AP Chemistry Electronic Structure of Atoms electronic structure


- Slides: 9

AP Chemistry Electronic Structure of Atoms electronic structure: the arrangement of electrons in an atom quantum mechanics: the physics that correctly describes atoms ?

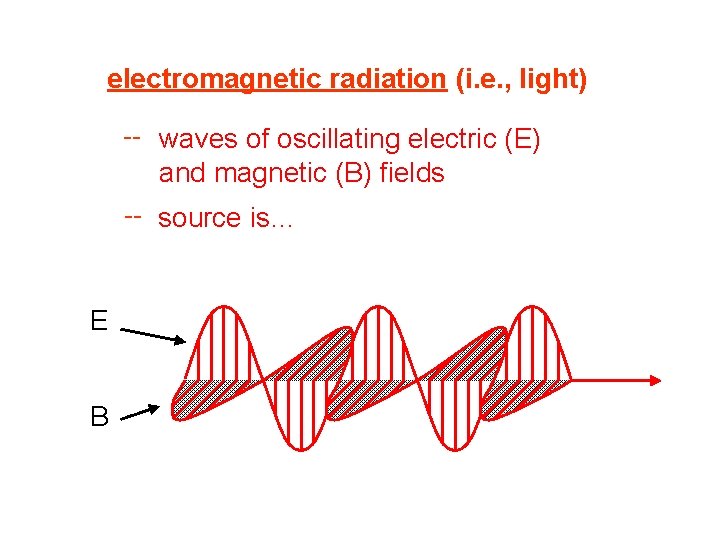
electromagnetic radiation (i. e. , light) -- waves of oscillating electric (E) and magnetic (B) fields -- source is… vibrating electric charges E B

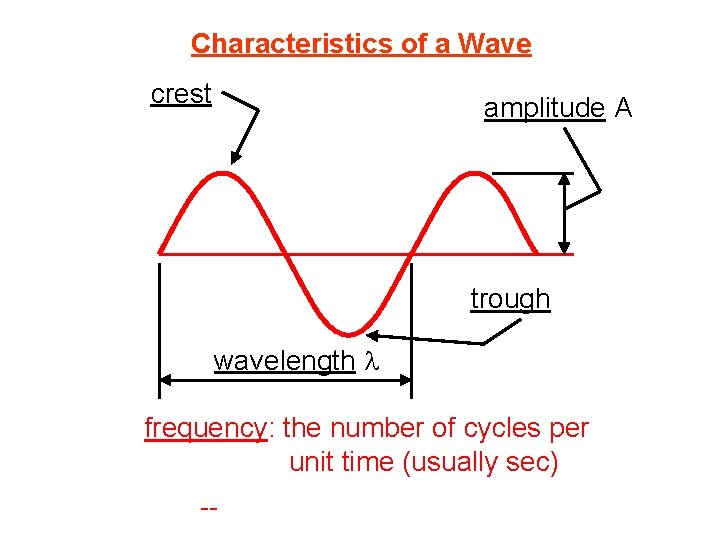
Characteristics of a Wave crest amplitude A trough wavelength l frequency: the number of cycles per unit time (usually sec) -- unit is Hz, or s– 1

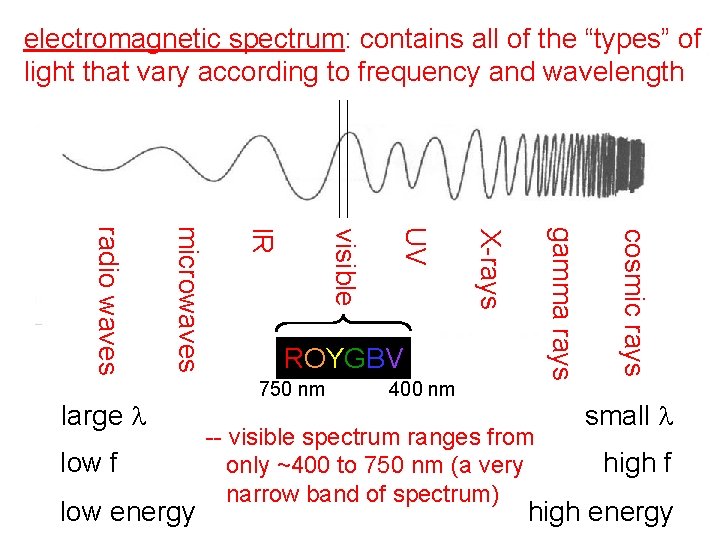
electromagnetic spectrum: contains all of the “types” of light that vary according to frequency and wavelength 750 nm 400 nm -- visible spectrum ranges from only ~400 to 750 nm (a very narrow band of spectrum) cosmic rays gamma rays X-rays UV low energy visible low f IR microwaves radio waves large l ROYGBV small l high f high energy

