Mole Calculations The Mole Mole measurement of the













- Slides: 31

Mole Calculations

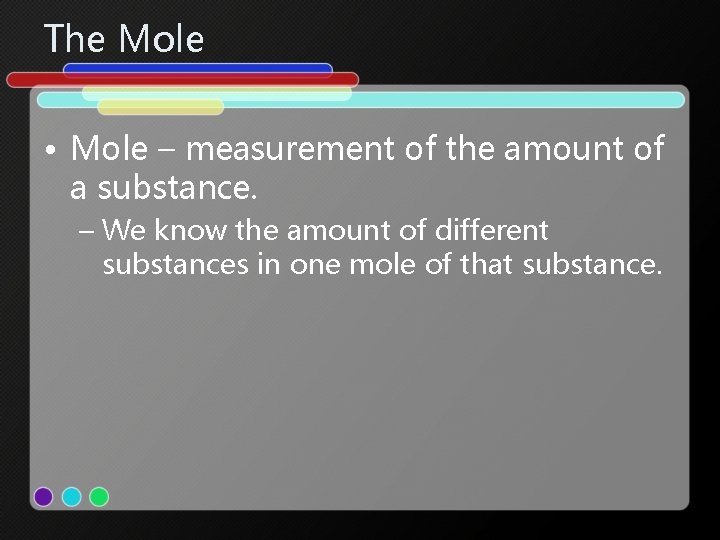
The Mole • Mole – measurement of the amount of a substance. – We know the amount of different substances in one mole of that substance.

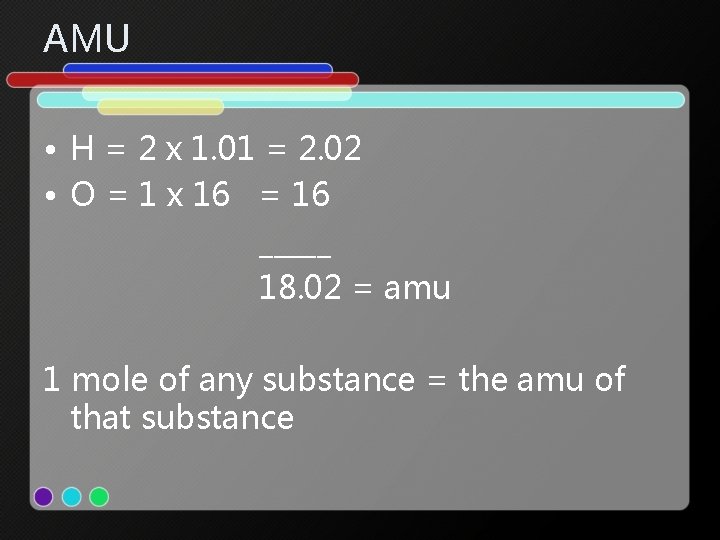
Atomic Mass Unit • Mass of 1 mole of compound • Found by adding the atomic weights of each atom of each element that makes up a compound. • H 2 O • There are 2 atoms of hydrogen and 1 atom of oxygen.

AMU • H = 2 x 1. 01 = 2. 02 • O = 1 x 16 = 16 _____ 18. 02 = amu 1 mole of any substance = the amu of that substance

AMU • Molar mass = the sum of the molar masses of atoms of the elements in the formula • Calculated the same way

The Mole • One mole of any substance has Avogadro’s number. • 6. 022 x 1023 atoms, molecules, ions

