SCIENCE 1206 UNIT 1 CHEMISTRY UNIT OUTLINE CHEMISTRY








- Slides: 25

SCIENCE 1206 – UNIT 1 CHEMISTRY

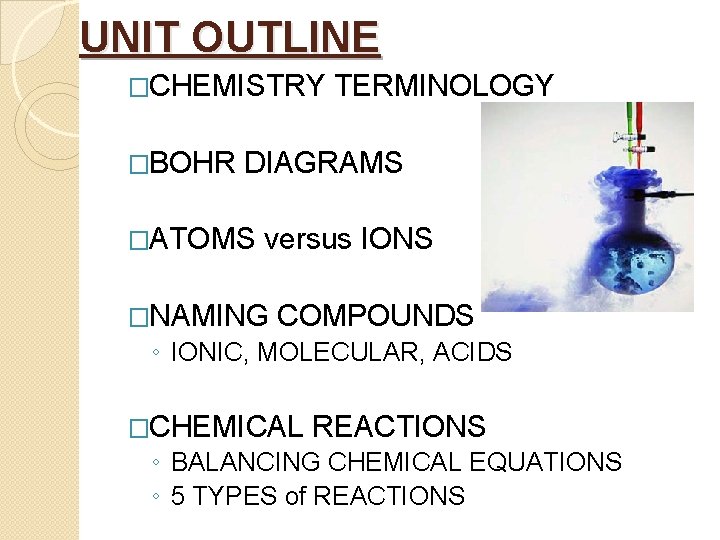
UNIT OUTLINE �CHEMISTRY �BOHR TERMINOLOGY DIAGRAMS �ATOMS versus IONS �NAMING COMPOUNDS ◦ IONIC, MOLECULAR, ACIDS �CHEMICAL REACTIONS ◦ BALANCING CHEMICAL EQUATIONS ◦ 5 TYPES of REACTIONS

IMPORTANT TO KNOW. . . �You �It will get a PERIODIC TABLE!!! is your best friend for this unit! �You will need it each and every day. �Take care of it, cherish it, appreciate it!

CHEMISTRY TERMINOLOGY Matter: Anything that has mass and volume (takes up space). What is not matter? �Energy Mass: The amount of matter an object contains, measured in grams, g.

3 STATES OF MATTER 1. SOLID ◦ Definite volume and shape 2. LIQUID ◦ Definite volume, indefinite shape 3. GAS ◦ Indefinite volume, indefinite shape �Chemistry ◦ ◦ Subscripts (s) - solid (l) - liquid (g) - gas (aq) - aqueous (dissolved in water)

WHAT IS CHEMISTRY? Chemistry is the study of the properties and chemical changes/reactions of matter. Examples of chemical reactions: ◦ Rusting ◦ Burning/Combustion

Physical Property - quality or characteristic of a substance that can be observed WITHOUT a chemical reaction. Examples: ◦ ◦ ◦ ◦ ◦ State of matter Hardness Colour Malleability Ductility Odor Solubility Brittleness Conductivity Melting and Boiling Points

Physical change - a change in state of matter of a substance. Examples: ◦ ◦ ◦ Melting/fusion (s → l) Freezing (l → s) Evaporation (l → g) Condensation (g → l) Sublimation (s → g) Deposition (g → s)

