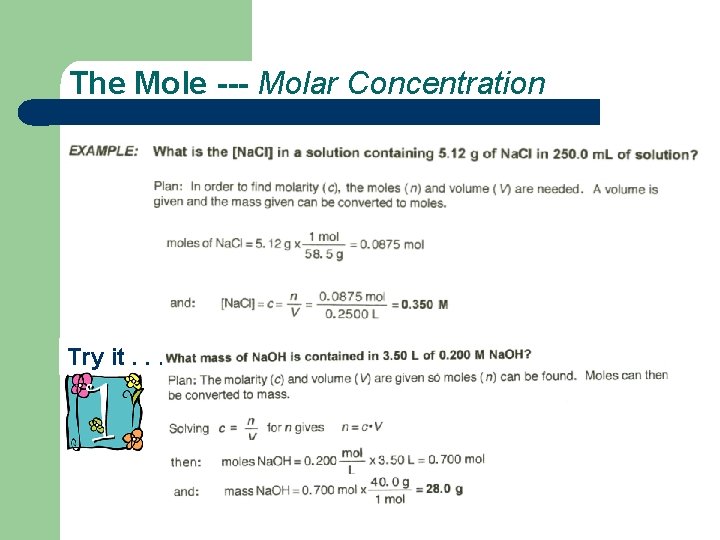
The Mole Try it. . .

The Mole Problem. . . Another scientist enters the arena: Joseph Gay-Lussac Yet, another scientist enters the arena: Amedeo Avagadro

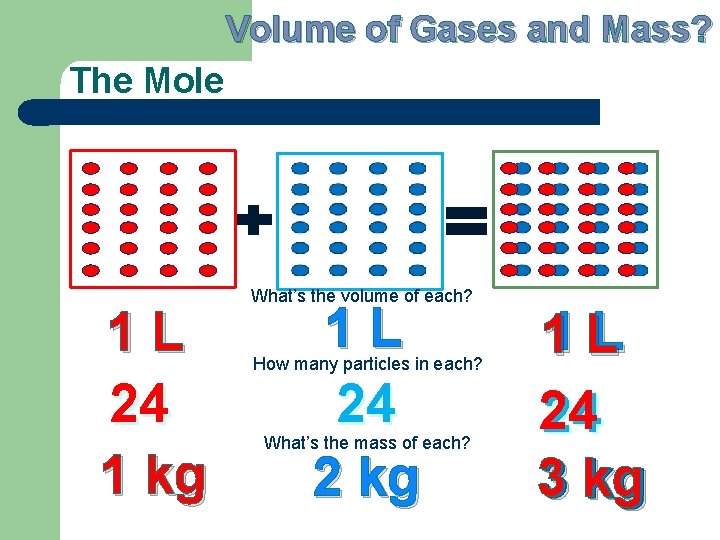
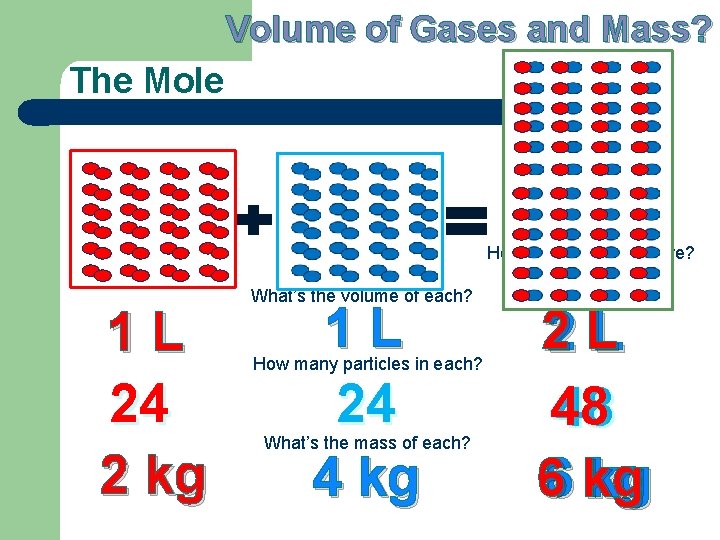
Volume of Gases and Mass? The Mole 1 L 24 1 kg What’s the volume of each? 1 L 24 2 kg How many particles in each? What’s the mass of each? L 11 L 24 24 3 kg

Volume of Gases and Mass? The Mole How many particles here? 1 L 24 2 kg What’s the volume of each? 1 L 24 4 kg How many particles in each? What’s the mass of each? 22 L L 48 48 kg 66 kg

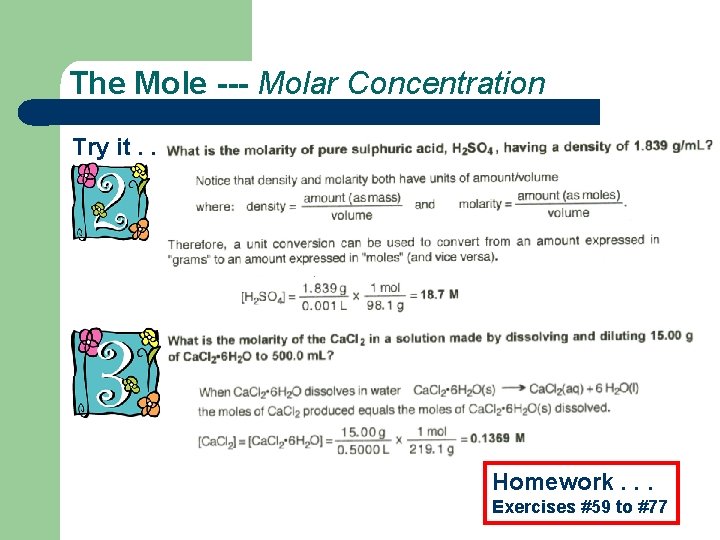
The Mole Try it. . .