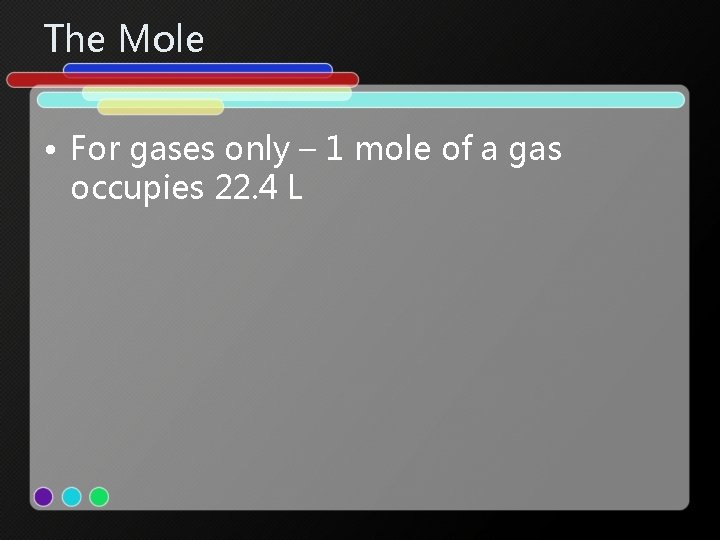
The Mole • For gases only – 1 mole of a gas occupies 22. 4 L

Converting Between Units amu 1 mole 6. 022 x 1023 atoms, mlc, ions (expressed in grams)

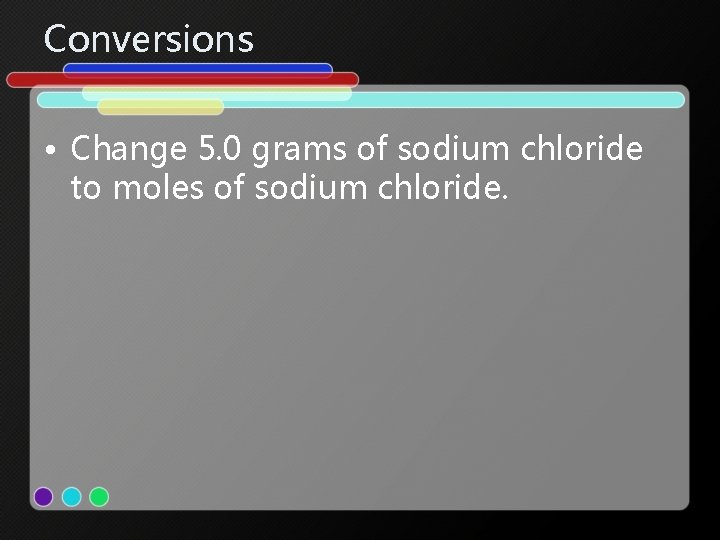
Conversions • Change 5. 0 grams of sodium chloride to moles of sodium chloride.

• Change 0. 45 moles of barium chlorate to grams of barium chlorate.

• Determine the number of atoms in 15 grams of water.

Empirical Formulas

Empirical Formulas • The simplest whole number ratio of moles of each element in the compound. • H 2 O • Na. Cl

Empirical Formulas • Usually given in percentages of each element in the compound. • Based on 100% of the compound. • Can be compared to a 100 gram sample of the substance. • 11. 2% Hydrogen • 88. 8% Oxygen

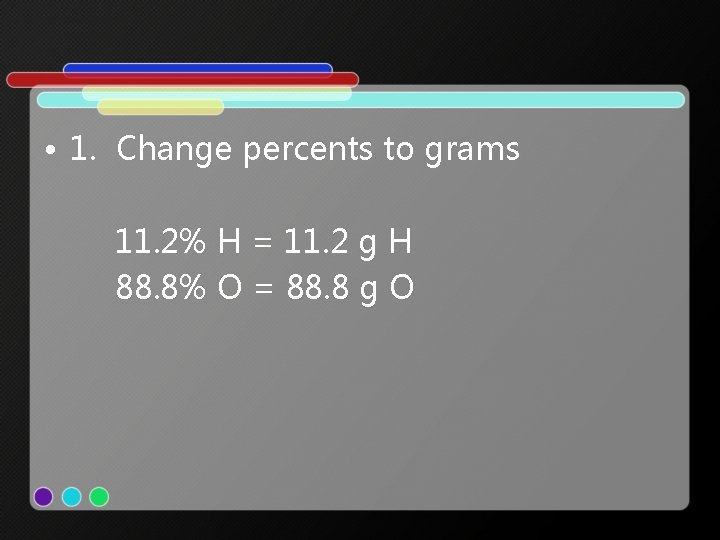
• 1. Change percents to grams 11. 2% H = 11. 2 g H 88. 8% O = 88. 8 g O