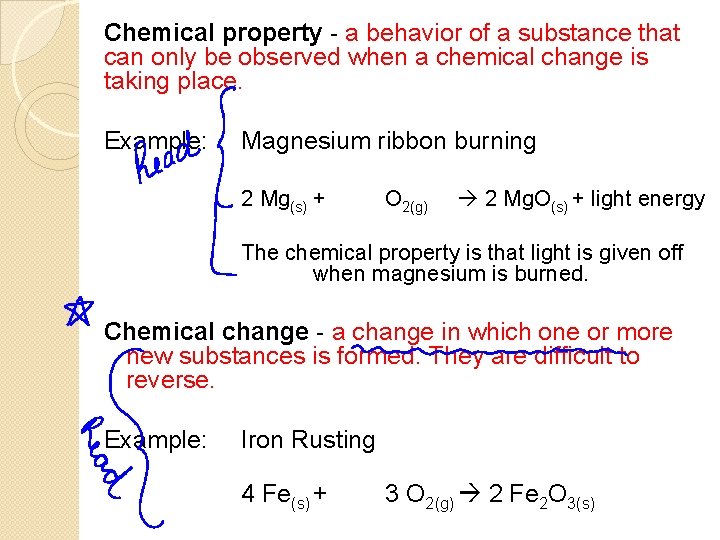
Chemical property - a behavior of a substance that can only be observed when a chemical change is taking place. Example: Magnesium ribbon burning 2 Mg(s) + O 2(g) 2 Mg. O(s) + light energy The chemical property is that light is given off when magnesium is burned. Chemical change - a change in which one or more new substances is formed. They are difficult to reverse. Example: Iron Rusting 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s)

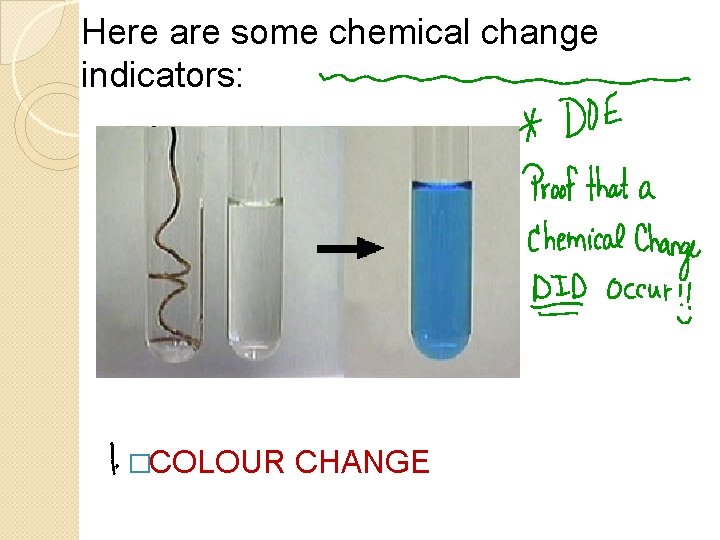
Here are some chemical change indicators: �COLOUR CHANGE