The Mole Think. . .

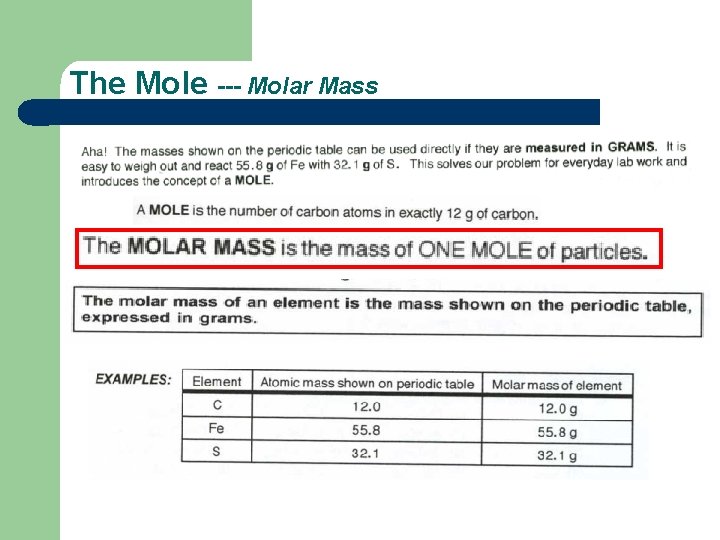
The Mole --- Molar Mass

The Mole --- Molar Mass Try it. . . Clue. . . Homework. . . Exercises 6 & 7

The Mole --- Molar Mass

The Mole --- Molar Mass Relating # of Moles and Mass Only use: mol

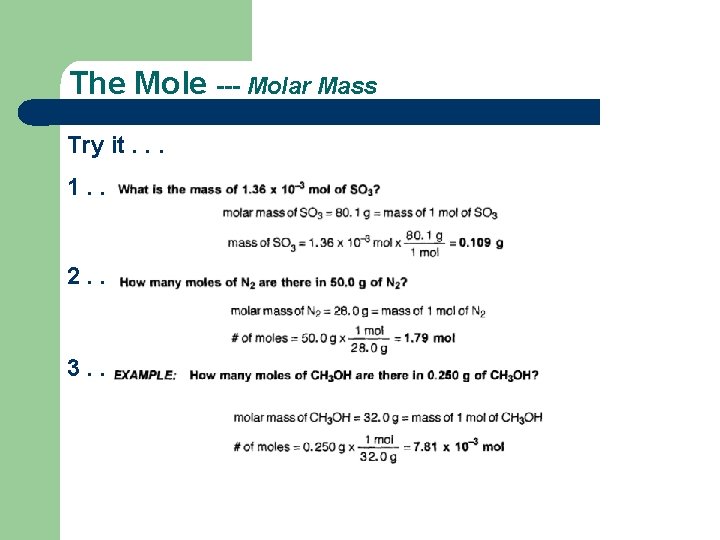
The Mole --- Molar Mass Try it. . . 1. . 2. . 3. .

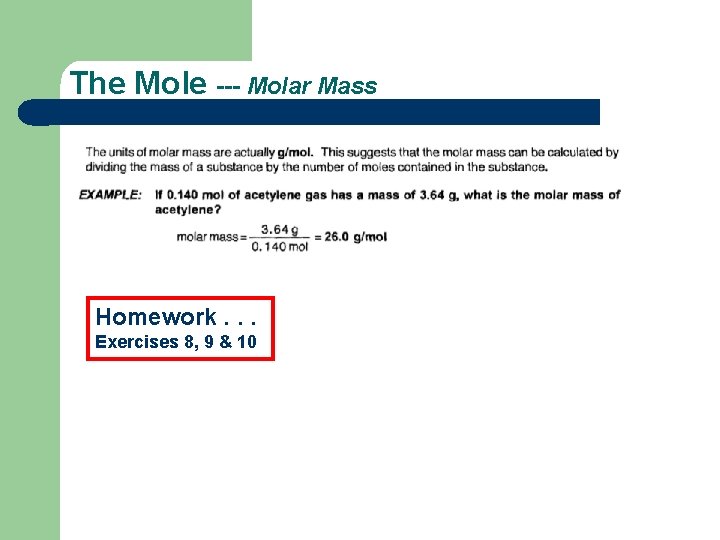
The Mole --- Molar Mass Homework. . . Exercises 8, 9 & 10