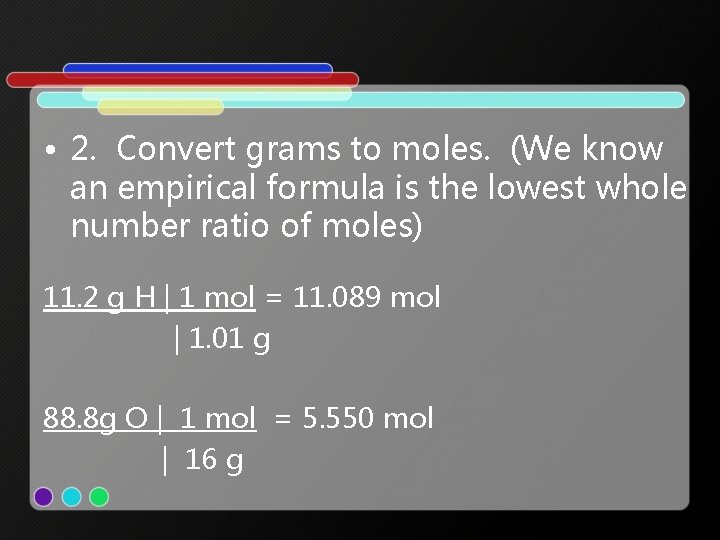
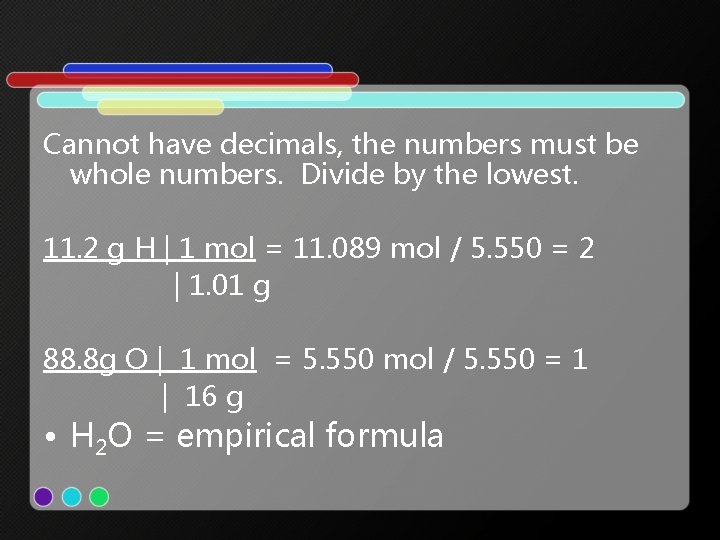
• 2. Convert grams to moles. (We know an empirical formula is the lowest whole number ratio of moles) 11. 2 g H | 1 mol = 11. 089 mol | 1. 01 g 88. 8 g O | 1 mol = 5. 550 mol | 16 g

Cannot have decimals, the numbers must be whole numbers. Divide by the lowest. 11. 2 g H | 1 mol = 11. 089 mol / 5. 550 = 2 | 1. 01 g 88. 8 g O | 1 mol = 5. 550 mol / 5. 550 = 1 | 16 g • H 2 O = empirical formula

