Chapter 7 Atomic Structure and Periodicity QUESTION Copyright






































- Slides: 44

Chapter 7 Atomic Structure and Periodicity

QUESTION Copyright © Houghton Mifflin Company. All rights reserved. 2

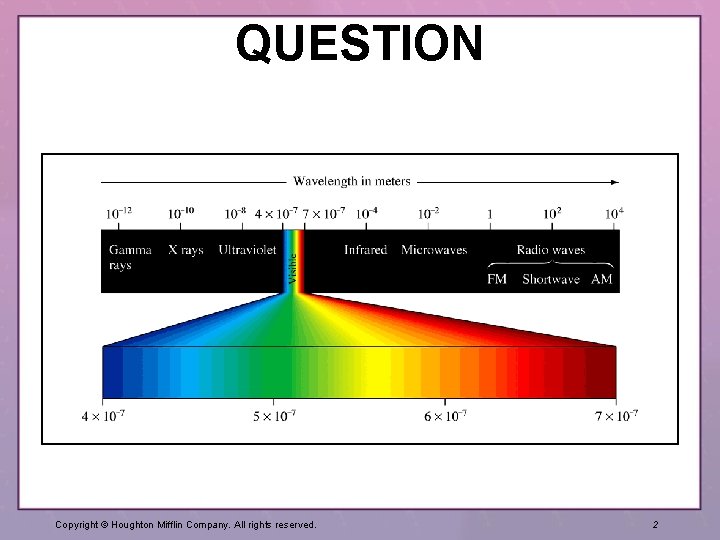
QUESTION (continued) Our understanding of electromagnetic radiation (EM) played a critical role in our understanding of atomic structure. Compare red light, blue light, and x-rays and arrange them in order so that the longest wavelength is first, highest frequency is second, and finally the highest energy is at the end of the list. 1. 2. 3. 4. X-ray; x-rays Red; x-rays; blue Blue; x-rays; red Red; x-rays Copyright © Houghton Mifflin Company. All rights reserved. 3

ANSWER Choice 4 provides the correct listing. Wavelength and frequency are inversely related. Energy is directly related to frequency. Section 7. 1: Electromagnetic Radiation Section 7. 2: The Nature of Matter Copyright © Houghton Mifflin Company. All rights reserved. 4

QUESTION Suppose that a microwave oven uses photons with an energy of 1. 42 10– 23 joules to provide you with a cooked popcorn snack. You have less than two minutes before the corn pops to determine the wavelength of the microwaves. (Note: use Planck’s constant as 6. 626 10– 34 Js. ) 1. 2. 3. 4. 1. 40 cm 2. 14 1010 cm 0. 714 cm This can’t be solved unless you are given the frequency. Copyright © Houghton Mifflin Company. All rights reserved. 5

ANSWER Choice 1 provides the correct response. The equation E = hn can also be written E = hc/l so that l can be determined from the given data. Section 7. 1: Electromagnetic Radiation Section 7. 2: The Nature of Matter Copyright © Houghton Mifflin Company. All rights reserved. 6

QUESTION The wavelength of the laser light that allows you to listen to your favorite tunes on a CD player lies in the red area of the visible spectra. If one mole of the photons delivers 1. 54 105 J, what is the frequency of this useful energy? 1. 2. 3. 4. 2. 59 10– 15 s– 1 3. 86 1014 s– 1 1. 56 1023 s– 1 I don’t know (I only listen to vinyl). Copyright © Houghton Mifflin Company. All rights reserved. 7

ANSWER Choice 2 correctly reports the frequency. The total energy for a mole must be converted to the energy for just one photon (divide by Avogadro’s number) then E = hn could be used to determine n. Section 7. 2: The Nature of Matter Section 7. 3: The Atomic Spectrum of Hydrogen Copyright © Houghton Mifflin Company. All rights reserved. 8

QUESTION While you are sitting in the dentist’s chair you are being hit with several forms of electromagnetic radiation. The bright light is sending energy in the visible region, the body heat of the dentist is emanating energy in the infrared region; perhaps a radio is playing and some energy in the radio waves region approach your ears; and you are about to receive an x-ray. Which arrangement places the EM waves in proper order with respect to increasing energy per photon? 1. 2. 3. 4. Visible; infrared, radio; x-rays X-rays; visible; radio; infrared Infrared; radio; visible; x-rays Radio; infrared; visible; x-rays Copyright © Houghton Mifflin Company. All rights reserved. 9