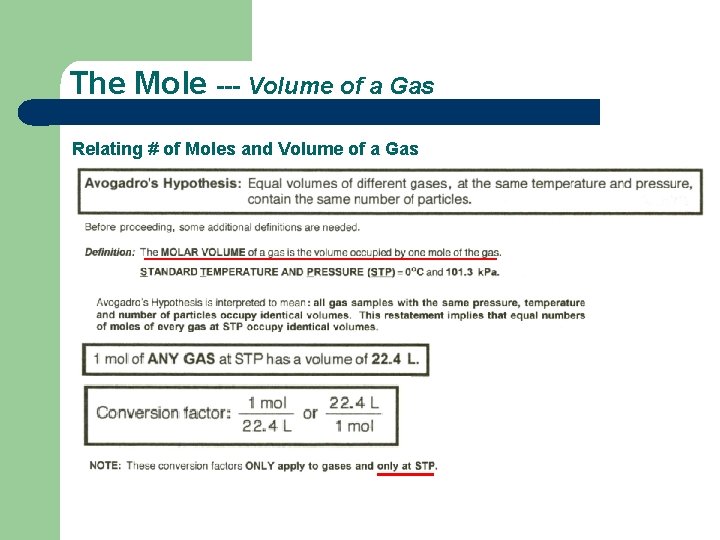
The Mole --- Volume of a Gas Relating # of Moles and Volume of a Gas

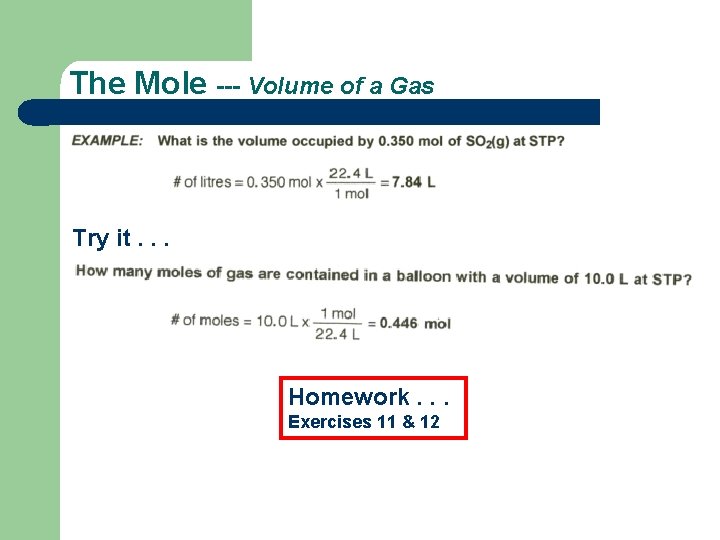
The Mole --- Volume of a Gas Try it. . . Homework. . . Exercises 11 & 12

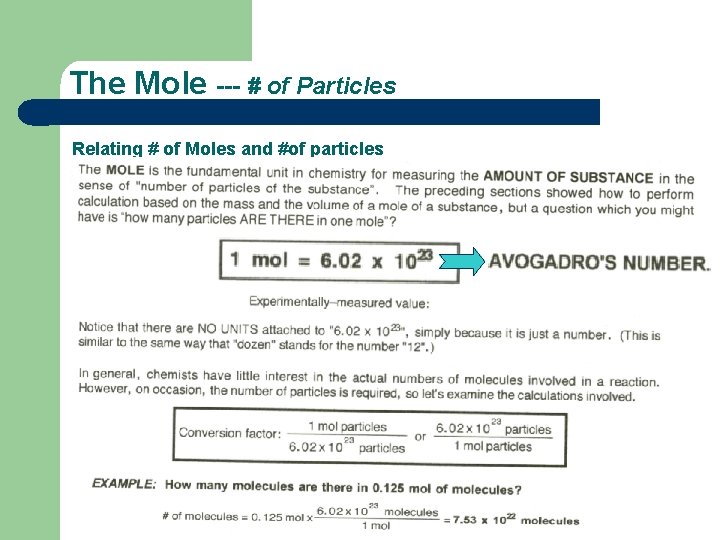
The Mole --- # of Particles Relating # of Moles and #of particles

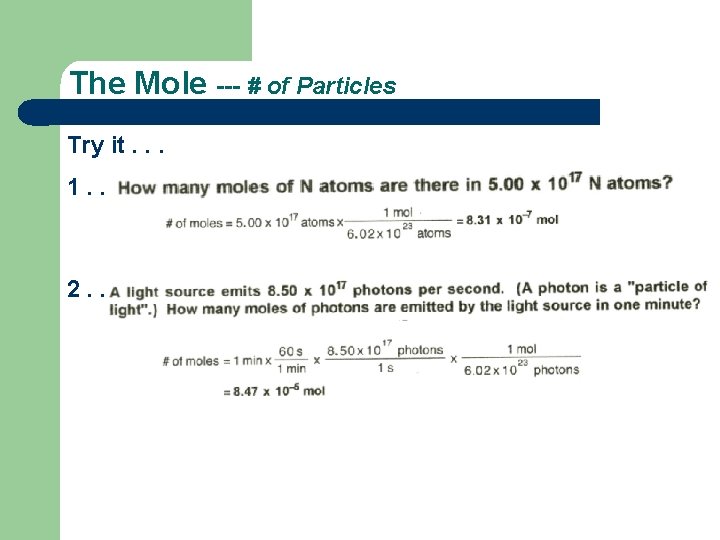
The Mole --- # of Particles Try it. . . 1. . 2. .