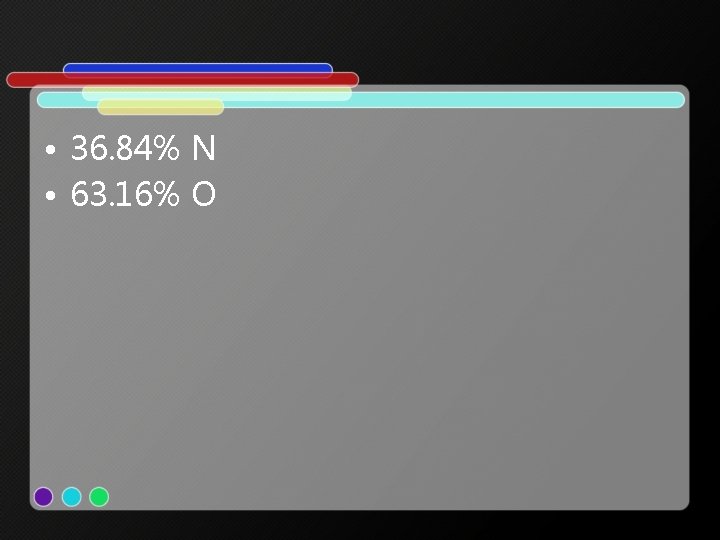
• 36. 84% N • 63. 16% O

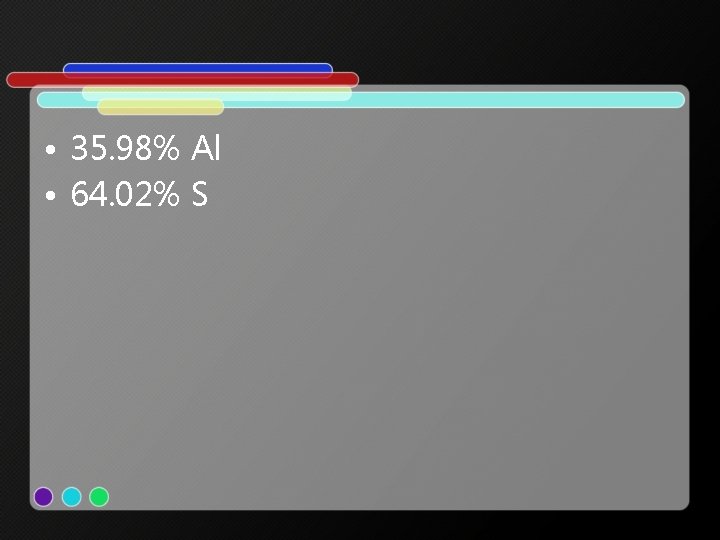
• 35. 98% Al • 64. 02% S

Molecular Formula

• Specifies the actual number of atoms of each element in one molecule or formula unit of the substance.

• The molar mass of acetylene is 26. 04 g/mol and the mass of the empirical formula, CH, is 13. 02 g/mol

• 1. The problem will give you a molar mass of the compound. • 2. Calculate the empirical formula as usual.

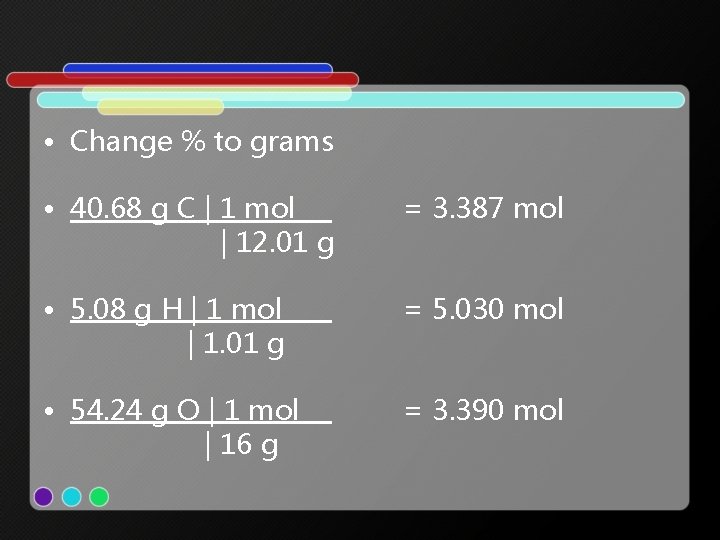
• 40. 68% carbon • 5. 08% hydrogen • 54. 24% oxygen • Molar mass = 118. 1 g/mol