�BUBBLES OF GAS

�SOLID (PRECIPITATE) FORMATION

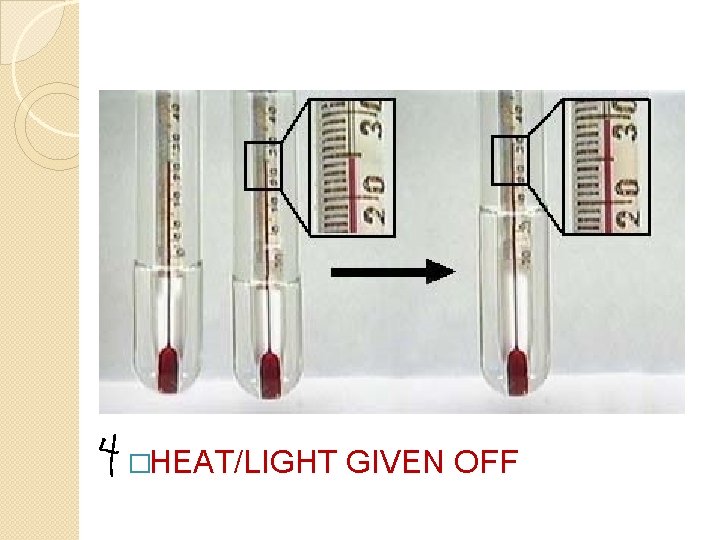
�HEAT/LIGHT GIVEN OFF

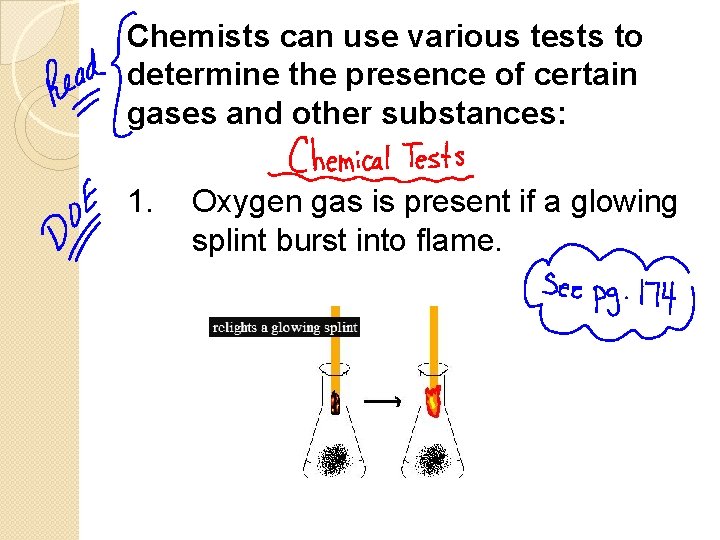
Chemists can use various tests to determine the presence of certain gases and other substances: 1. Oxygen gas is present if a glowing splint burst into flame.

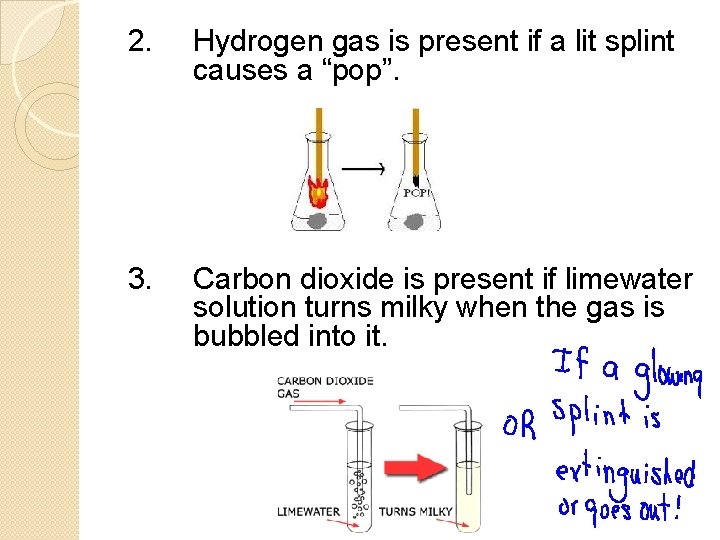
2. Hydrogen gas is present if a lit splint causes a “pop”. 3. Carbon dioxide is present if limewater solution turns milky when the gas is bubbled into it.

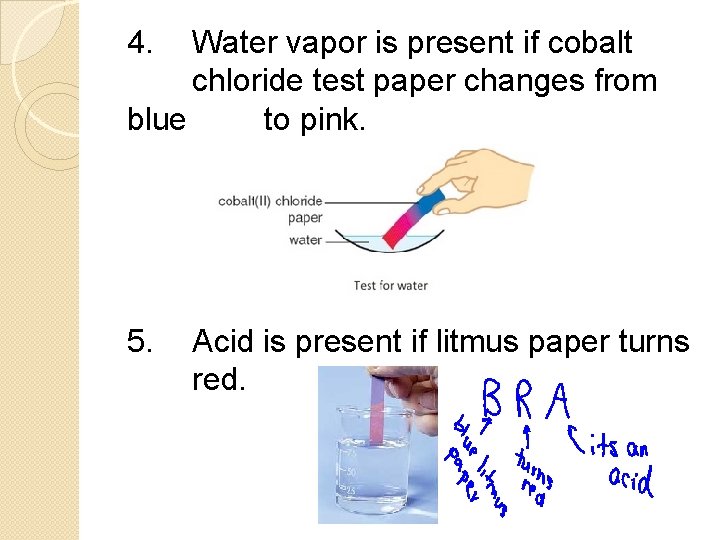
4. Water vapor is present if cobalt chloride test paper changes from blue to pink. 5. Acid is present if litmus paper turns red.