The Mole --- # of Particles --- Molar Mass Homework. . . Exercises 15 to 20

The Mole --- # of Particles, Mass, & Volume Multiple Conversions Between Moles, Mass, Volume and #of particles Try it. . . Homework. . . Exercises 21

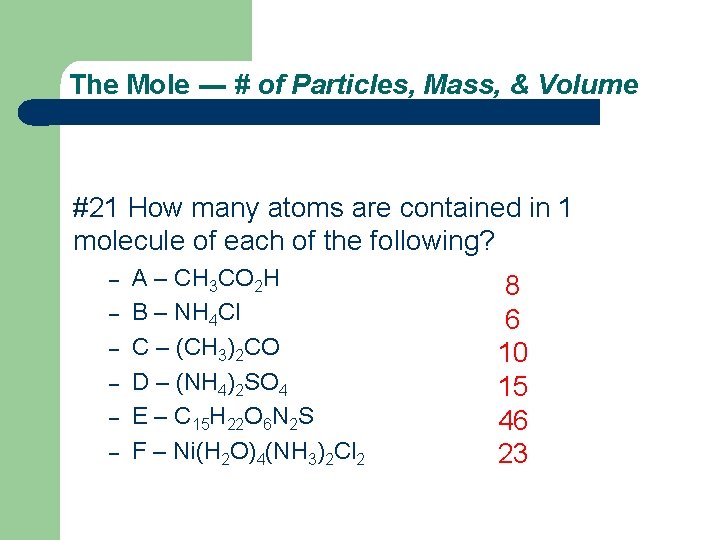
The Mole --- # of Particles, Mass, & Volume #21 How many atoms are contained in 1 molecule of each of the following? – – – A – CH 3 CO 2 H B – NH 4 Cl C – (CH 3)2 CO D – (NH 4)2 SO 4 E – C 15 H 22 O 6 N 2 S F – Ni(H 2 O)4(NH 3)2 Cl 2 8 6 10 15 46 23

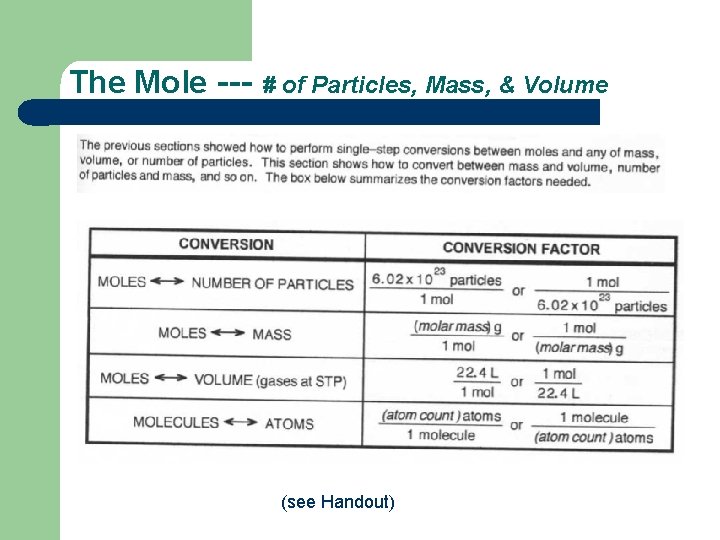
The Mole --- # of Particles, Mass, & Volume (see Handout)

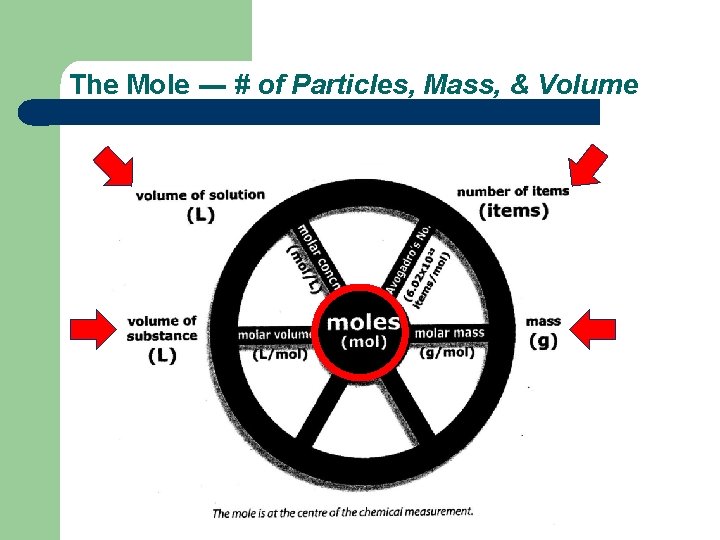
The Mole --- # of Particles, Mass, & Volume

