GRADE 10 CHEMISTRY TOPIC GRADE 9 REVIEW START














- Slides: 17

GRADE 10: CHEMISTRY TOPIC: GRADE 9 REVIEW & START OF GRADE 10 MATERIAL Date: Tuesday, March 19 th, 2013

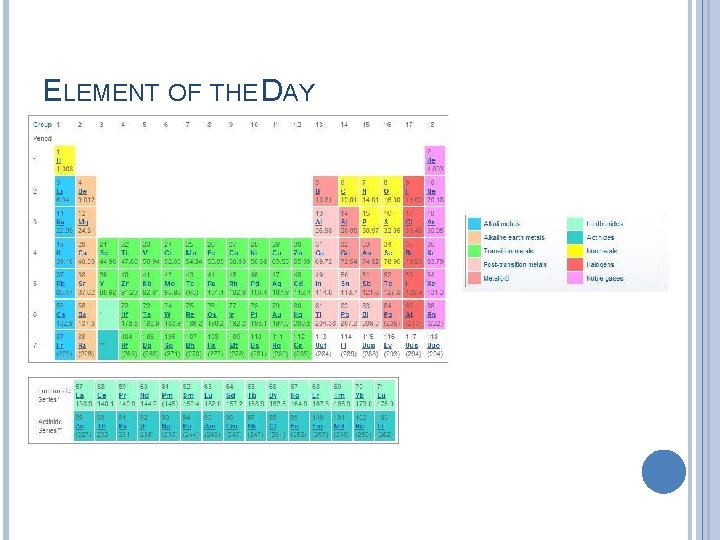
ELEMENT OF THE DAY

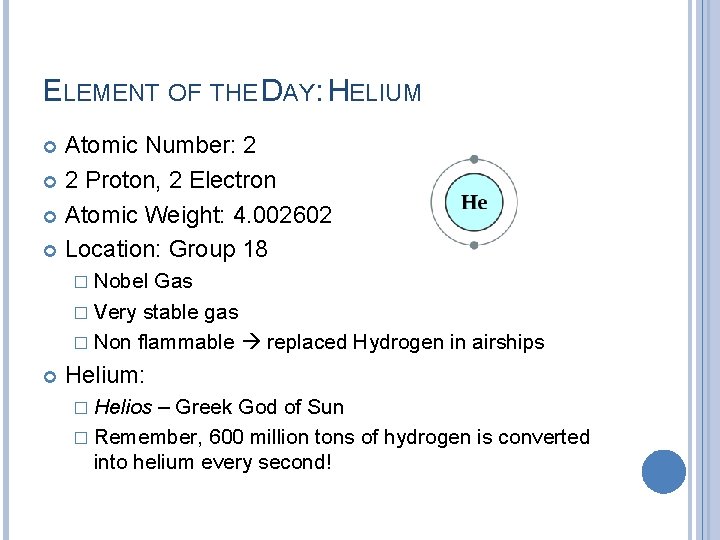
ELEMENT OF THE DAY: HELIUM Atomic Number: 2 2 Proton, 2 Electron Atomic Weight: 4. 002602 Location: Group 18 � Nobel Gas � Very stable gas � Non flammable replaced Hydrogen in airships Helium: � Helios – Greek God of Sun � Remember, 600 million tons of hydrogen is converted into helium every second!

ELEMENT OF THE DAY: HELIUM Helium Video: � Watch until 3: 39: � http: //www. youtube. com/watch? v=a 8 FJEi. I 5 e 6 Q

LESSON OVERVIEW Jeopardy Game Grade 10 Introductory Experiment Chemical Reactions Video Extra Help with Ionic Bonds

JEOPARDY FOR GRADE 9 REVIEW Rules � Two teams � The team that asked the question continues until the other team steals. � If the team answers correctly, they get the allotted points. � If the team answers incorrectly, they lose the allotted points. � Teams cycle through until all questions have been answered. � Stealing in this game is encouraged! � Surprise for the winning team!

GRADE 10 INTRODUCTORY EXPERIMENT Experiment: Mixing Baking Soda, Citric Acid, and Water to see the reaction Chapter 4 Power Point – slide 13 ON Science 10 Textbook – page 137 Students will work in their row/pod groups and given the following roles: � 1: Reading the instructions � 1: Gathering the materials � 1 -2: Pouring the materials � 1: Recording observations � Optional: Group leader to keep group on track & to help clean up

GRADE 10 INTRODUCTORY EXPERIMENT Make a hypothesis about what will happen � Observations � � Our group believes _______ will happen because _______________________. Record everything that happens Examples: Colour, time reaction takes, what is formed, texture, consistency, etc. What type of reaction was it? How do you know it was this type of reaction? Make your conclusions & relate back to what you know about physical and chemical reactions (from grade 9 review) **Put all group members’ names on your lab report and hand in for communication marks.