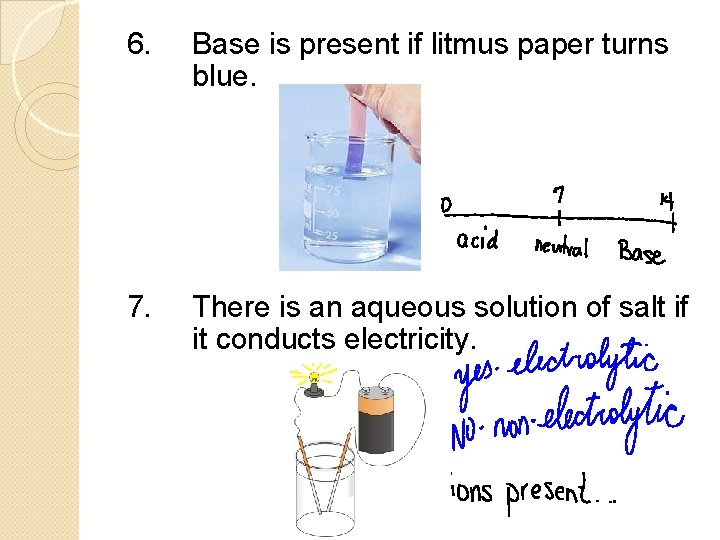
6. Base is present if litmus paper turns blue. 7. There is an aqueous solution of salt if it conducts electricity.

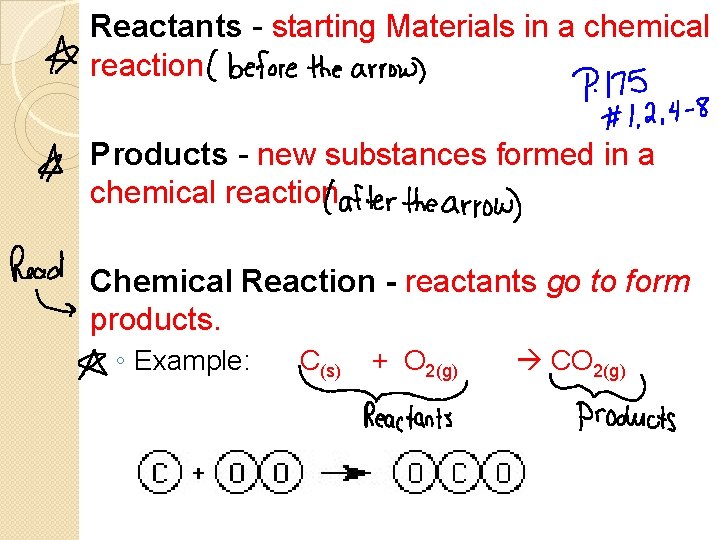
Reactants - starting Materials in a chemical reaction Products - new substances formed in a chemical reaction Chemical Reaction - reactants go to form products. ◦ Example: C(s) + O 2(g) CO 2(g)