The Mole --- # of Particles, Mass, & Volume

The Mole --- # of Particles, Mass, & Volume Homework. . . Exercises 22, 23 & 24 (see Handout)

Homework. . . The Mole --- Try it. . . 1. . 2. . Exercises 22, 23 & 24 # of Particles, Mass, & Volume

The Mole --- # of Particles, Mass, & Volume Show video: The Mole – 10 min -- library

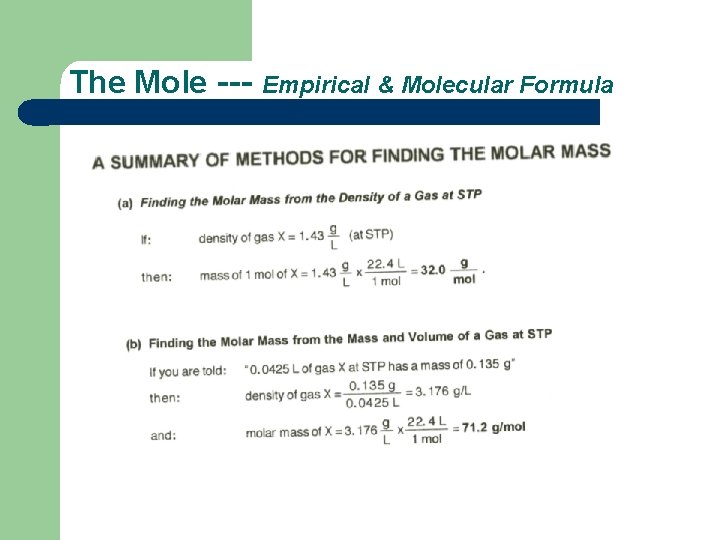
The Mole --- # of Particles, Mass, & Volume Try it. . . 1. . Clues . . . 2. . density = mass of 1 mol = 32. 0 g = 1. 43 g/L V vol. of 1 mol 22. 4 L

Homework. . . Exercises 25, to 34 The Mole --Try it. . . 3. . 4. . Go to Molarity # of Particles, Mass, & Volume

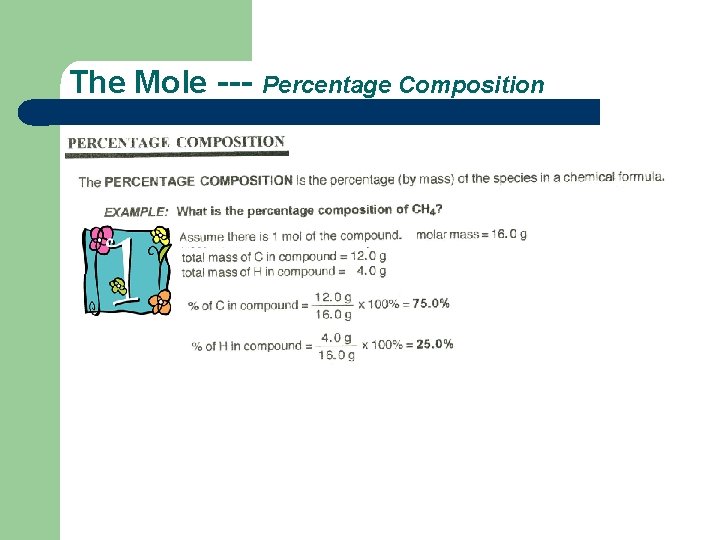
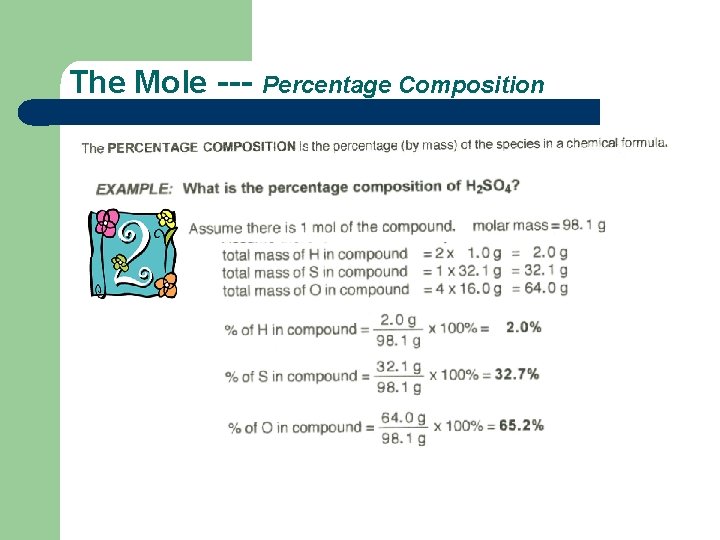
The Mole --- Percentage Composition