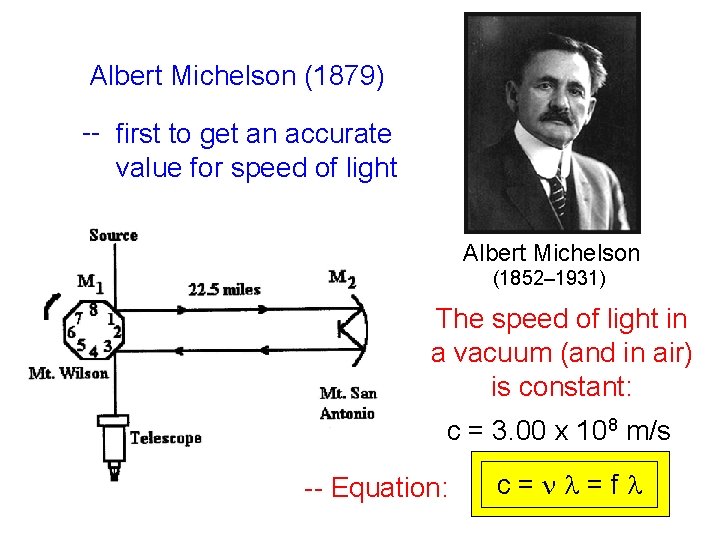
Albert Michelson (1879) -- first to get an accurate value for speed of light Albert Michelson (1852– 1931) The speed of light in a vacuum (and in air) is constant: c = 3. 00 x 108 m/s -- Equation: c=nl=fl

In 1900, Max Planck assumed that energy can be absorbed or released only in certain discrete amounts, which he called quanta. Later, Albert Einstein dubbed a light “particle” that carried a quantum of energy a photon. -- Equation: Max Planck (1858– 1947) E=hn=hf E = energy, in J h = Planck’s constant = 6. 63 x 10– 34 J-s (i. e. , J/Hz) Albert Einstein (1879– 1955)

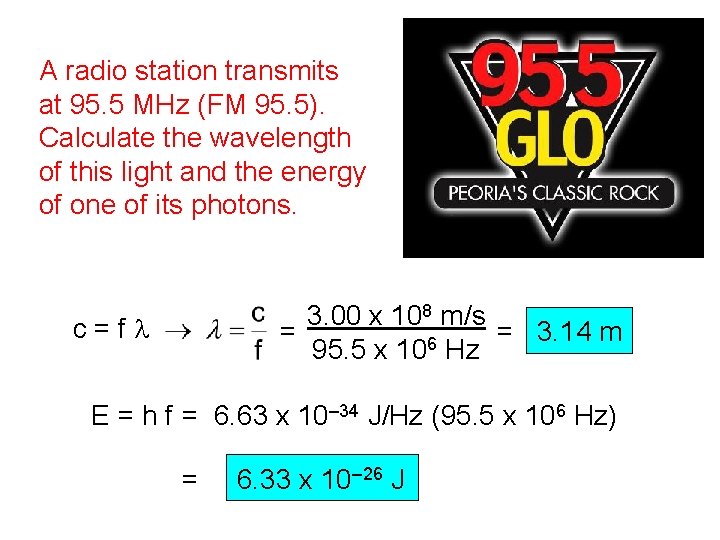
A radio station transmits at 95. 5 MHz (FM 95. 5). Calculate the wavelength of this light and the energy of one of its photons. 3. 00 x 108 m/s = 3. 14 m = 6 95. 5 x 10 Hz c=fl E = h f = 6. 63 x 10– 34 J/Hz (95. 5 x 106 Hz) = 6. 33 x 10– 26 J

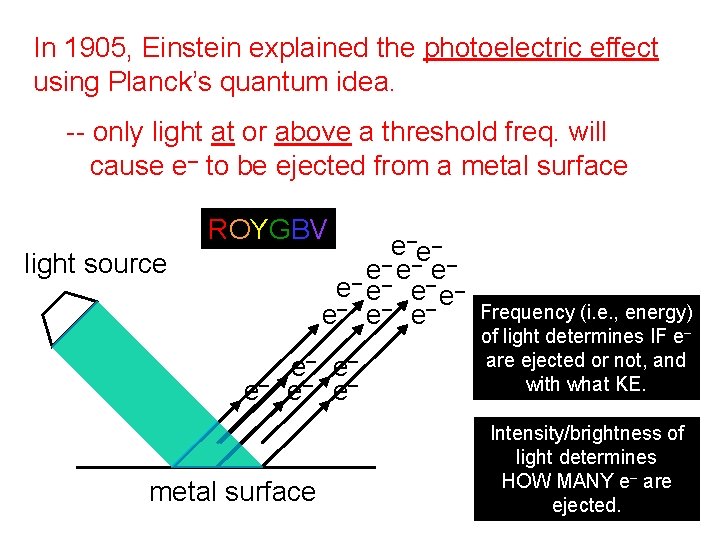
In 1905, Einstein explained the photoelectric effect using Planck’s quantum idea. -- only light at or above a threshold freq. will cause e– to be ejected from a metal surface ROYGBV e–e– – e– e– e– light source e– e– e– metal surface Frequency (i. e. , energy) of light determines IF e– are ejected or not, and with what KE. Intensity/brightness of light determines HOW MANY e– are ejected.

Einstein also expanded Planck’s idea, saying that energy exists only in quanta. Light has both wavelike and particle-like qualities, and. . . so does matter. ?