ANSWER Choice 4 sorts the EM in order of increasing energy per photon. Energy is directly proportional to frequency. The arrangement is also in order of increasing frequency. Section 7. 1: Electromagnetic Radiation Section 7. 2: The Nature of Matter Copyright © Houghton Mifflin Company. All rights reserved. 10

QUESTION Diffraction is known to be a characteristic of waves. Yet, when electrons, with mass, pass through the openings in crystals a diffraction pattern appears. Which of the following is consistent with this information? Copyright © Houghton Mifflin Company. All rights reserved. 11

QUESTION (continued) 1. Diffraction patterns are caused as the waves exhibit destructive interference only. Electrons annihilate each other. 2. Electrons and EM radiation produce diffraction patterns meaning mass must have wavelike properties and waves must have mass. 3. Electrons can exhibit diffraction patterns because as they pass through the regular patterns of openings in crystals they get lodged in the crystal causing light to be emitted. 4. Mass and wavelength are directly related. The small mass of an electron allows it to have a small enough wavelength to cause diffraction Copyright © Houghton Mifflin Company. All rights reserved. 12

ANSWER Choice 2 is consistent with known observations. The work of De. Broglie, Davisson and Germer helped to develop the concept of wave-particle duality. Section 7. 2: The Nature of Matter Copyright © Houghton Mifflin Company. All rights reserved. 13

QUESTION If you dropped your textbook, you might claim that “ it is difficult to hold on to waves” with some validity. After all, mass does possess wave-like properties. However, what would be the wavelength of a 855 g textbook moving 9. 8 m/s? 1. 2. 3. 4. 7. 91 10– 38 m 7. 75 10– 37 m 7. 91 10– 35 m I don’t see how something with mass could have a wavelength. Copyright © Houghton Mifflin Company. All rights reserved. 14

ANSWER Choice 3 provides the correct value. The wave-particle duality equation developed by De. Broglie should be used here after the mass has been converted to kg ( 855 1/1 000). Section 7. 2: The Nature of Matter Copyright © Houghton Mifflin Company. All rights reserved. 15

QUESTION The familiar rainbow of colors often seen following a rain storm has some important aspects in common with the visible light detected when a sample of H 2 is excited by high voltage electricity. Of the following statements, which provides an accurate comparison? 1. Both consist of EM radiation, and both can provide all the visible colors detected by humans. 2. Rainbows are formed from visible EM radiation, however the spectrum in rainbows does have small gaps while the spectrum from hydrogen is continuous. 3. The visible H 2 spectrum is discontinuous unless the electricity is high enough voltage to cause more excitation that allows the spectrum to become continuous. 4. Rainbows provide a continuous spectrum, while H 2 provides a discontinuous spectrum. Both are in the visible EM range. Copyright © Houghton Mifflin Company. All rights reserved. 16

ANSWER Choice 4 provides an accurate comparison of the visible spectra for rainbows and excited H 2. The cause of the discontinuous nature of the H 2 spectra was a major observation connected to the quantum theory of atomic structure. Section 7. 3: The Atomic Spectrum of Hydrogen Copyright © Houghton Mifflin Company. All rights reserved. 17

QUESTION Of the following, which provides an accurate statement about the importance of the discrete line spectrum of H 2? 1. Some aspect of hydrogen’s structure must allow electrons to be attracted to the nucleus in such a way to have energy available from the visible spectrum. 2. The discontinuous nature of the spectrum indicates that electrons somehow have destructive interference. 3. Since only certain wavelengths are observed, electrons must only have certain allowed energy levels around the nucleus. 4. The quanta of energy associated with an electron changing energy levels must be indirectly related to its frequency. Copyright © Houghton Mifflin Company. All rights reserved. 18

ANSWER Choice 3 is the hypothesis scientists formed as a result of their observations. The difference in the energy levels was shown by the light emitted. Since the light was emitted only in discrete wavelengths it indicated that electron energy levels are quantized. Section 7. 3: The Atomic Spectrum of Hydrogen Copyright © Houghton Mifflin Company. All rights reserved. 19

QUESTION According to Bohr’s calculation, the energy for an electron in a hydrogen atom’s first energy level is – 2. 178 10– 18 J. What would be the energy of an electron, of the same electron, in the third level? 1. 2. 3. 4. – 6. 534 10– 18 J – 2. 420 10– 19 J – 1. 960 10– 17 J Without more information I am unable to solve this. Copyright © Houghton Mifflin Company. All rights reserved. 20

ANSWER Choice 2 indicates that the energy of the electron in the third level is higher (less negative) by 9 times. The squared value of the energy level is inversely related to the energy of an electron in the atom. Section 7. 4: The Bohr Model Copyright © Houghton Mifflin Company. All rights reserved. 21

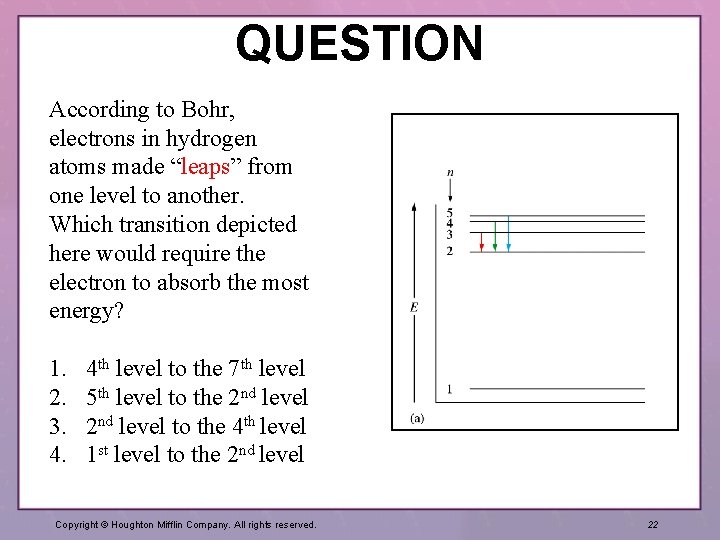
QUESTION According to Bohr, electrons in hydrogen atoms made “leaps” from one level to another. Which transition depicted here would require the electron to absorb the most energy? 1. 2. 3. 4. 4 th level to the 7 th level 5 th level to the 2 nd level to the 4 th level 1 st level to the 2 nd level Copyright © Houghton Mifflin Company. All rights reserved. 22

ANSWER Choice 4 relates the correct relationship between energy level number and gaps between levels. Note that the differences between energy level decreases as the level number increases. Section 7. 4: The Bohr Model Copyright © Houghton Mifflin Company. All rights reserved. 23

QUESTION Schrodinger and Heisenberg interpreted electron distribution in atoms with a quantum momentum and probability. This revolutionary view could be summarized accurately by which of the following? 1. The probability for finding an electron (e-) is greatest near the nucleus, but the radial probability increases to a maximum then decreases. 2. The orbital of an electron must replace the term orbit due to uncertainties in its position. The orbital is where the e– may be found 50% of the time. 3. The volume of a particular sphere of e– movement around the nucleus increases with distance; therefore, the total e– probability must decrease. 4. Like Heisenberg, I am uncertain. Copyright © Houghton Mifflin Company. All rights reserved. 24

ANSWER Choice 1 contains the correct reasoning in this revolutionary treatment of e– and atoms. Note that as the distance from the nucleus increases the probability of finding an e– at a certain position does decrease. However, if we sum the probabilities over that volume, the total probability reaches a maximum (basically the atomic radius), then decreases. Note the view shown in Figure 7. 12 on page 293. Section 7. 5: The Quantum Mechanical Model of the Atom Copyright © Houghton Mifflin Company. All rights reserved. 25

QUESTION One key result of the Schrodinger equation for general chemistry is the ability to use quantum numbers to discuss and predict the distribution of energy among an atom’s electrons. This is done through a series of quantum numbers. How many total orbitals should be present for electrons in the fourth energy level? 1. 2. 3. 4. 4 7 16 32 Copyright © Houghton Mifflin Company. All rights reserved. 26

ANSWER Choice 3 correctly predicts the value, although that does not mean that all would be occupied in a given atom or ion. Section 7. 6: Quantum Numbers Copyright © Houghton Mifflin Company. All rights reserved. 27

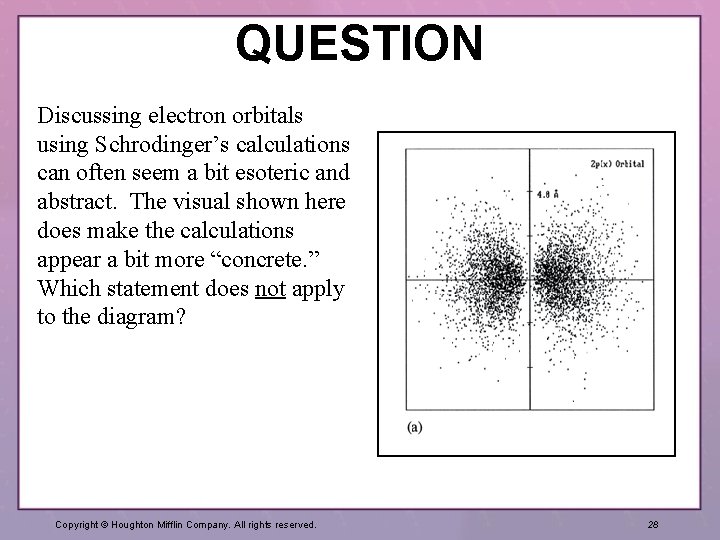
QUESTION Discussing electron orbitals using Schrodinger’s calculations can often seem a bit esoteric and abstract. The visual shown here does make the calculations appear a bit more “concrete. ” Which statement does not apply to the diagram? Copyright © Houghton Mifflin Company. All rights reserved. 28

QUESTION (continued) 1. The dots shown represent the probability for one electron (each dot is not an e–). 2. Although the diagram shows two lobes, it is still just one orbital. 3. The representation has a single node. 4. This diagram depicts an orbital that is degenerate with a 3 p orbital. Copyright © Houghton Mifflin Company. All rights reserved. 29

ANSWER Choice 4 does not match known information pertinent to this diagram. Degenerate refers to orbitals that have the same energy. With hydrogen, the single electron would have to have more energy to occupy the 3 p orbital. Degenerate orbitals in hydrogen all have the same value for n. Section 7. 7: Orbital Shapes and Energies Copyright © Houghton Mifflin Company. All rights reserved. 30

QUESTION Two electrons in an atom of helium have the same value of n, l, and m. It follows then, that… 1. the two electrons can only be in the first level (1 s). 2. Heisenberg’s uncertainty principle works because we can never simultaneously know both the exact position and momentum of electrons. So, with the small level of uncertainty both electrons can have those three values. 3. The two electrons must have an opposite spin. 4. The electrons must now be in separate orbitals. Copyright © Houghton Mifflin Company. All rights reserved. 31

ANSWER Choice 3 is correct, based on Pauli’s Exclusion Principle. No two electrons in atom may have the same two quantum numbers. The quality of spin (either assigned +½ or –½) allows the fourth quantum number to distinguish the two electrons. Section 7. 8: Electron Spin and the Pauli Principle Copyright © Houghton Mifflin Company. All rights reserved. 32

QUESTION Quantum numbers, of course, are applied to atoms other than hydrogen. When examining electron energy levels and their distribution in polyelectronic atoms, what key precept must be kept in mind? 1. The penetration effect predicts that the value of n determines how close an e– may be to the nucleus. 2. The penetration toward the nucleus for an e– is the same as its most probable distance from the nucleus. 3. The electron treatment should take into account the nuclear pull and the other electron repulsions. 4. The electron treatment is based on penetration toward the nucleus, which is based on its four quantum numbers. Copyright © Houghton Mifflin Company. All rights reserved. 33

ANSWER Choice 3 takes into account the two major factors for the energy of an electron and the electron’s attraction toward the nucleus. Penetration toward the + nucleus is a key factor, but other negative electrons will repel each electron. The diagram in Figure 7. 21 may be helpful to review. Section 7. 9: Polyelectronic Atoms Copyright © Houghton Mifflin Company. All rights reserved. 34

QUESTION Using Hund’s rule and the Aufbau principle, compare the number of unpaired electrons in one atom each of C, O and Ne. 1. All are in the same row on the periodic table so they have the same number of unpaired electrons. 2. C has the fewest; O has the most, and Ne has none 3. O has two; C and Ne have none 4. C and O have two; Ne has none Copyright © Houghton Mifflin Company. All rights reserved. 35

ANSWER Choice 4 produces the correct comparison. Note that carbon’s two “p” electrons, based on Hund’s rule would be in separate orbitals thus they would both be unpaired. Oxygen also has two unpaired “p” electrons, since three orbitals must accommodate six electrons before electrons enter a new level. Section 7. 11: The Aufbau Principle and the Periodic Table Copyright © Houghton Mifflin Company. All rights reserved. 36

QUESTION Arrange the following metals in order from most unpaired electrons to the least considering only their 3 d sublevel: Cu; Mn; Cr; Sc 1. 2. 3. 4. Cu; Mn; Cr; Sc Cr and Mn are the same; then Sc, and then Cu Mn; Cr; then Cu and Sc which have fewer but are the same Cr and Mn are the same; then Sc and Cu, which have fewer but are the same Copyright © Houghton Mifflin Company. All rights reserved. 37

ANSWER Choice 2 provides the proper sorting from most unpaired electrons in the 3 d to least among those four metals. Remember that the question for both Cr and Cu must be examined carefully due to the 4 s electron being so close in energy to the 3 d and the increased stability of half-filled sublevels. Section 7. 11: The Aufbau Principle and the Periodic Table Copyright © Houghton Mifflin Company. All rights reserved. 38

QUESTION Sodium and aluminum both have one unpaired electron in their neutral ground state atoms. Why is the first ionization energy of Na lower than Al, but the second ionization of energy of Al lower than the second ionization energy of Na? 1. The smaller size of Al makes it difficult to ionize at first, but after losing one e–, the other atoms can expand more and make it easier to ionize a second e–. 2. The 3 s electrons of Na does a better job of shielding than the 3 p electron of Al. 3. The second e– taken from Na must be taken from a new lower level; the second e– from Al is in same level as before. 4. The first e– ionized from both is single, but the second one in Na is paired, while the second in Al is still single. Copyright © Houghton Mifflin Company. All rights reserved. 39

ANSWER Choice 3 is correct. When removing the first e– from the larger Na atom, less energy is needed than the smaller Al atom. However, removing the next electron from Na means taking an e– from the second energy level. This is closer to the nucleus making it more difficult than Al’s second e–, which is still in the third energy level. Section 7. 12: Periodic Trends in Atomic Properties Copyright © Houghton Mifflin Company. All rights reserved. 40

QUESTION Which of the following statements correctly interprets the relationship between ionization energy (IE) atomic radius and electron affinity when comparing two atoms? (Answer this thinking about the general trends rather than some exceptions. ) 1. When atomic radius increases, IE decreases and electron affinity becomes less negative. 2. When atomic radius increases, IE increases and electron affinity becomes more negative. 3. When atomic radius increases, IE decreases and electron affinity becomes more negative. 4. When atomic radius increases, IE increases and electron affinity becomes less negative. Copyright © Houghton Mifflin Company. All rights reserved. 41

ANSWER Choice 1 indicates the proper relationship when comparing two atoms. As atomic radius increases the negative electron is further from the positive nucleus, which decreases their attraction, thus lowering the IE. Since electron affinity also deals with the attraction of the nucleus for electrons (new ones), a large size would generally decrease the attraction, thus causing the affinity to be less exothermic (less negative. ) Section 7. 12: Periodic Trends in Atomic Properties Copyright © Houghton Mifflin Company. All rights reserved. 42

QUESTION Chemical reducing agents tend to give up electrons easily. Based on their first ionization energy Na should give up its outer e– more easily than Li. In fact, this is true unless the two are being compared in aqueous solution. What is the best explanation for this apparent anomaly? 1. Lithium ions are larger than sodium ions. 2. In water, Li+ ion behaves like He and evaporates away thus not having the opportunity to regain its electron. Na+ stay in the water and can regain their electron. 3. The larger charge density of the Li+ attracts water stronger than Na+ thus enhancing the reaction that leads to forming the ion. 4. This still does not seem logical, am I missing something? Copyright © Houghton Mifflin Company. All rights reserved. 43

ANSWER Choice 3 provides the subtle reasoning behind this apparent anomaly. Both Li and Na become +1 ions, but the charge in a Li ion is in a much smaller volume. Thus, the greater charge density attracts water (hydration. ) Since this is greater for Li than Na, it increases the reaction going from atom to ion. Section 7. 13: The Properties of a Group: The Alkali Metals Copyright © Houghton Mifflin Company. All rights reserved. 44