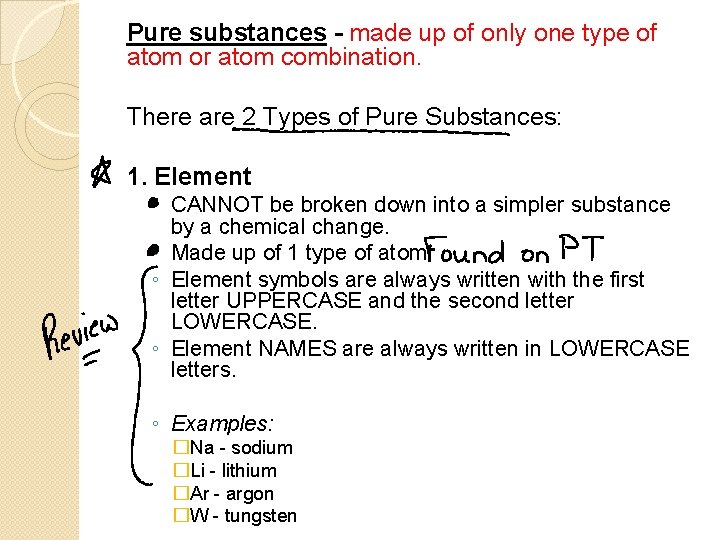
Pure substances - made up of only one type of atom or atom combination. There are 2 Types of Pure Substances: 1. Element ◦ CANNOT be broken down into a simpler substance by a chemical change. ◦ Made up of 1 type of atom. ◦ Element symbols are always written with the first letter UPPERCASE and the second letter LOWERCASE. ◦ Element NAMES are always written in LOWERCASE letters. ◦ Examples: �Na - sodium �Li - lithium �Ar - argon �W - tungsten

2. Compound ◦ CAN be broken down into its elements with a chemical change. . ◦ Made up of two or more different elements are chemically joined together in fixed proportions. ◦ Examples: �Na. Cl �C 12 H 22 O 11 �CH 4 �H 2 O ◦

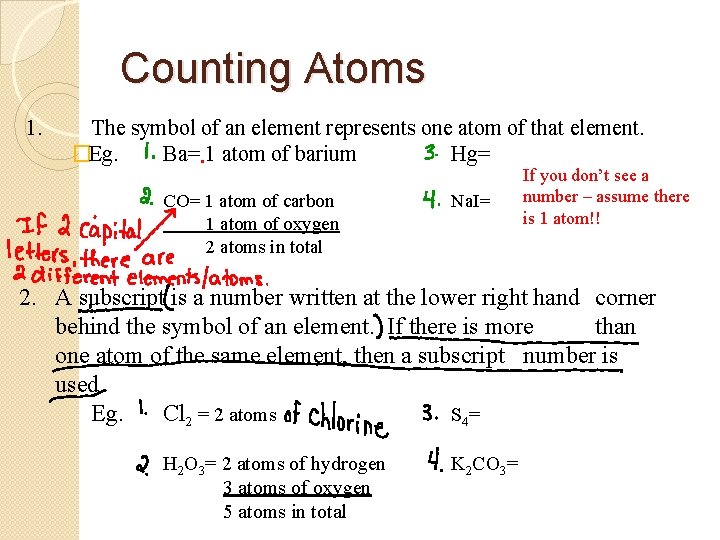
Counting Atoms 1. The symbol of an element represents one atom of that element. �Eg. Ba= 1 atom of barium Hg= CO= 1 atom of carbon 1 atom of oxygen 2 atoms in total Na. I= If you don’t see a number – assume there is 1 atom!! 2. A subscript is a number written at the lower right hand corner behind the symbol of an element. If there is more than one atom of the same element, then a subscript number is used. Eg. Cl 2 = 2 atoms S 4 = H 2 O 3= 2 atoms of hydrogen 3 atoms of oxygen 5 atoms in total K 2 CO 3=

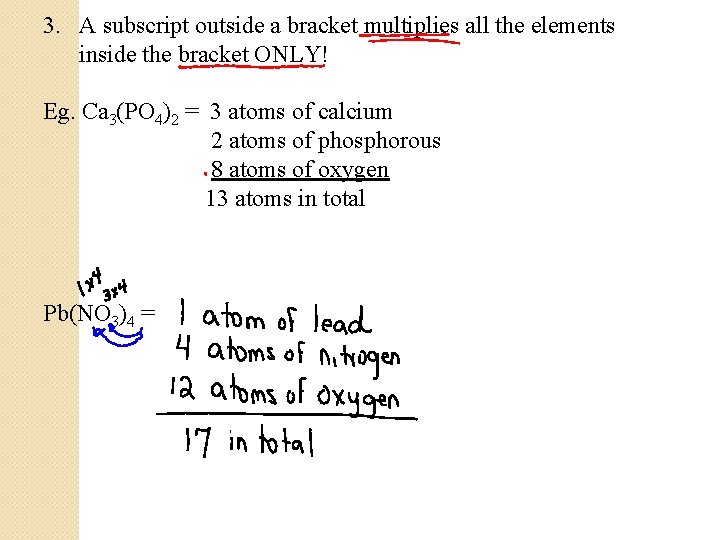
3. A subscript outside a bracket multiplies all the elements inside the bracket ONLY! Eg. Ca 3(PO 4)2 = 3 atoms of calcium 2 atoms of phosphorous 8 atoms of oxygen 13 atoms in total Pb(NO 3)4 =