EXPLANATION OF EXPERIMENT What happened? ? � Reaction absorbed heat bag feels cold Endothermic Reaction Absorbing heat from your hand, which makes your hand feel cold � Balanced Formula for Chemical Reaction Occurring: � Carbon Dioxide (CO 2) gas was formed and bubbles H 3 C 6 H 5 O 7(aq) + 3 Na. HCO 3(s) → 3 CO 2(g) + 3 H 2 O(l) + Na 3 C 6 H 5 O 7(aq) Summarizing what happens: Don’t need sound! � http: //www. youtube. com/watch? v=b. PSy 5 Nk. Pk. Jk �

CHEMICAL REACTIONS VIDEO Bill Nye Video: Chemical Reactions � http: //www. youtube. com/watch? v=Plwuxp. Mh 8 nk

FORMING IONIC COMPOUNDS Extra Help with Forming Ionic Compounds: � Review � What is an ionic bond? � Physical Properties � Common Ionic Bonds � http: //www. education. uoit. ca/lordec/ID_LORDEC/ionic_c ompounds/law_ionic_compounds. swf

SUPPORTING DOCUMENTS ON Science 10 Textbook � Pages: 134 -135, 137 Chapter 4 Power Point for ON Science 10 � Slides: 3 -13 Interactive Videos: ‘Subatomic Particles and Isotopes’ Chapter 4 Notebook - pages 3 -7 Websites: http: //www. education. uoit. ca/lordec/ID_LORDEC/ionic_compoun ds/law_ionic_compounds. swf

HOMEWORK ON Science 10 Textbook � Page #: 134 -135 � Questions: 1 -8

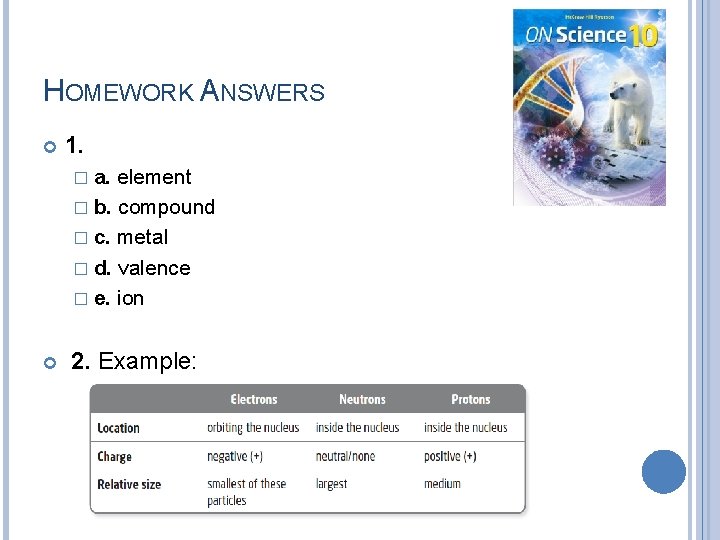
HOMEWORK ANSWERS 1. � a. element � b. compound � c. metal � d. valence � e. ion 2. Example:

HOMEWORK ANSWERS 3. � a. Be � b. 2 � c. 4 � d. 4 � e. 4 � f. 5 4. � a. physical property, qualitative (words, descriptions) � b. chemical property � c. physical property, quantitative (numbers, concrete) � d. chemical property

HOMEWORK ANSWERS 5. � a. 1) potassium and chlorine, 2) oxygen and hydrogen � b. Compound 1 is ionic since an electron is transferred between atoms, and compound 2 is covalent since the atoms share electrons. � c. 1) potassium chloride, KCl, 2) dihydrogen monoxide (water), H 2 O 6. The yellow spheres need to be equal on both sides (e. g. , two more yellow spheres on the right/product side).

HOMEWORK ANSWERS 7. Aluminum, 13 � density = mass/volume � = 10/1. 12 � = 8. 93 8. Example: Three facts—red water caused by nickel tailings that contain iron; mining pollution killed vegetation around Sudbury; chemicals helped rehabilitate the area