The Mole --- Percentage Composition

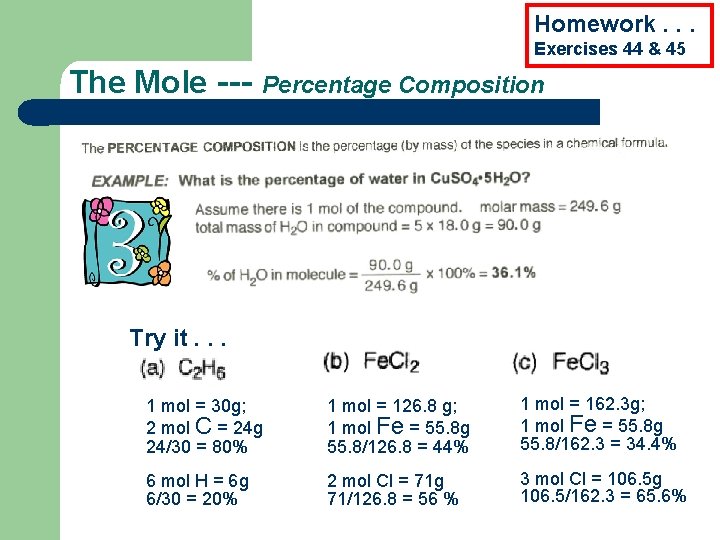
Homework. . . Exercises 44 & 45 The Mole --- Percentage Composition Try it. . . 1 mol = 30 g; 2 mol C = 24 g 24/30 = 80% 1 mol = 126. 8 g; 1 mol Fe = 55. 8 g 55. 8/126. 8 = 44% 1 mol = 162. 3 g; 1 mol Fe = 55. 8 g 55. 8/162. 3 = 34. 4% 6 mol H = 6 g 6/30 = 20% 2 mol Cl = 71 g 71/126. 8 = 56 % 3 mol Cl = 106. 5 g 106. 5/162. 3 = 65. 6%

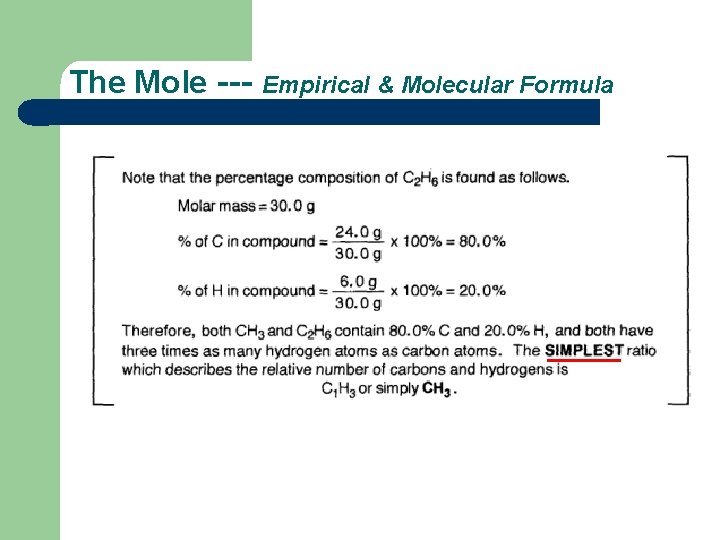
The Mole --- Empirical & Molecular Formula

The Mole --- Empirical & Molecular Formula 1 more point to make. .

The Mole --- Empirical & Molecular Formula

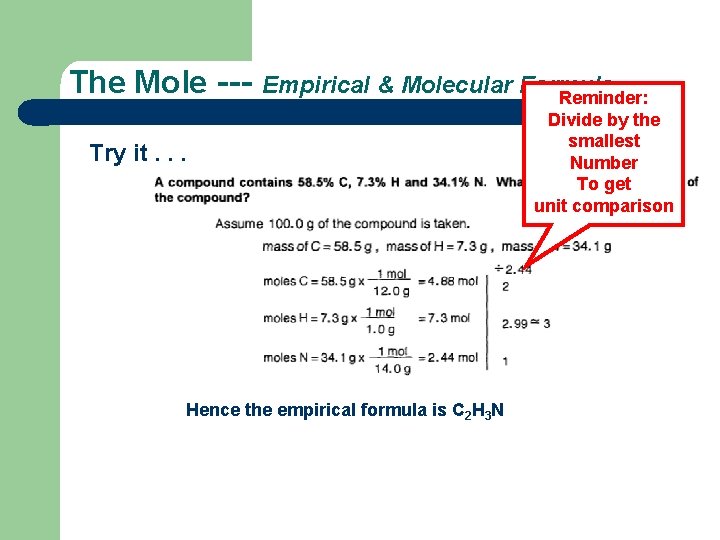
The Mole --- Empirical & Molecular Formula Reminder: Try it. . . Hence the empirical formula is C 2 H 3 N Divide by the smallest Number To get unit comparison

The Mole --Try it. . . Empirical & Molecular Formula

The Mole --- Empirical & Molecular Formula

The Mole --- Empirical & Molecular Formula Try it. . . Homework. . . Exercises #46