• Change % to grams • 40. 68 g C | 1 mol | 12. 01 g = 3. 387 mol • 5. 08 g H | 1 mol | 1. 01 g = 5. 030 mol • 54. 24 g O | 1 mol | 16 g = 3. 390 mol

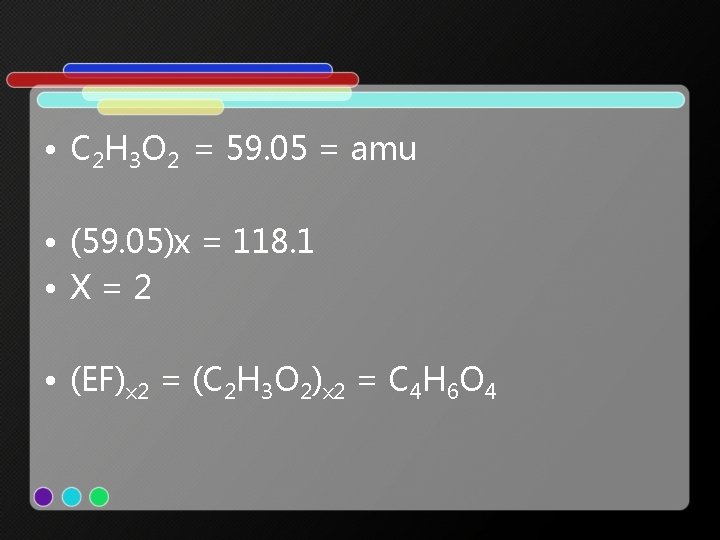
• 3. 387 mol / 3. 387 mol = 1 x 2 = 2 • 5. 030 mol / 3. 387 mol = 1. 5 x 2 = 3 • 3. 390 mol / 3. 387 mol = 1 x 2 = 2 • Empirical Formula = C 2 H 3 O 2

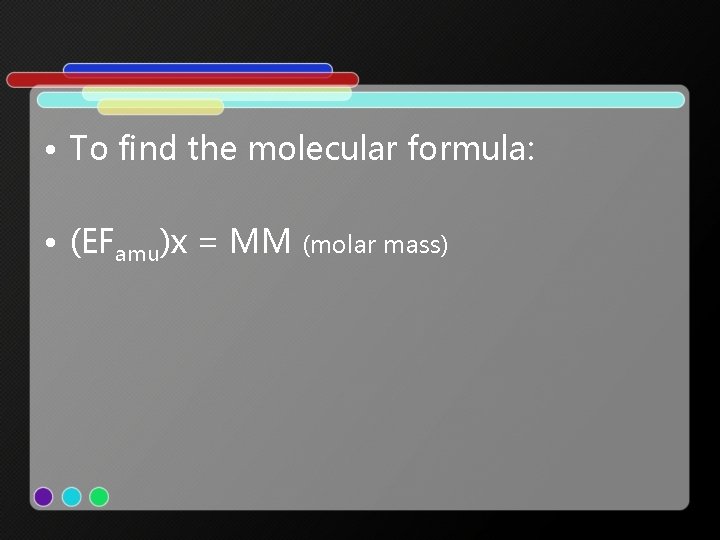
• To find the molecular formula: • (EFamu)x = MM (molar mass)

• C 2 H 3 O 2 = 59. 05 = amu • (59. 05)x = 118. 1 • X=2 • (EF)x 2 = (C 2 H 3 O 2)x 2 = C 4 H 6 O 4