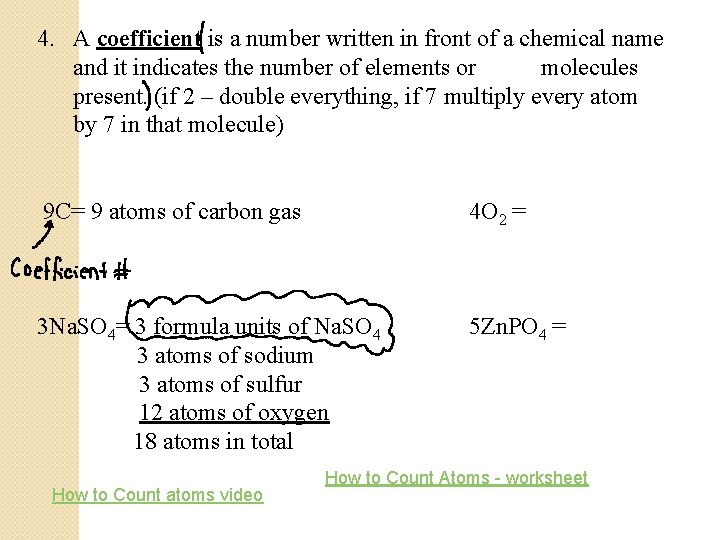
4. A coefficient is a number written in front of a chemical name and it indicates the number of elements or molecules present. (if 2 – double everything, if 7 multiply every atom by 7 in that molecule) 9 C= 9 atoms of carbon gas 4 O 2 = 3 Na. SO 4= 3 formula units of Na. SO 4 3 atoms of sodium 3 atoms of sulfur 12 atoms of oxygen 18 atoms in total 5 Zn. PO 4 = How to Count atoms video How to Count Atoms - worksheet

Remember - Diatomic Molecules �There are 7 elements that are diatomic, or found in pairs, in their natural state. �These are: Meaning if you are counting atoms – you need to remember if these molecules are mentioned you have to remember there are 2, 4 or 8!! ◦ ◦ ◦ ◦ H 2, O 2, F 2, Br 2, I 2, N 2, Cl 2, Also P 4 and S 8 �Memory tool: P S H O F Br I N Cl

Worksheet Counting atoms Quiz # 1 – all chem notes, worksheets, homework questions up to this point!
 Science 1206
Science 1206 1206-1368
1206-1368 1368-1206
1368-1206 Ferret mongols
Ferret mongols A word 1206
A word 1206 Msc circular 1206
Msc circular 1206 Reglamento 1206/01
Reglamento 1206/01 My favourite subject is english
My favourite subject is english What is a quote sandwich
What is a quote sandwich Lesson 2 measurement and scientific tools
Lesson 2 measurement and scientific tools Outline of social science
Outline of social science Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers Gz science chemistry
Gz science chemistry Environmental chemistry science olympiad
Environmental chemistry science olympiad Social science vs natural science
Social science vs natural science Mind map of branches of science
Mind map of branches of science Natural and physical science
Natural and physical science Applied science vs pure science
Applied science vs pure science Rapid change
Rapid change Science fusion digital lessons
Science fusion digital lessons Rule of 70 population growth
Rule of 70 population growth Julie lundquist
Julie lundquist Hard science and soft science
Hard science and soft science Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test