The Mole --- Empirical & Molecular Formula

The Mole --- Empirical & Molecular Formula

The Mole --- Empirical & Molecular Formula

The Mole --Try it. . . Empirical & Molecular Formula

The Mole --Try it. . . Empirical & Molecular Formula

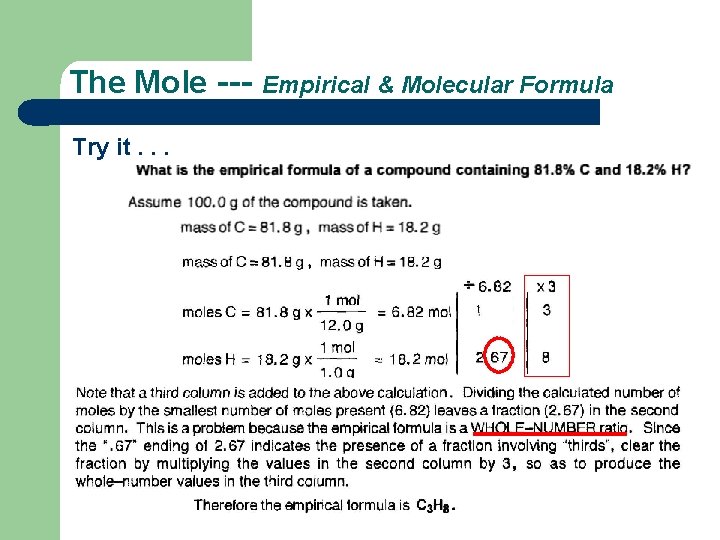
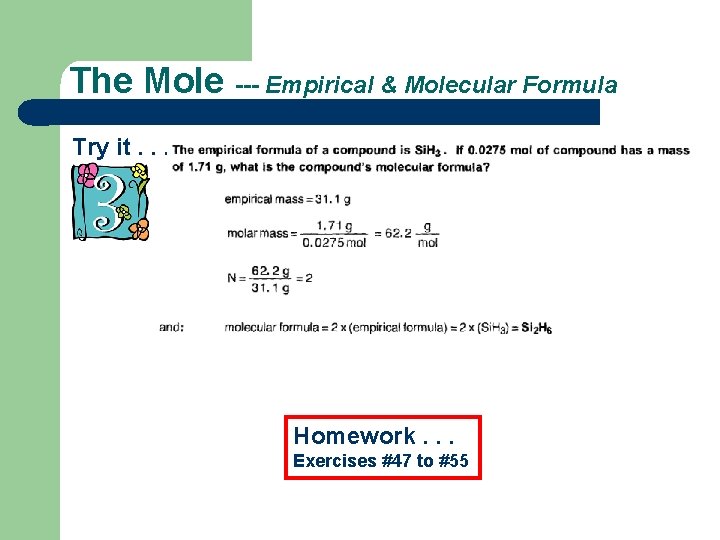
The Mole --- Empirical & Molecular Formula Try it. . . Homework. . . Exercises #47 to #55

The Mole --- Molar Concentration Knowing the concentration of a solution provides a way to find how much of a particular substance exists in a given volume of the solution.

The Mole --- Molar Concentration

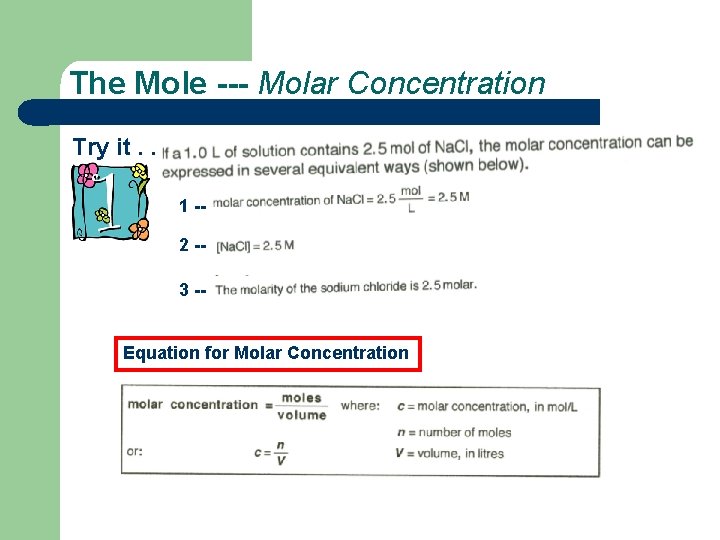
The Mole --- Molar Concentration Try it. . . 1 -2 -3 -Equation for Molar Concentration

The Mole --- Molar Concentration Try it. . .

The Mole --- Molar Concentration Try it. . . Homework. . . Exercises #59 to #77

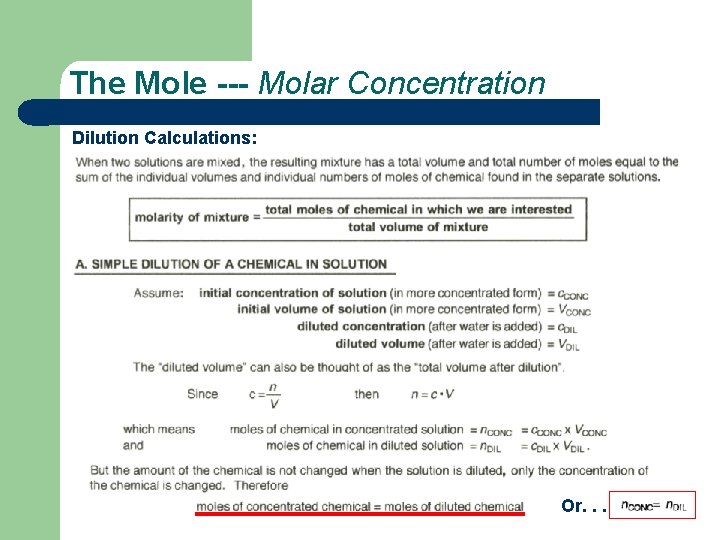
The Mole --- Molar Concentration Dilution Calculations: Or. . .