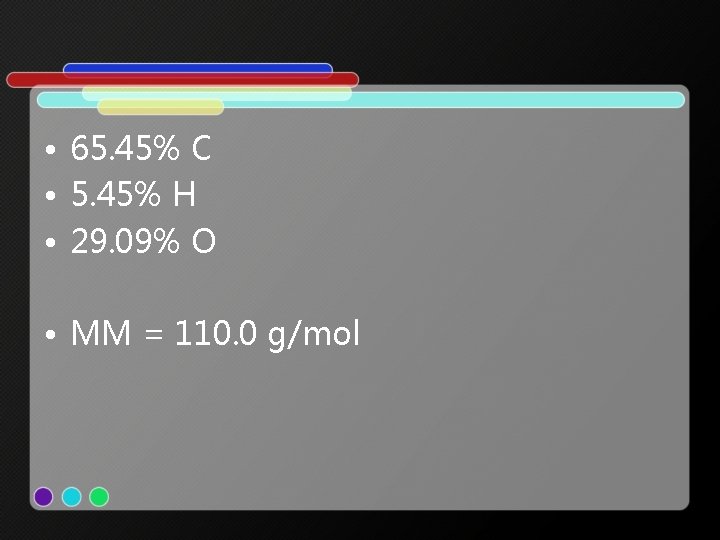
• 65. 45% C • 5. 45% H • 29. 09% O • MM = 110. 0 g/mol

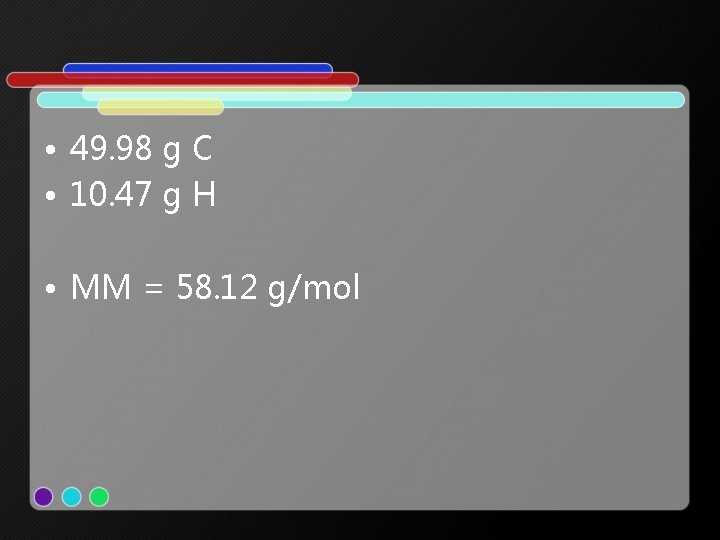
• 49. 98 g C • 10. 47 g H • MM = 58. 12 g/mol

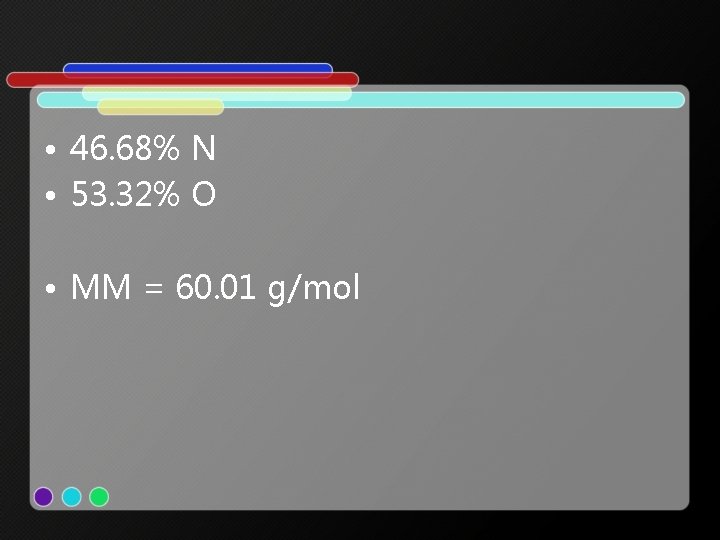
• 46. 68% N • 53. 32% O • MM = 60. 01 g/mol