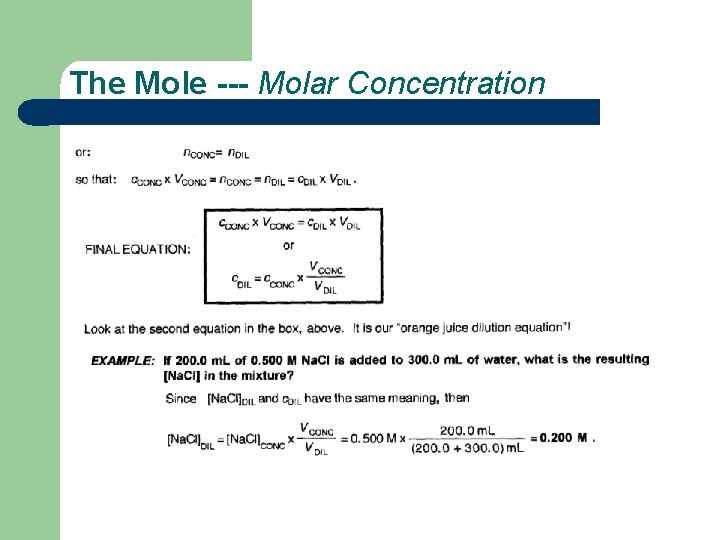
The Mole --- Molar Concentration

Handout: Dilution Calculations The Mole --- Molar Concentration

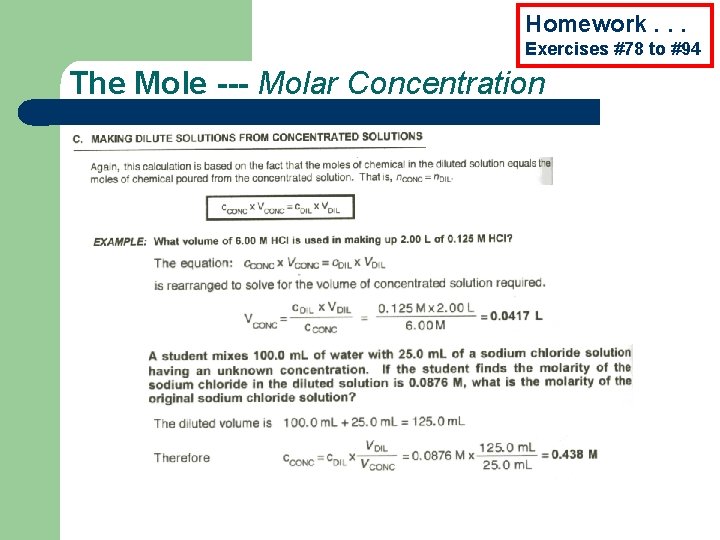
Homework. . . Exercises #78 to #94 The Mole --- Molar Concentration

The Mole --- Molar Concentration of Ions Go to Dissolved Ions

Quiz: Concentration Review The Mole --- Summary Handout: Molarity Summary Handout: Practice Problems ALSO: Mole Test Review Homework. . . Exercises #95 to #102

The Mole --- The End
 Mole-mass-volume relationships
Mole-mass-volume relationships Molecular mass of sucrose
Molecular mass of sucrose Mole problem
Mole problem Stoichiometry mole-mole
Stoichiometry mole-mole Mole mole factor
Mole mole factor Grams to mass formula
Grams to mass formula Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Mole bridge chemistry
Mole bridge chemistry Mols to grams
Mols to grams Mole hill chemistry
Mole hill chemistry Calculate the grams of nitrogen in 125g of each fertilizer
Calculate the grams of nitrogen in 125g of each fertilizer Stoichiometry cookie lab
Stoichiometry cookie lab What's a mole chemistry
What's a mole chemistry Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 No of moles formula
No of moles formula Mole bridge chemistry
Mole bridge chemistry Mole cookies chemistry
Mole cookies chemistry Formula mole
Formula mole Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Frameset trong html5
Frameset trong html5 Các số nguyên tố là gì
Các số nguyên tố là gì đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Phối cảnh
Phối cảnh Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Tư thế worm breton
Tư thế worm breton Sơ đồ cơ thể người
Sơ đồ cơ thể người ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Bổ thể
Bổ thể Tư thế ngồi viết
Tư thế ngồi viết Ví dụ về giọng cùng tên
Ví dụ về giọng cùng tên Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Hát lên người ơi
Hát lên người ơi Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Phép trừ bù
Phép trừ bù Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Lời thề hippocrates
Lời thề hippocrates đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng