Atomic Structure and Periodicity Chapter 7 1 Copyright


![Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+ Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+](https://slidetodoc.com/presentation_image_h2/1e8f4a105f9a091d86bf4284a4b51273/image-47.jpg)
- Slides: 66

Atomic Structure and Periodicity Chapter 7 1 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

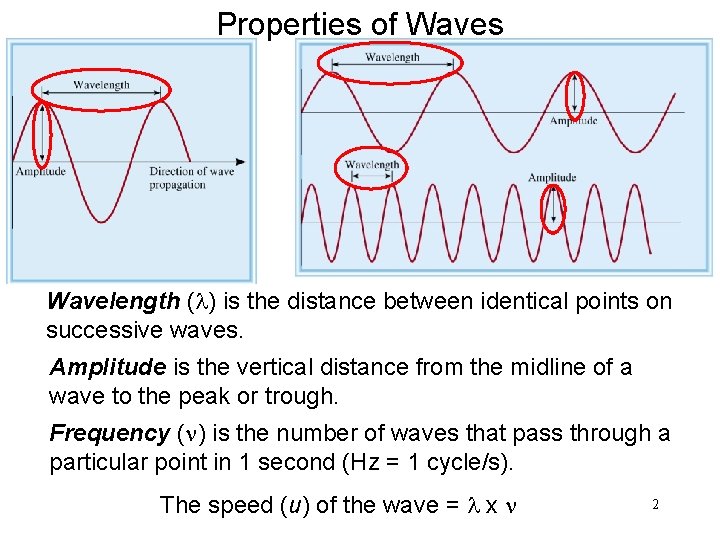
Properties of Waves Wavelength (l) is the distance between identical points on successive waves. Amplitude is the vertical distance from the midline of a wave to the peak or trough. Frequency (n) is the number of waves that pass through a particular point in 1 second (Hz = 1 cycle/s). The speed (u) of the wave = l x n 2

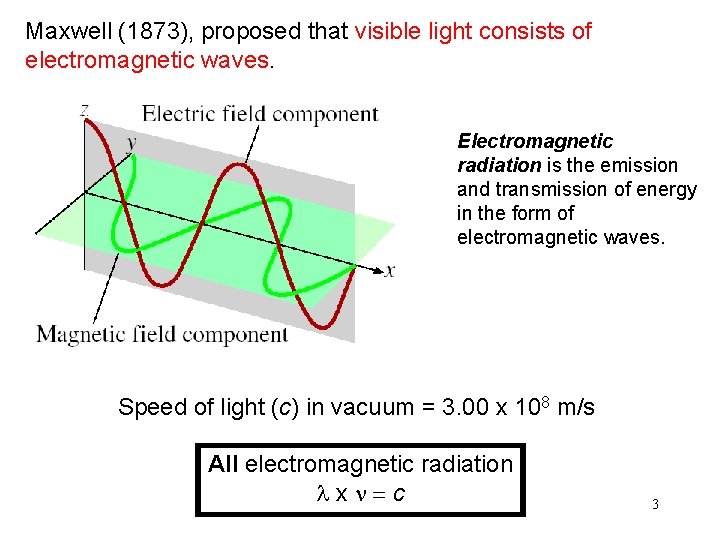
Maxwell (1873), proposed that visible light consists of electromagnetic waves. Electromagnetic radiation is the emission and transmission of energy in the form of electromagnetic waves. Speed of light (c) in vacuum = 3. 00 x 108 m/s All electromagnetic radiation lxn=c 3

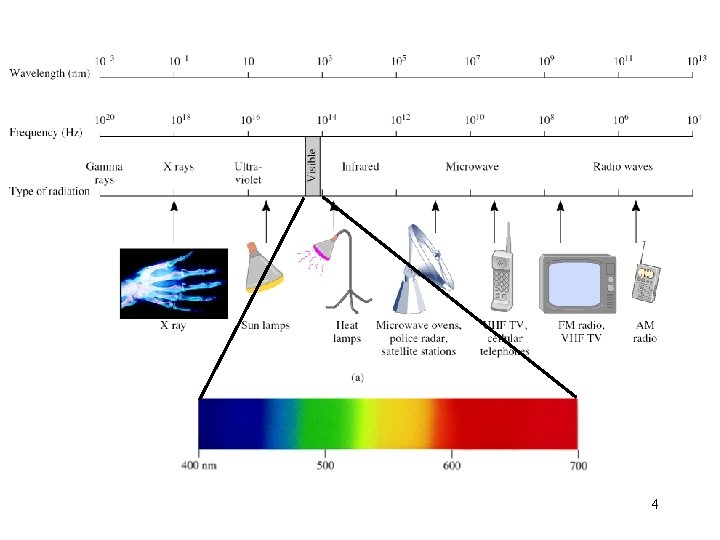
4

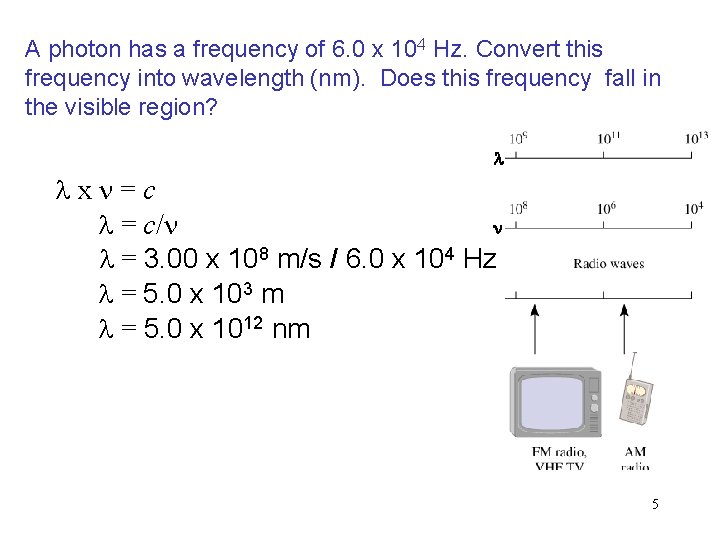
A photon has a frequency of 6. 0 x 104 Hz. Convert this frequency into wavelength (nm). Does this frequency fall in the visible region? l lxn=c n l = c/n l = 3. 00 x 108 m/s / 6. 0 x 104 Hz l = 5. 0 x 103 m l = 5. 0 x 1012 nm 5

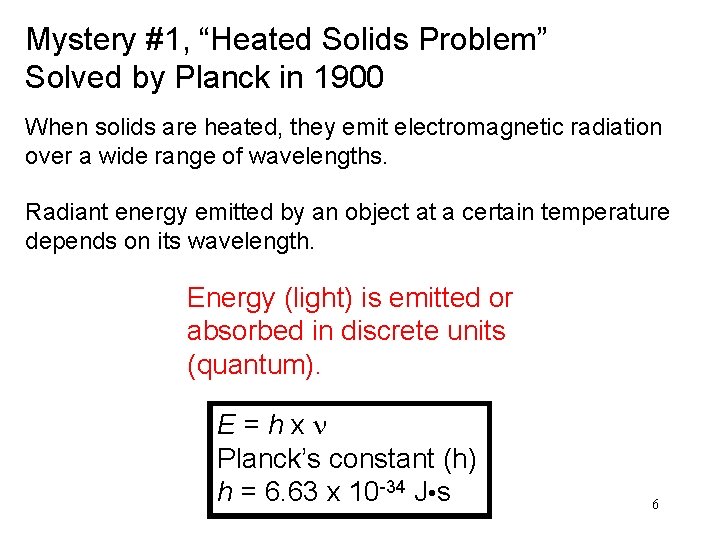
Mystery #1, “Heated Solids Problem” Solved by Planck in 1900 When solids are heated, they emit electromagnetic radiation over a wide range of wavelengths. Radiant energy emitted by an object at a certain temperature depends on its wavelength. Energy (light) is emitted or absorbed in discrete units (quantum). E=hxn Planck’s constant (h) h = 6. 63 x 10 -34 J • s 6

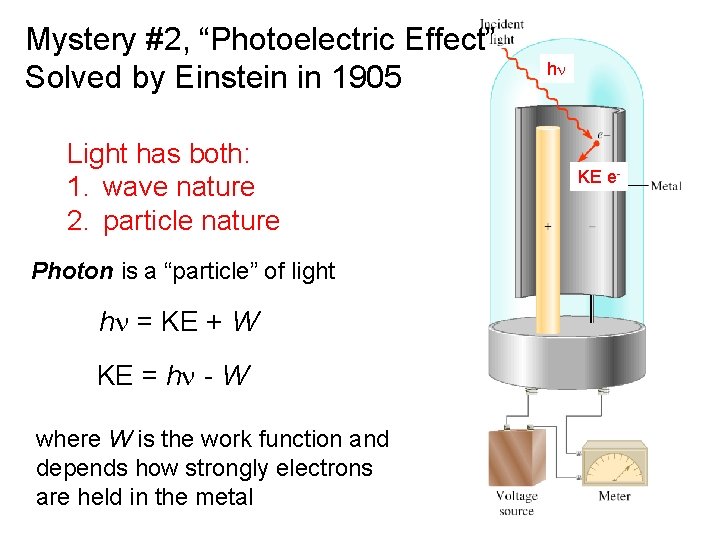
Mystery #2, “Photoelectric Effect” Solved by Einstein in 1905 Light has both: 1. wave nature 2. particle nature hn KE e- Photon is a “particle” of light hn = KE + W KE = hn - W where W is the work function and depends how strongly electrons are held in the metal 7

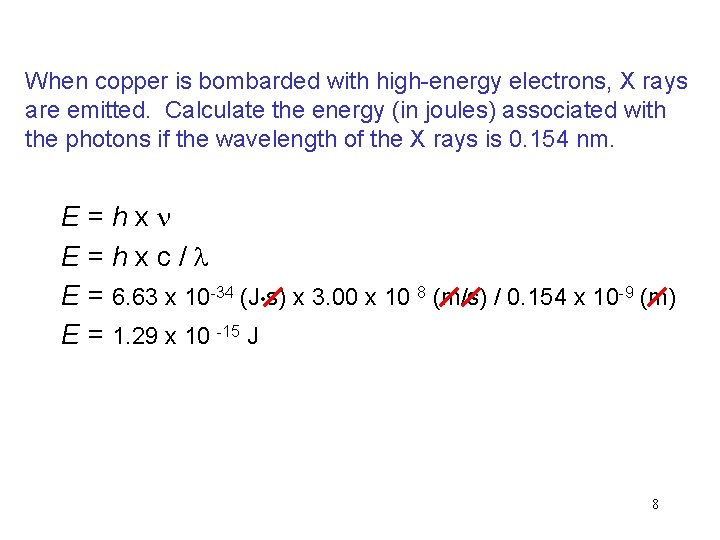
When copper is bombarded with high-energy electrons, X rays are emitted. Calculate the energy (in joules) associated with the photons if the wavelength of the X rays is 0. 154 nm. E=hxn E=hxc/l E = 6. 63 x 10 -34 (J • s) x 3. 00 x 10 8 (m/s) / 0. 154 x 10 -9 (m) E = 1. 29 x 10 -15 J 8

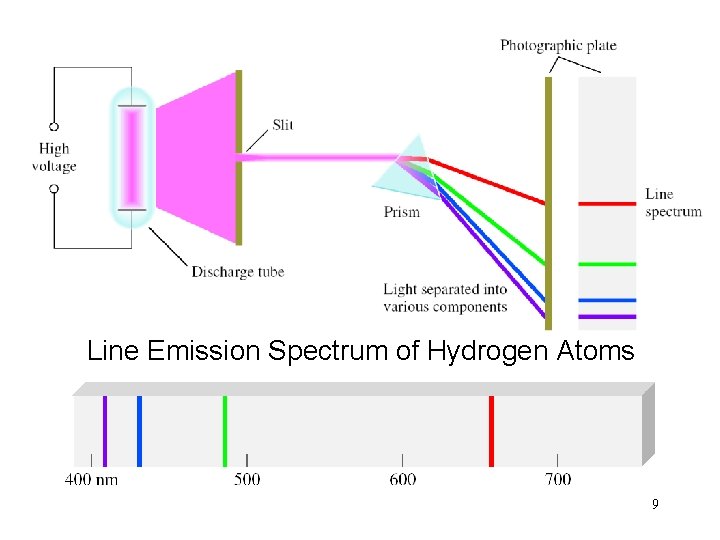
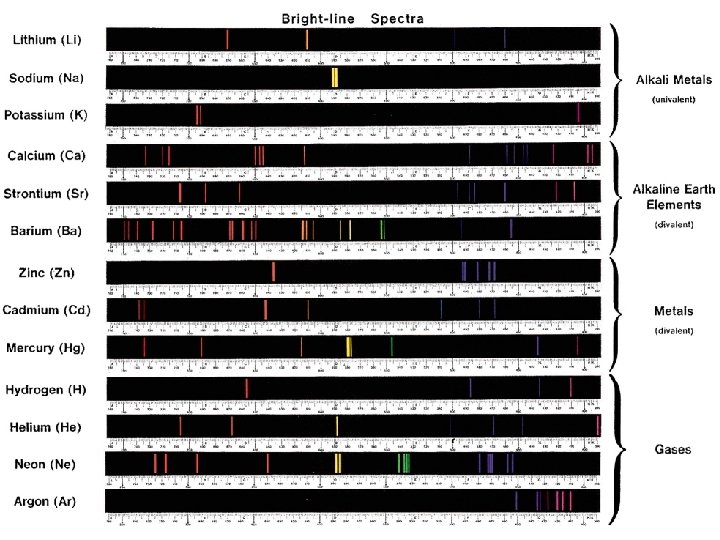
Line Emission Spectrum of Hydrogen Atoms 9

10

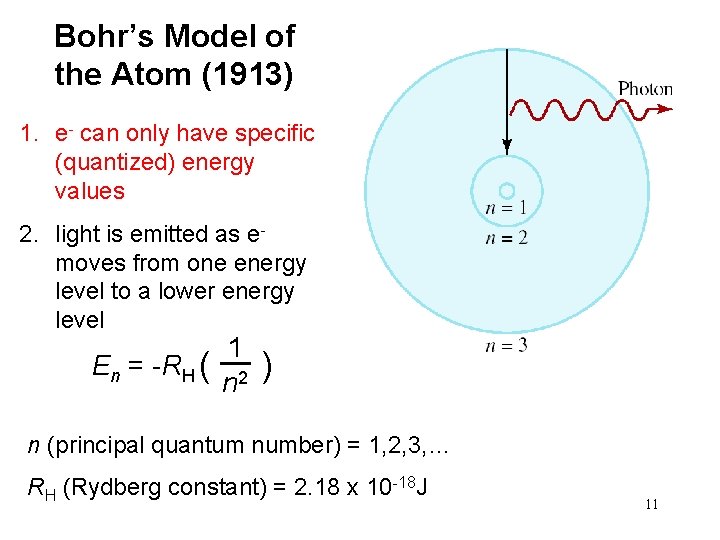
Bohr’s Model of the Atom (1913) 1. e- can only have specific (quantized) energy values 2. light is emitted as emoves from one energy level to a lower energy level En = -RH ( 1 n 2 ) n (principal quantum number) = 1, 2, 3, … RH (Rydberg constant) = 2. 18 x 10 -18 J 11

E = hn 12

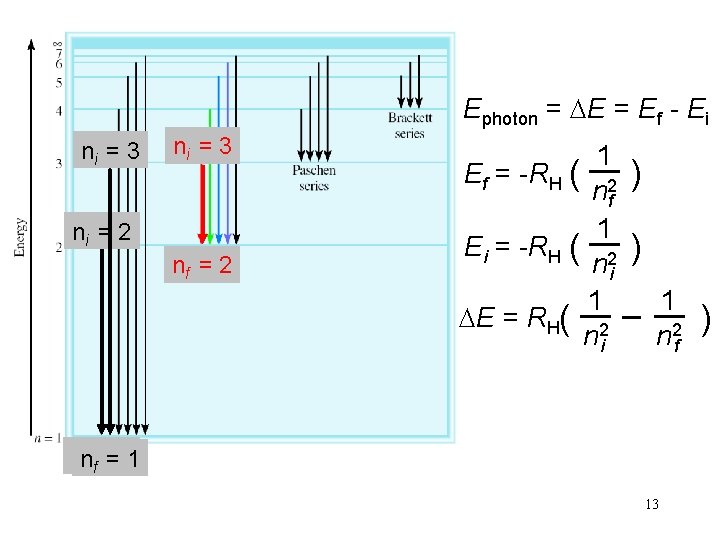
Ephoton = DE = Ef - Ei ni = 3 ni = 2 nf = 2 1 Ef = -RH ( 2 nf 1 Ei = -RH ( 2 ni 1 DE = RH( 2 ni ) ) 1 n 2 f nnf f==11 13 )

14

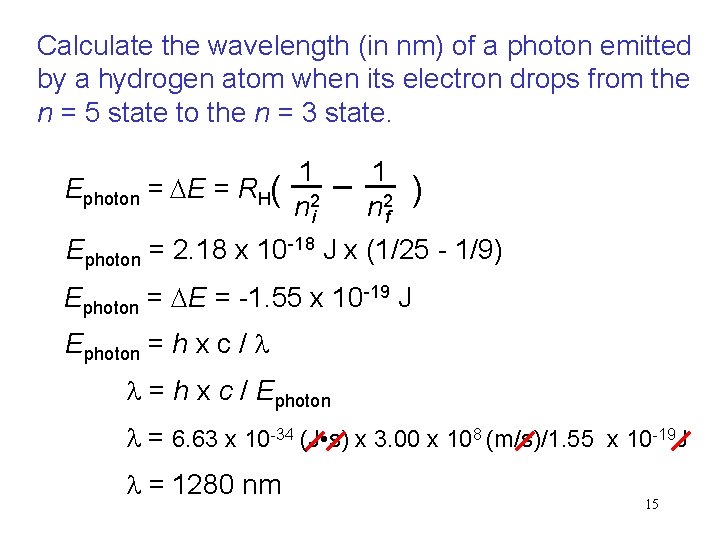
Calculate the wavelength (in nm) of a photon emitted by a hydrogen atom when its electron drops from the n = 5 state to the n = 3 state. Ephoton = DE = RH( 1 n 2 i 1 n 2 f ) Ephoton = 2. 18 x 10 -18 J x (1/25 - 1/9) Ephoton = DE = -1. 55 x 10 -19 J Ephoton = h x c / l l = h x c / Ephoton l = 6. 63 x 10 -34 (J • s) x 3. 00 x 108 (m/s)/1. 55 x 10 -19 J l = 1280 nm 15

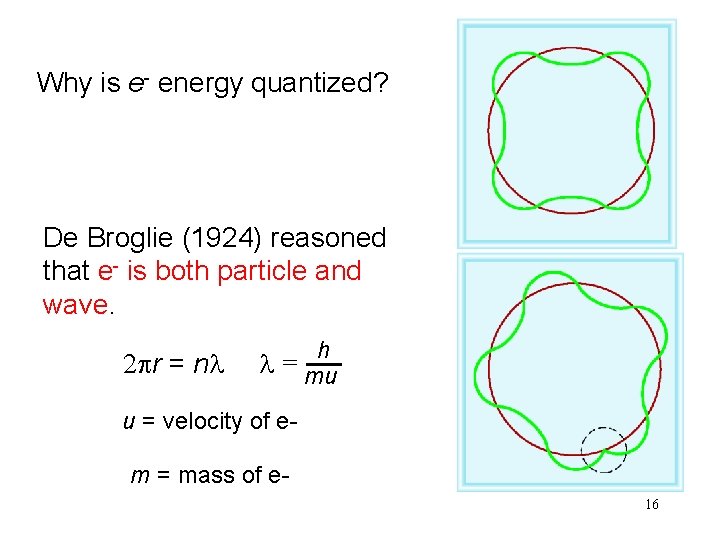
Why is e- energy quantized? De Broglie (1924) reasoned that e- is both particle and wave. 2 pr = nl h l = mu u = velocity of em = mass of e 16

What is the de Broglie wavelength (in nm) associated with a 2. 5 g Ping-Pong ball traveling at 15. 6 m/s? l = h/mu h in J • s m in kg u in (m/s) l = 6. 63 x 10 -34 / (2. 5 x 10 -3 x 15. 6) l = 1. 7 x 10 -32 m = 1. 7 x 10 -23 nm 17

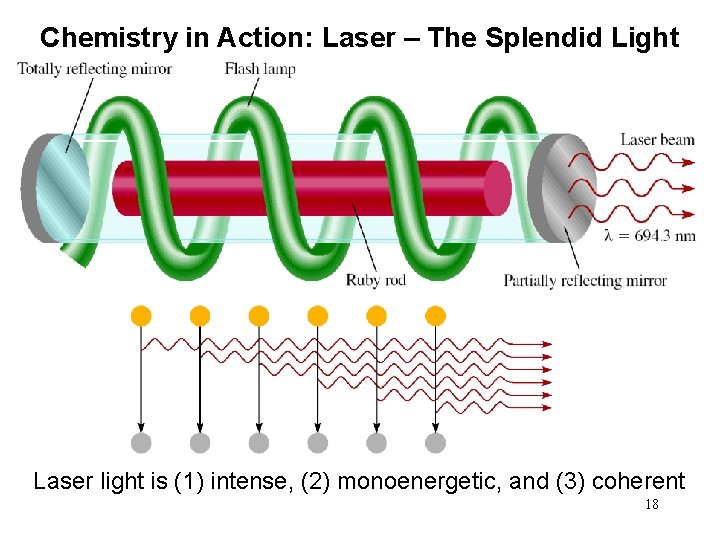
Chemistry in Action: Laser – The Splendid Light Laser light is (1) intense, (2) monoenergetic, and (3) coherent 18

Chemistry in Action: Electron Microscopy le = 0. 004 nm Electron micrograph of a normal red blood cell and a sickled red blood cell from the same person STM image of iron atoms on copper surface 19

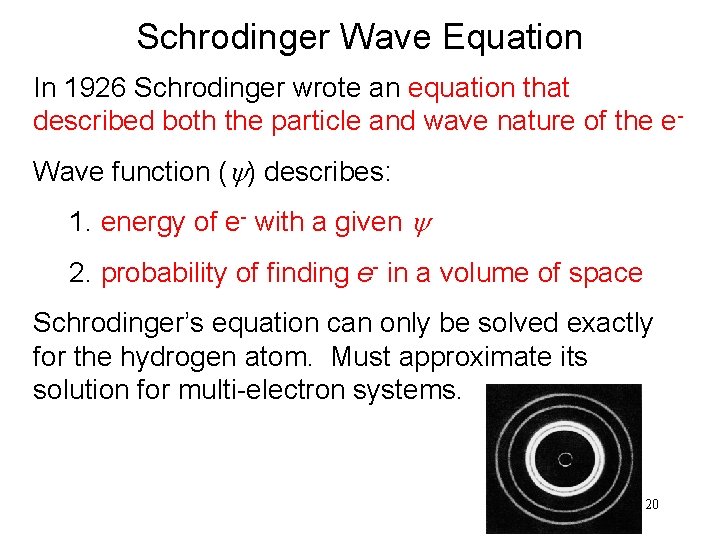
Schrodinger Wave Equation In 1926 Schrodinger wrote an equation that described both the particle and wave nature of the e. Wave function (y) describes: 1. energy of e- with a given y 2. probability of finding e- in a volume of space Schrodinger’s equation can only be solved exactly for the hydrogen atom. Must approximate its solution for multi-electron systems. 20

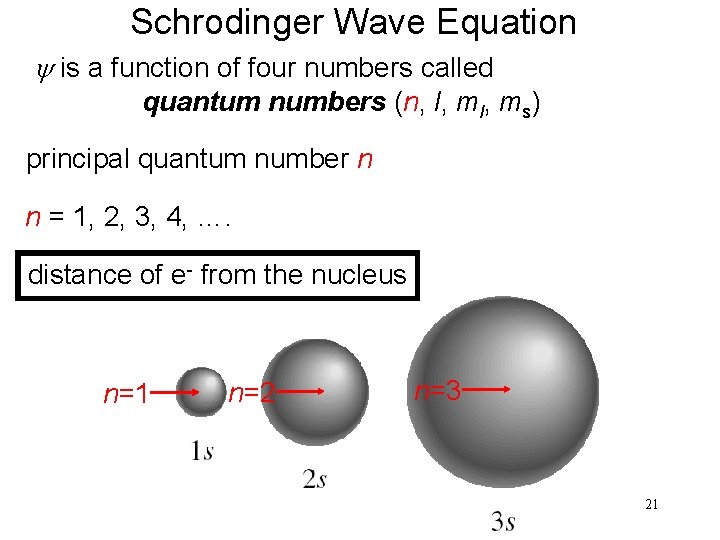
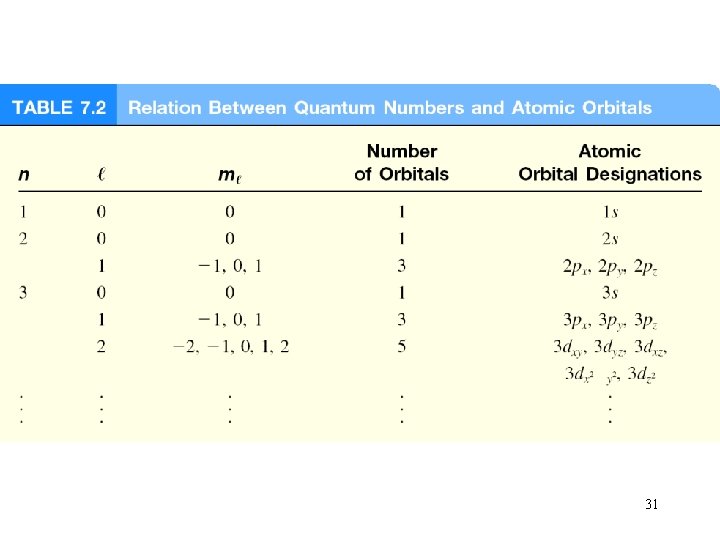
Schrodinger Wave Equation y is a function of four numbers called quantum numbers (n, l, ms) principal quantum number n n = 1, 2, 3, 4, …. distance of e- from the nucleus n=1 n=2 n=3 21

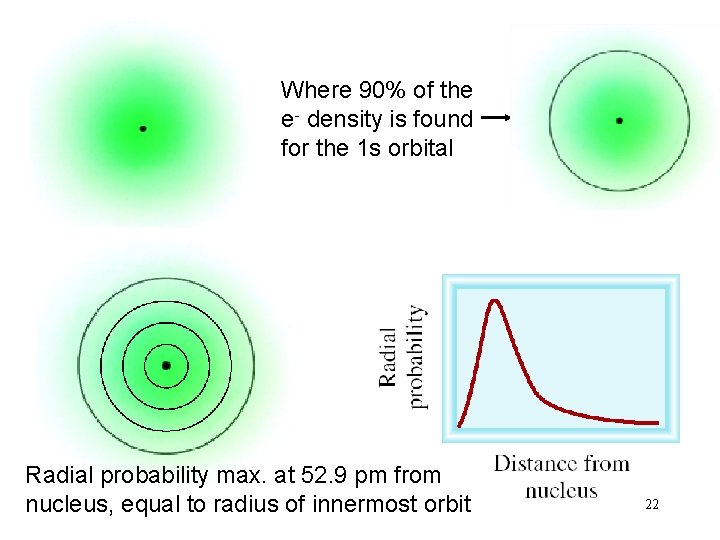
Where 90% of the e- density is found for the 1 s orbital Radial probability max. at 52. 9 pm from nucleus, equal to radius of innermost orbit 22

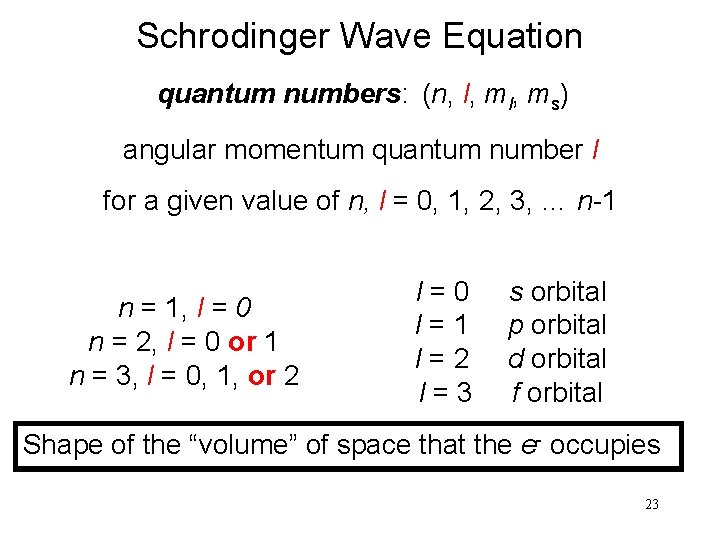
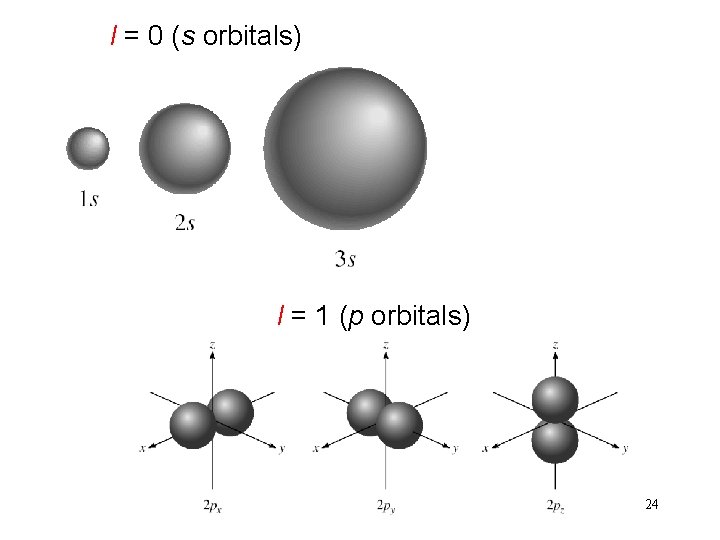
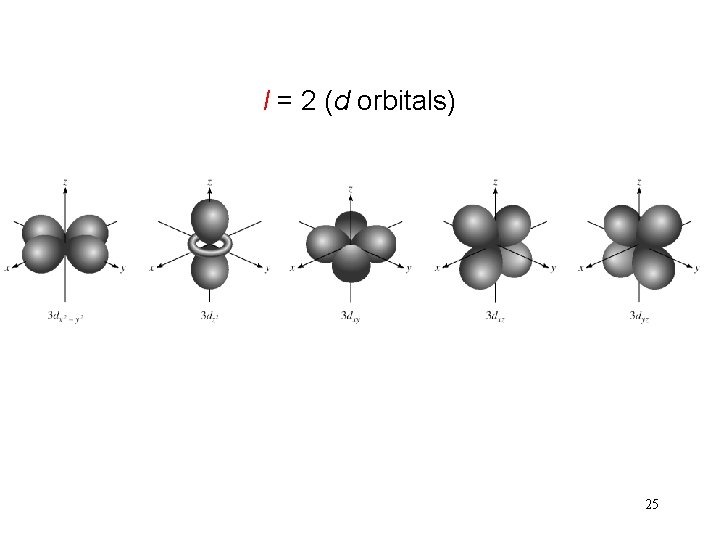
Schrodinger Wave Equation quantum numbers: (n, l, ms) angular momentum quantum number l for a given value of n, l = 0, 1, 2, 3, … n-1 n = 1, l = 0 n = 2, l = 0 or 1 n = 3, l = 0, 1, or 2 l=0 l=1 l=2 l=3 s orbital p orbital d orbital f orbital Shape of the “volume” of space that the e- occupies 23

l = 0 (s orbitals) l = 1 (p orbitals) 24

l = 2 (d orbitals) 25

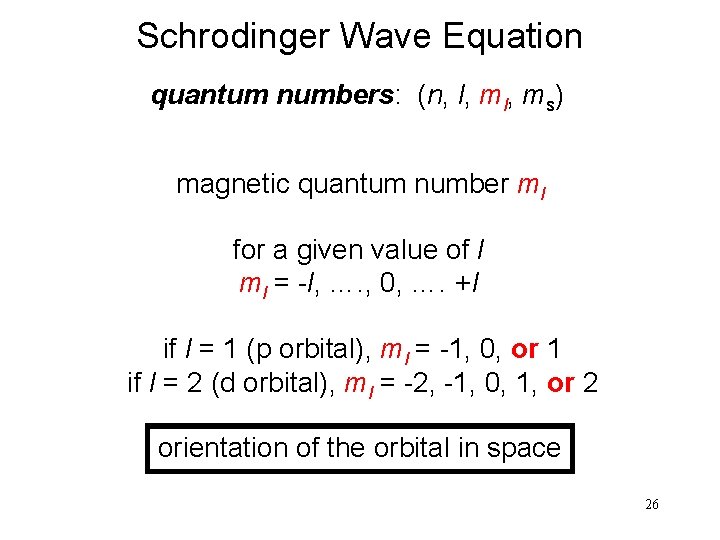
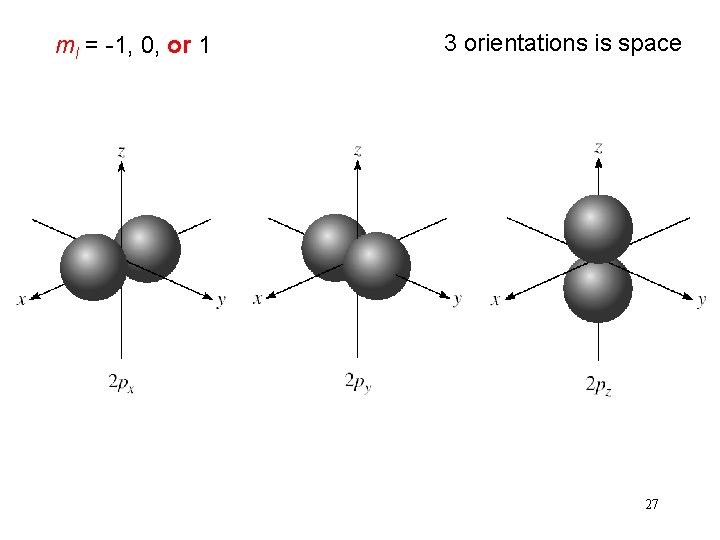
Schrodinger Wave Equation quantum numbers: (n, l, ms) magnetic quantum number ml for a given value of l ml = -l, …. , 0, …. +l if l = 1 (p orbital), ml = -1, 0, or 1 if l = 2 (d orbital), ml = -2, -1, 0, 1, or 2 orientation of the orbital in space 26

ml = -1, 0, or 1 3 orientations is space 27

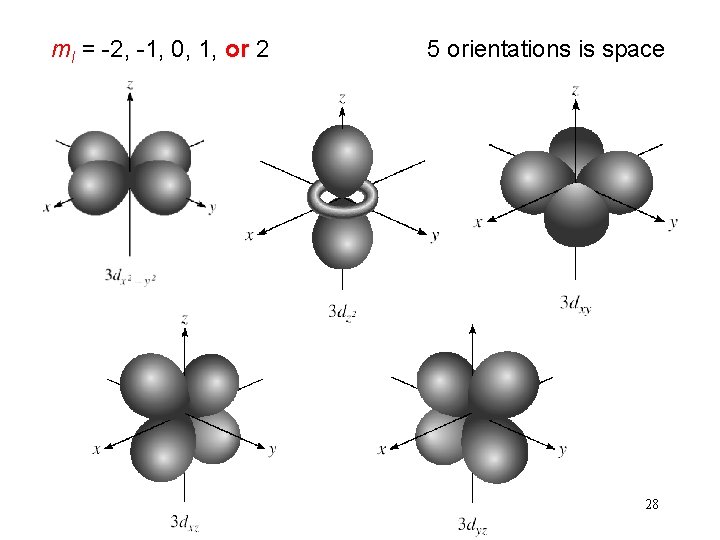
ml = -2, -1, 0, 1, or 2 5 orientations is space 28

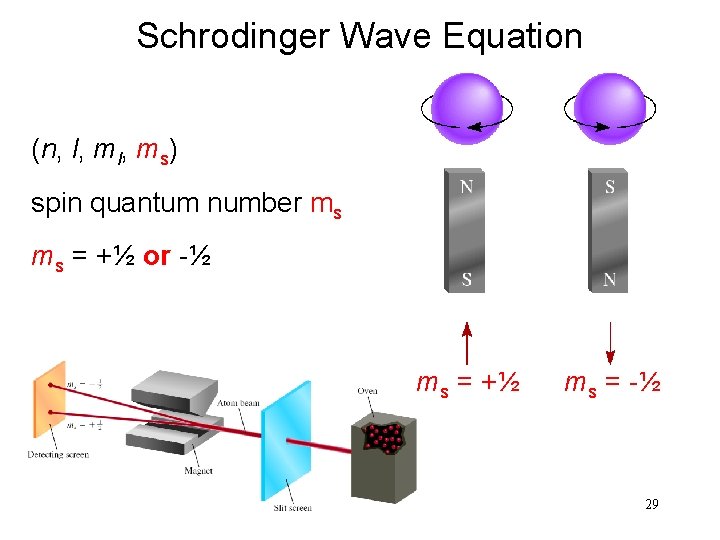
Schrodinger Wave Equation (n, l, ms) spin quantum number ms ms = +½ or -½ ms = +½ ms = -½ 29

Schrodinger Wave Equation quantum numbers: (n, l, ms) Existence (and energy) of electron in atom is described by its unique wave function y. Pauli exclusion principle - no two electrons in an atom can have the same four quantum numbers. Each seat is uniquely identified (E, R 12, S 8) Each seat can hold only one individual at a time 30

31

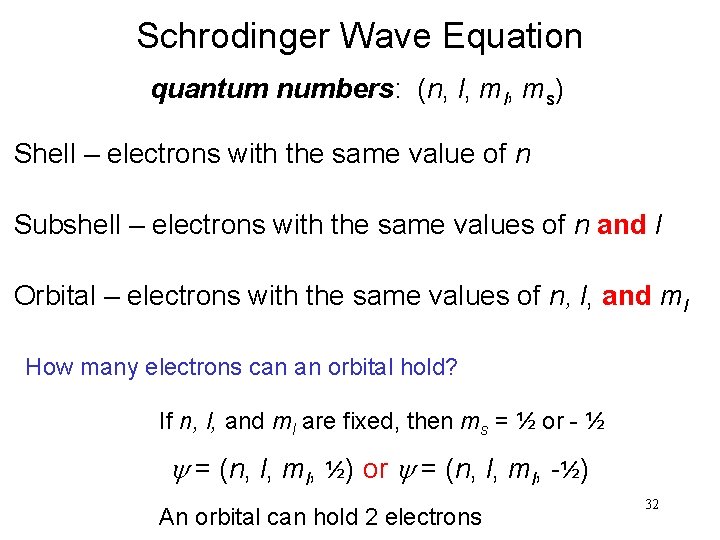
Schrodinger Wave Equation quantum numbers: (n, l, ms) Shell – electrons with the same value of n Subshell – electrons with the same values of n and l Orbital – electrons with the same values of n, l, and ml How many electrons can an orbital hold? If n, l, and ml are fixed, then ms = ½ or - ½ y = (n, l, ml, ½) or y = (n, l, ml, -½) An orbital can hold 2 electrons 32

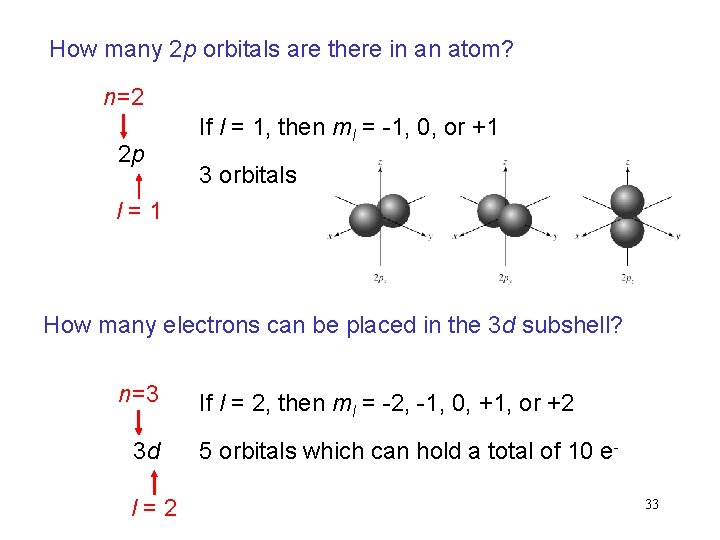
How many 2 p orbitals are there in an atom? n=2 2 p If l = 1, then ml = -1, 0, or +1 3 orbitals l=1 How many electrons can be placed in the 3 d subshell? n=3 3 d l=2 If l = 2, then ml = -2, -1, 0, +1, or +2 5 orbitals which can hold a total of 10 e 33

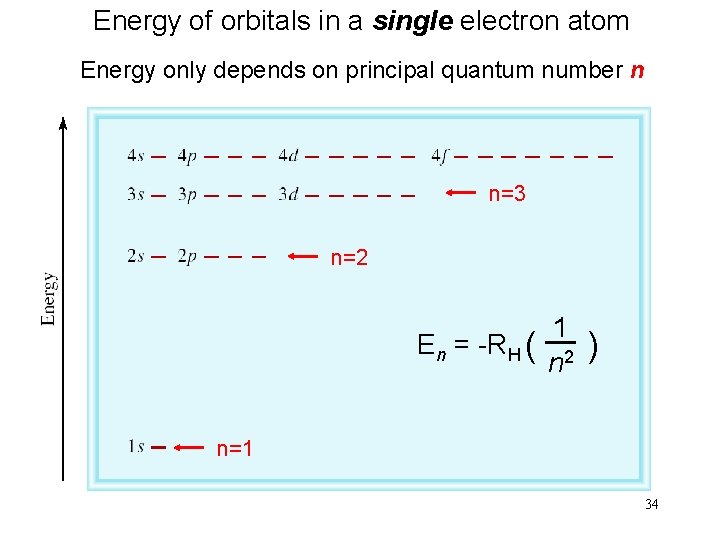
Energy of orbitals in a single electron atom Energy only depends on principal quantum number n n=3 n=2 En = -RH ( 1 n 2 ) n=1 34

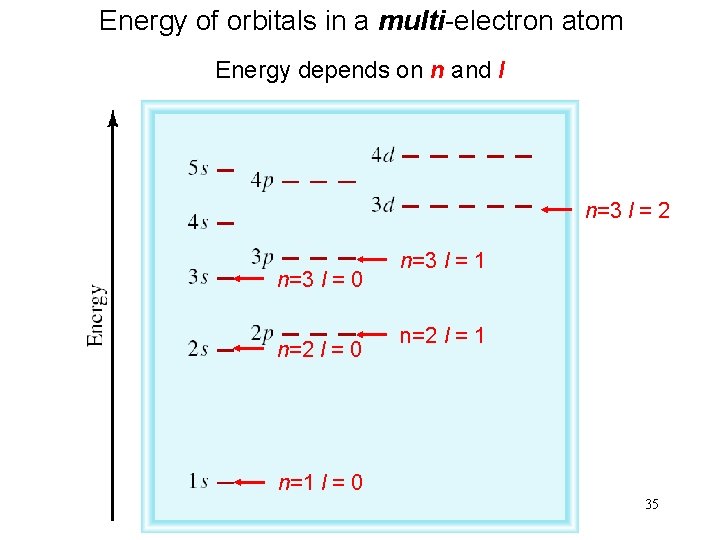
Energy of orbitals in a multi-electron atom Energy depends on n and l n=3 l = 2 n=3 l = 0 n=2 l = 0 n=3 l = 1 n=2 l = 1 n=1 l = 0 35

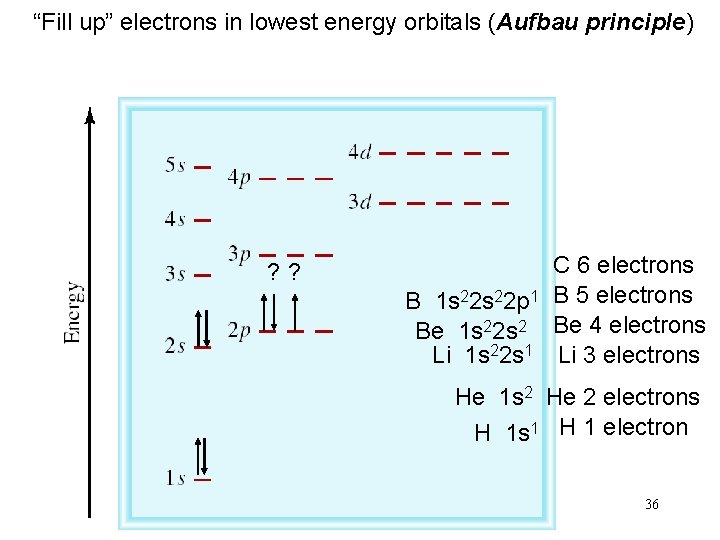
“Fill up” electrons in lowest energy orbitals (Aufbau principle) ? ? C 6 electrons B 1 s 22 p 1 B 5 electrons Be 1 s 22 s 2 Be 4 electrons Li 1 s 22 s 1 Li 3 electrons He 1 s 2 He 2 electrons H 1 s 1 H 1 electron 36

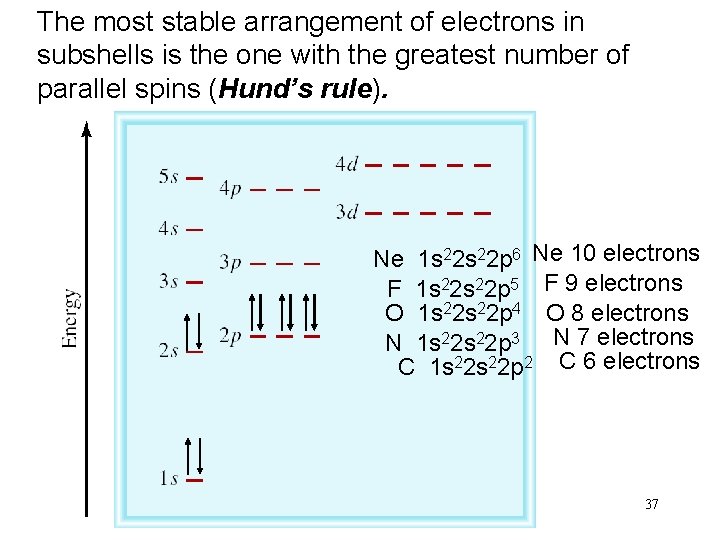
The most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins (Hund’s rule). Ne 1 s 22 p 6 Ne 10 electrons F 1 s 22 p 5 F 9 electrons O 1 s 22 p 4 O 8 electrons N 1 s 22 p 3 N 7 electrons C 1 s 22 p 2 C 6 electrons 37

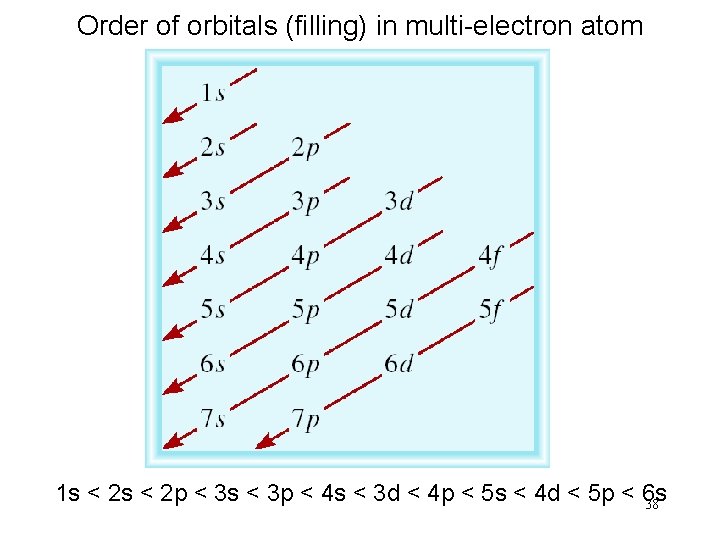
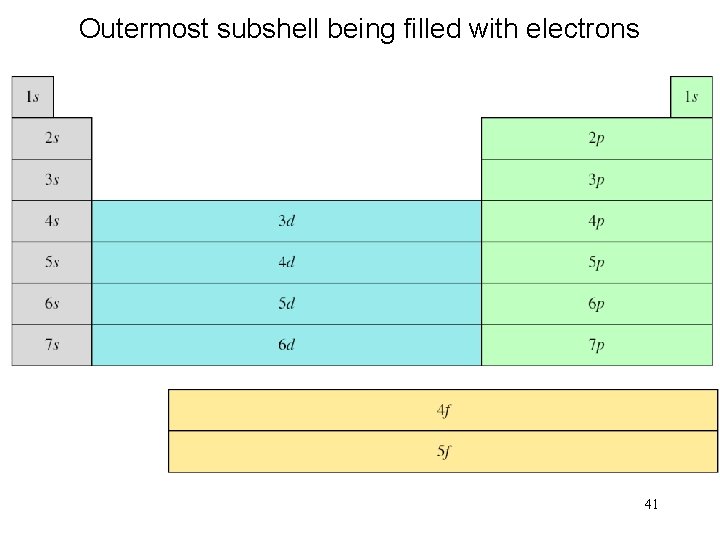
Order of orbitals (filling) in multi-electron atom 1 s < 2 p < 3 s < 3 p < 4 s < 3 d < 4 p < 5 s < 4 d < 5 p < 6 s 38

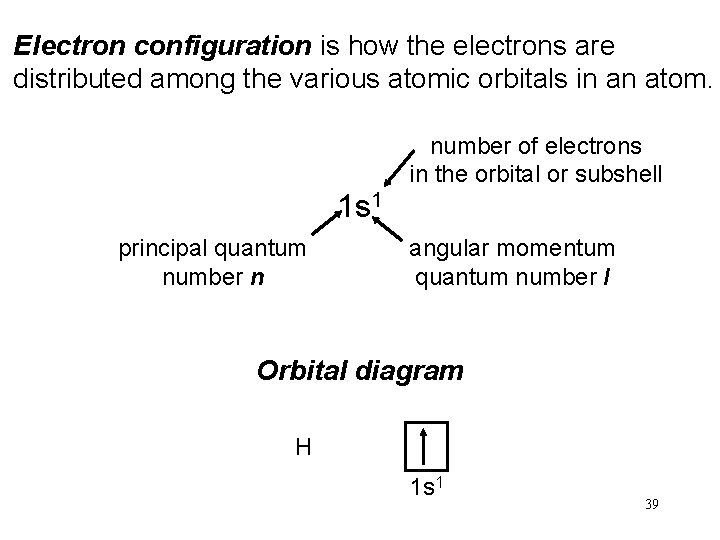
Electron configuration is how the electrons are distributed among the various atomic orbitals in an atom. number of electrons in the orbital or subshell 1 s 1 principal quantum number n angular momentum quantum number l Orbital diagram H 1 s 1 39

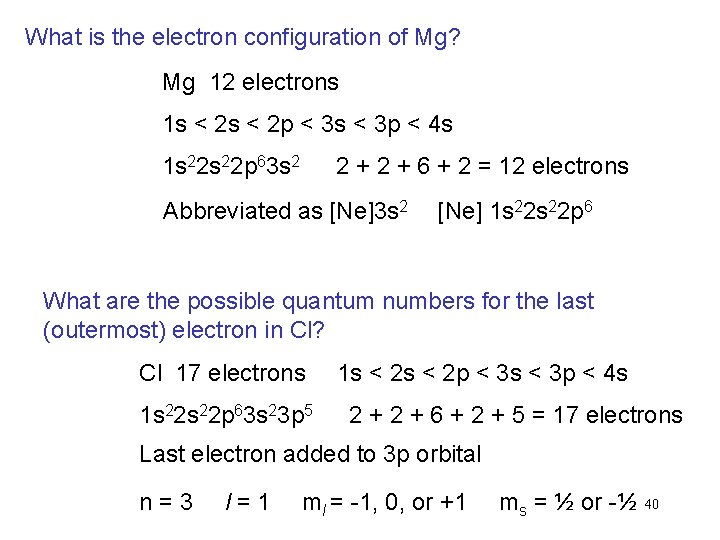
What is the electron configuration of Mg? Mg 12 electrons 1 s < 2 p < 3 s < 3 p < 4 s 1 s 22 p 63 s 2 2 + 6 + 2 = 12 electrons Abbreviated as [Ne]3 s 2 [Ne] 1 s 22 p 6 What are the possible quantum numbers for the last (outermost) electron in Cl? Cl 17 electrons 1 s 22 p 63 s 23 p 5 1 s < 2 p < 3 s < 3 p < 4 s 2 + 6 + 2 + 5 = 17 electrons Last electron added to 3 p orbital n=3 l=1 ml = -1, 0, or +1 ms = ½ or -½ 40

Outermost subshell being filled with electrons 41

42

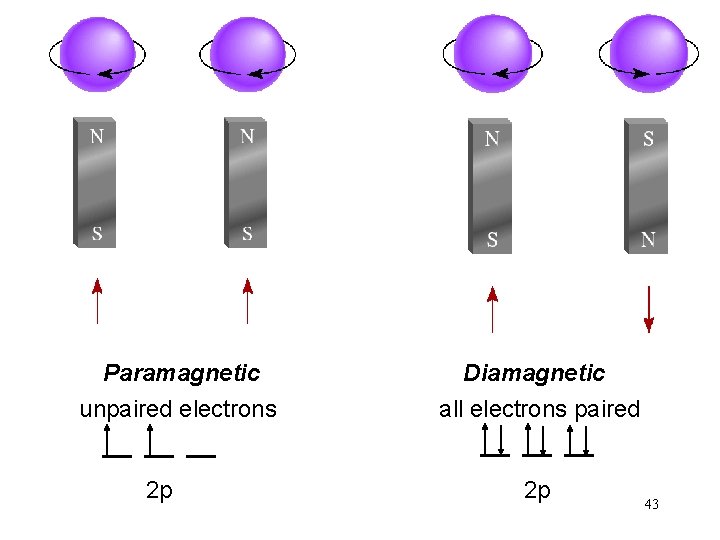
Paramagnetic unpaired electrons 2 p Diamagnetic all electrons paired 2 p 43

When the Elements Were Discovered 44

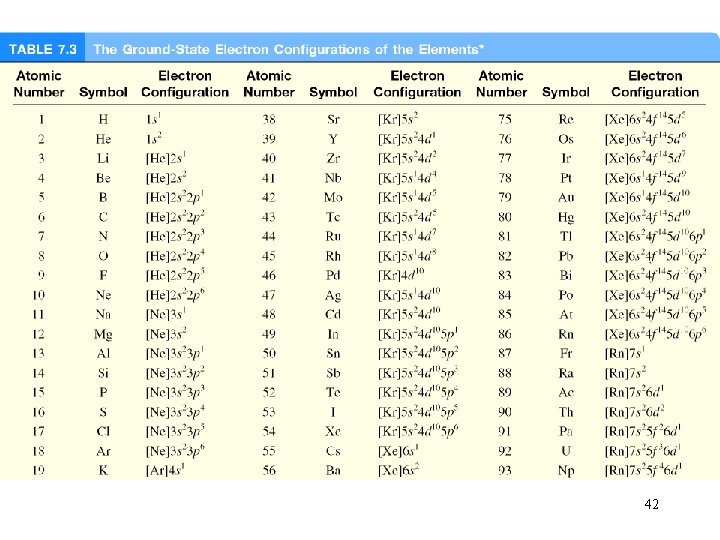
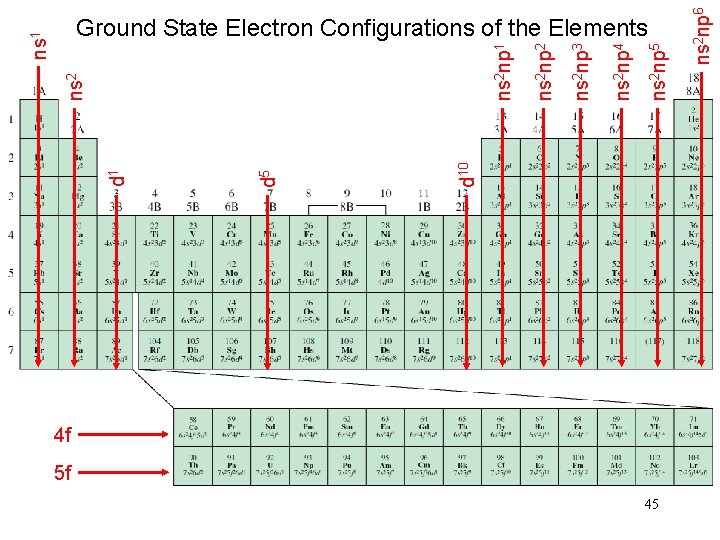
4 f 5 f 45 ns 2 np 6 ns 2 np 5 ns 2 np 4 ns 2 np 3 ns 2 np 2 ns 2 np 1 d 10 d 5 d 1 ns 2 ns 1 Ground State Electron Configurations of the Elements

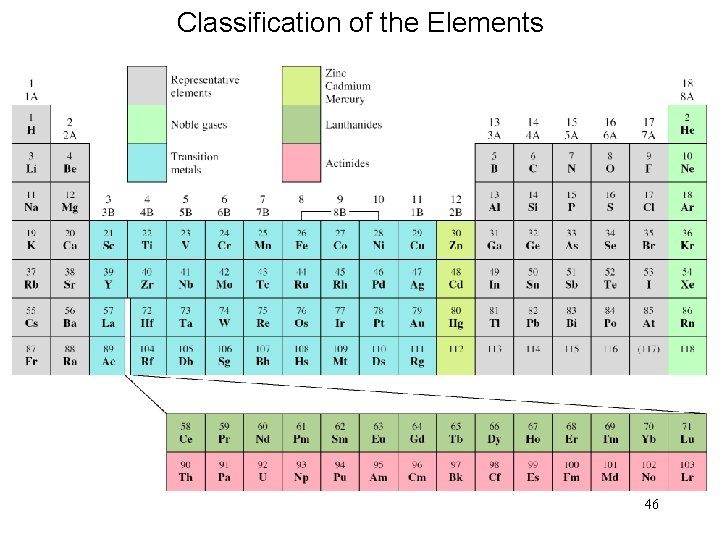
Classification of the Elements 46
![Electron Configurations of Cations and Anions Of Representative Elements Na Ne3 s 1 Na Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+](https://slidetodoc.com/presentation_image_h2/1e8f4a105f9a091d86bf4284a4b51273/image-47.jpg)
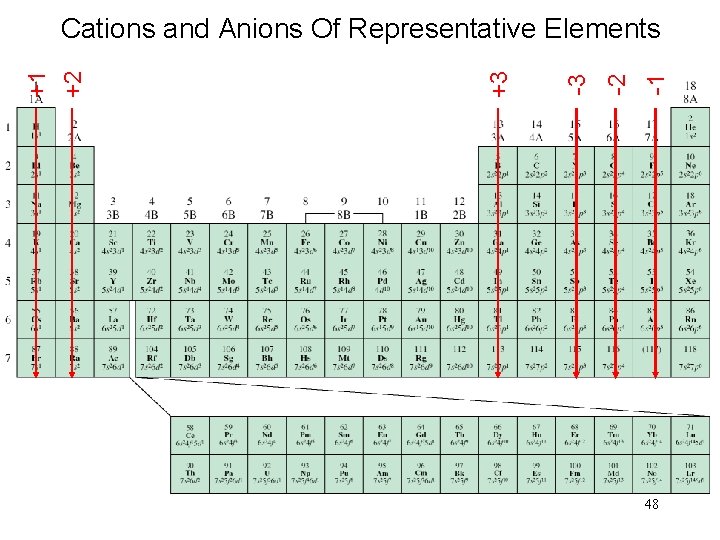
Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+ [Ne] Ca [Ar]4 s 2 Ca 2+ [Ar] Al [Ne]3 s 23 p 1 Al 3+ [Ne] Atoms gain electrons so that anion has a noblegas outer electron configuration. Atoms lose electrons so that cation has a noble-gas outer electron configuration. H 1 s 1 H- 1 s 2 or [He] F 1 s 22 p 5 F- 1 s 22 p 6 or [Ne] O 1 s 22 p 4 O 2 - 1 s 22 p 6 or [Ne] N 1 s 22 p 3 N 3 - 1 s 22 p 6 or [Ne] 47

-1 -2 -3 +3 +1 +2 Cations and Anions Of Representative Elements 48

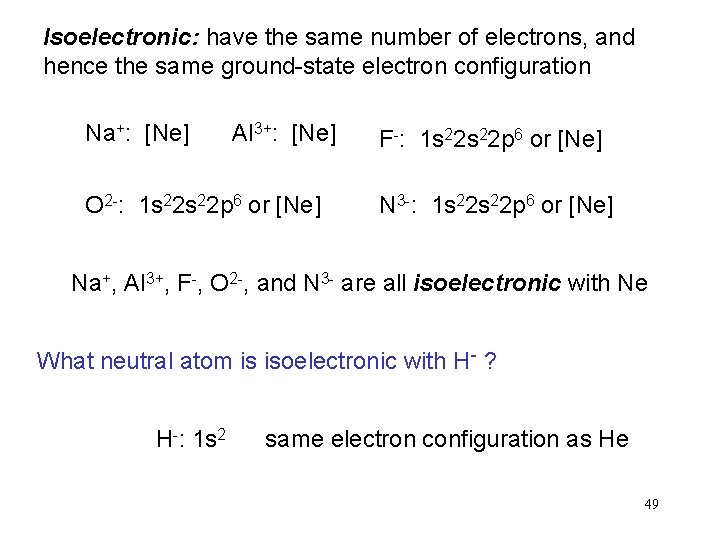
Isoelectronic: have the same number of electrons, and hence the same ground-state electron configuration Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or [Ne] F-: 1 s 22 p 6 or [Ne] N 3 -: 1 s 22 p 6 or [Ne] Na+, Al 3+, F-, O 2 -, and N 3 - are all isoelectronic with Ne What neutral atom is isoelectronic with H- ? H-: 1 s 2 same electron configuration as He 49

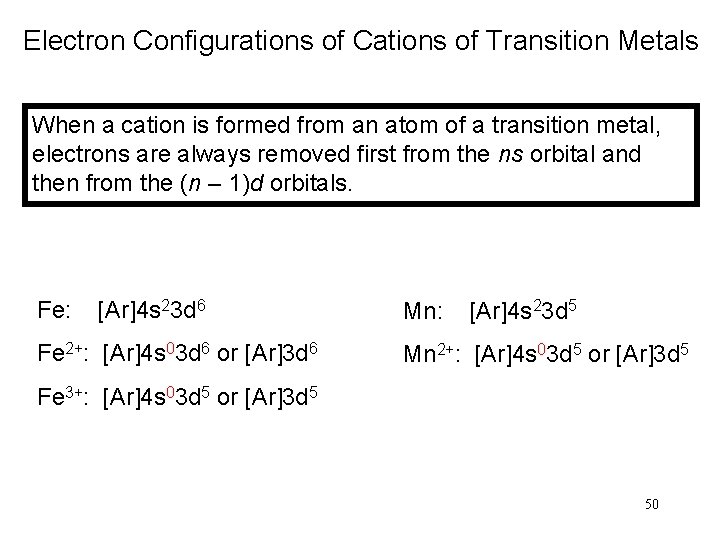
Electron Configurations of Cations of Transition Metals When a cation is formed from an atom of a transition metal, electrons are always removed first from the ns orbital and then from the (n – 1)d orbitals. Fe: [Ar]4 s 23 d 6 Fe 2+: [Ar]4 s 03 d 6 or [Ar]3 d 6 Mn: [Ar]4 s 23 d 5 Mn 2+: [Ar]4 s 03 d 5 or [Ar]3 d 5 Fe 3+: [Ar]4 s 03 d 5 or [Ar]3 d 5 50

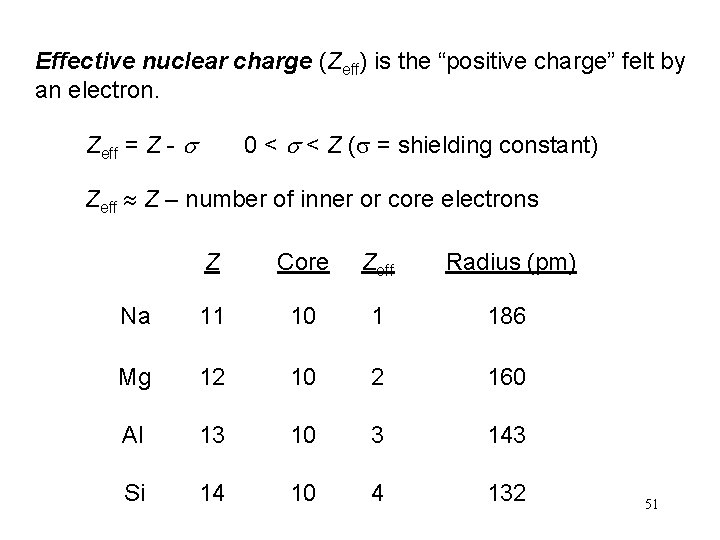
Effective nuclear charge (Zeff) is the “positive charge” felt by an electron. Zeff = Z - s 0 < s < Z (s = shielding constant) Zeff Z – number of inner or core electrons Z Core Zeff Radius (pm) Na 11 10 1 186 Mg 12 10 2 160 Al 13 10 3 143 Si 14 10 4 132 51

Effective Nuclear Charge (Zeff) increasing Zeff 52

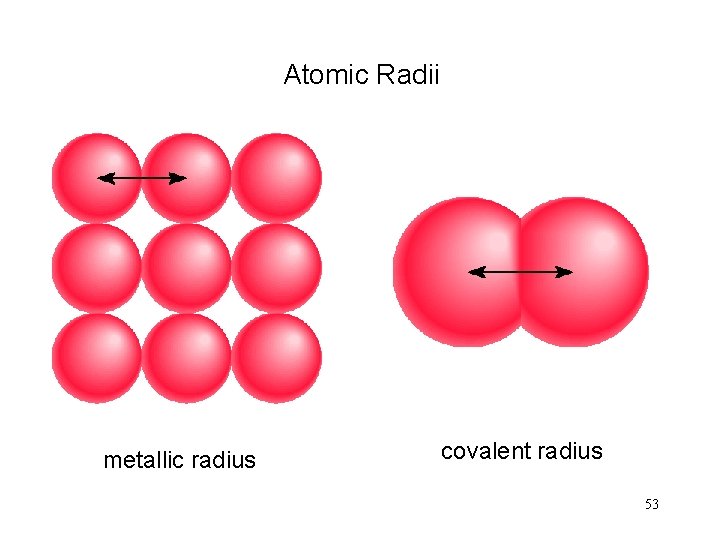
Atomic Radii metallic radius covalent radius 53

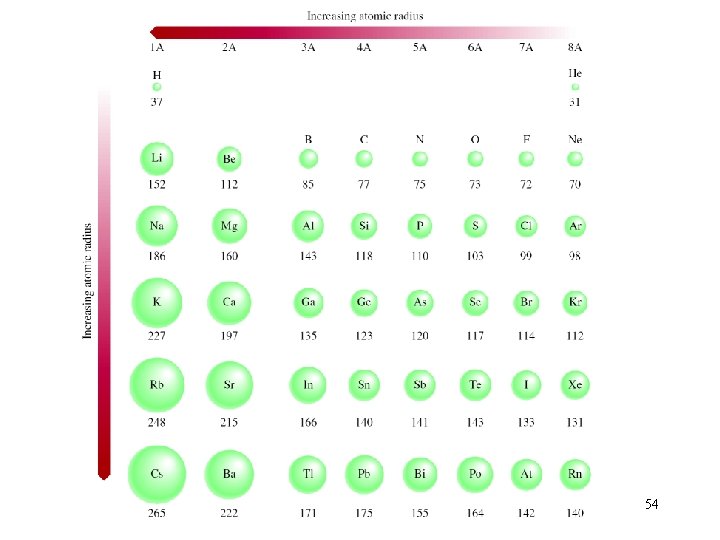
54

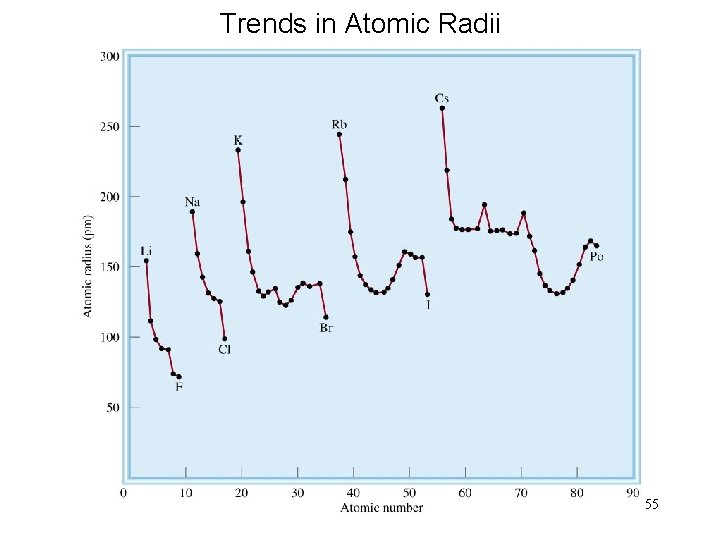
Trends in Atomic Radii 55

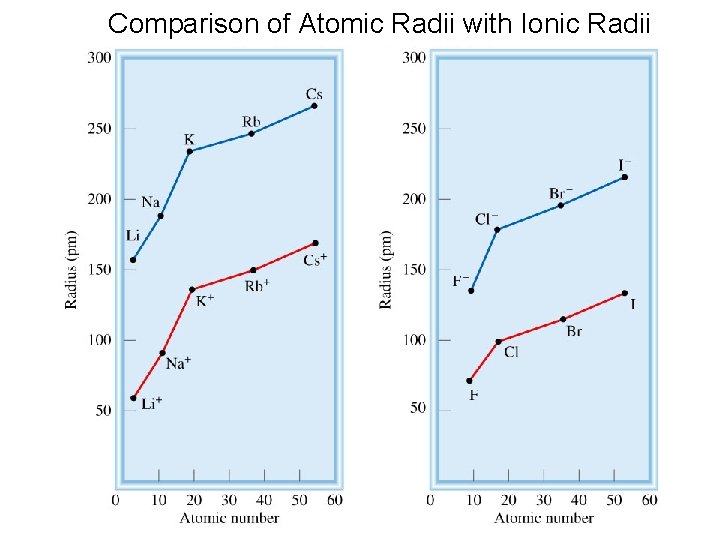
Comparison of Atomic Radii with Ionic Radii 56

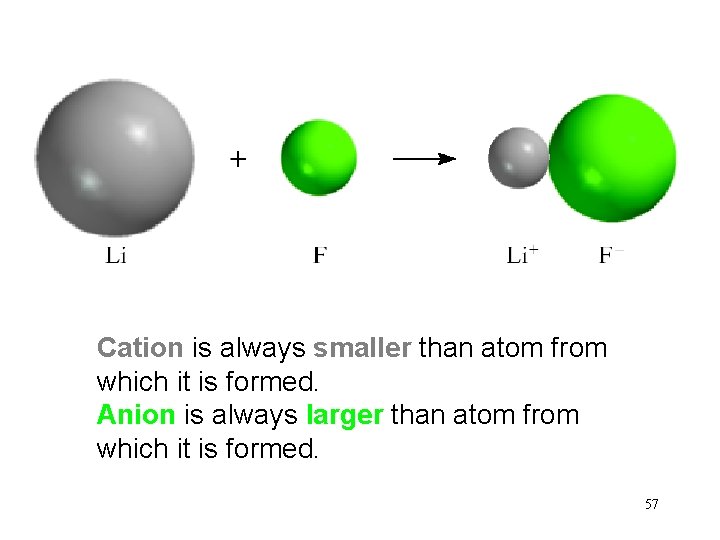
Cation is always smaller than atom from which it is formed. Anion is always larger than atom from which it is formed. 57

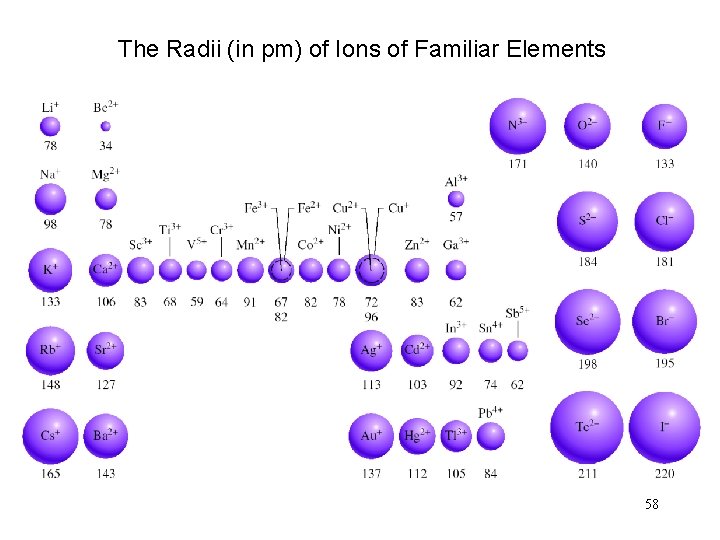
The Radii (in pm) of Ions of Familiar Elements 58

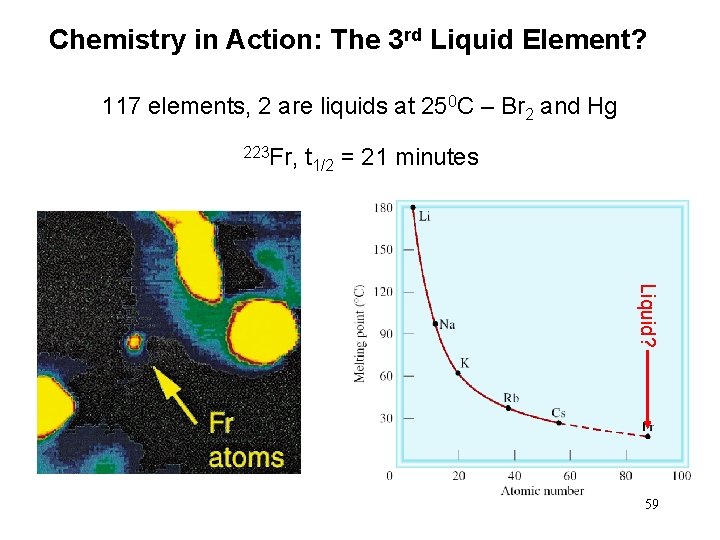
Chemistry in Action: The 3 rd Liquid Element? 117 elements, 2 are liquids at 250 C – Br 2 and Hg 223 Fr, t 1/2 = 21 minutes Liquid? 59

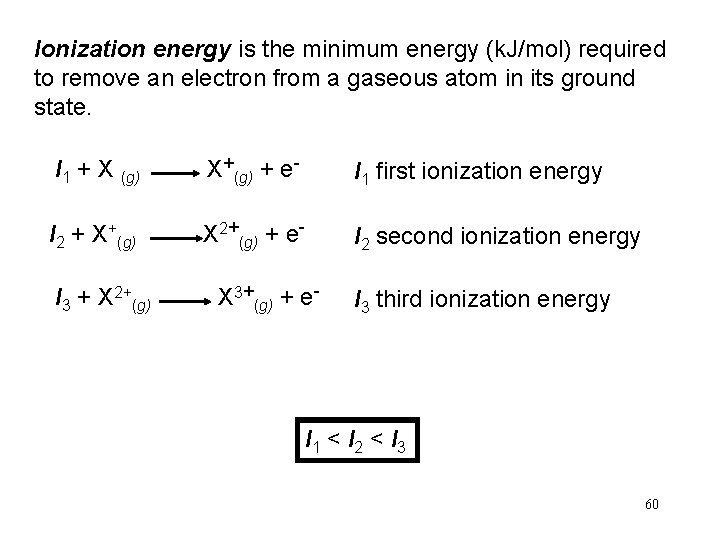
Ionization energy is the minimum energy (k. J/mol) required to remove an electron from a gaseous atom in its ground state. I 1 + X (g) X+(g) + e- I 1 first ionization energy I 2 + X+(g) X 2+(g) + e- I 2 second ionization energy I 3 + X 2+(g) X 3+(g) + e- I 3 third ionization energy I 1 < I 2 < I 3 60

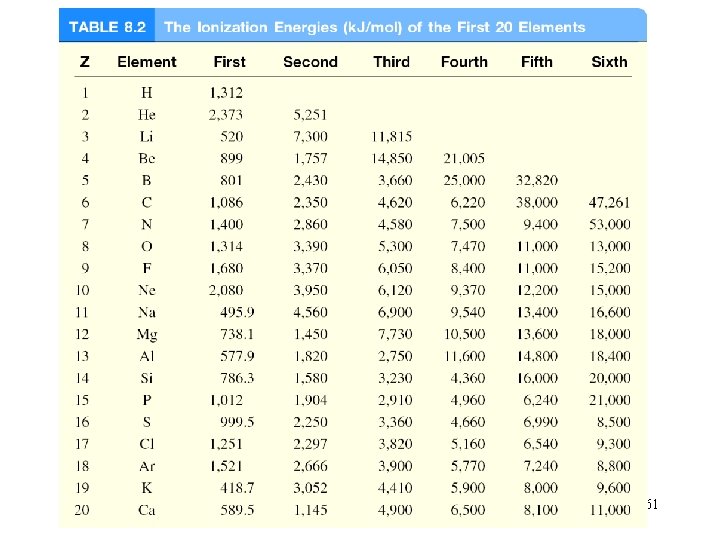
61

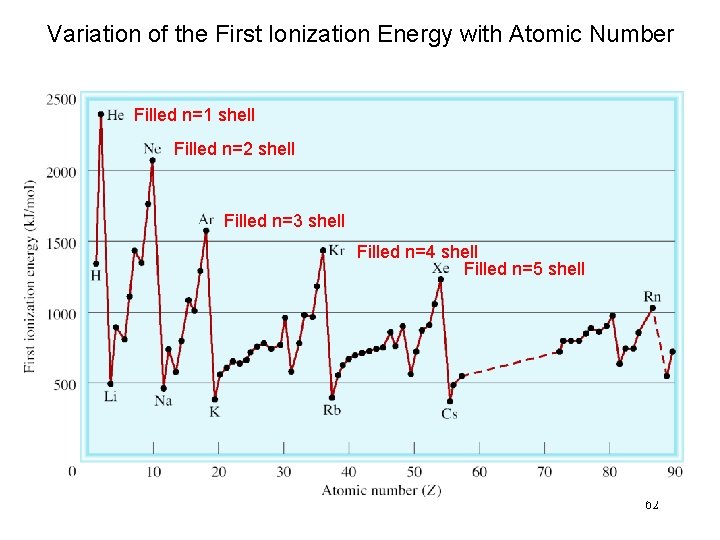
Variation of the First Ionization Energy with Atomic Number Filled n=1 shell Filled n=2 shell Filled n=3 shell Filled n=4 shell Filled n=5 shell 62

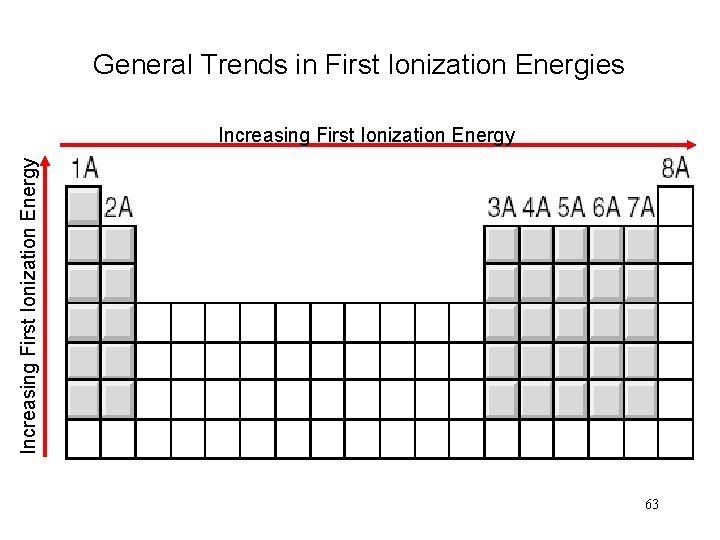
General Trends in First Ionization Energies Increasing First Ionization Energy 63

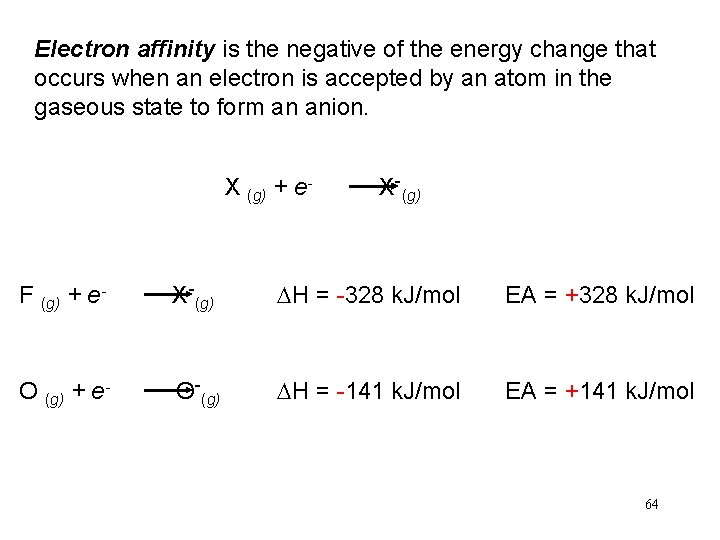
Electron affinity is the negative of the energy change that occurs when an electron is accepted by an atom in the gaseous state to form an anion. X (g) + e- X-(g) F (g) + e- X-(g) DH = -328 k. J/mol EA = +328 k. J/mol O (g) + e- O-(g) DH = -141 k. J/mol EA = +141 k. J/mol 64

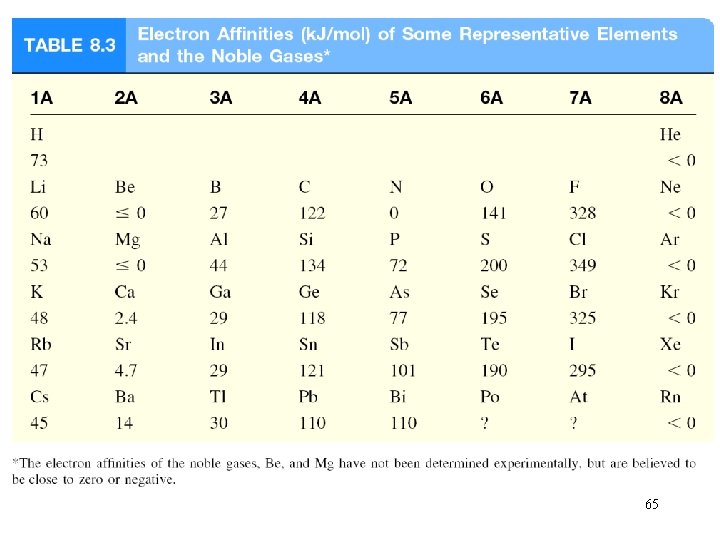
65

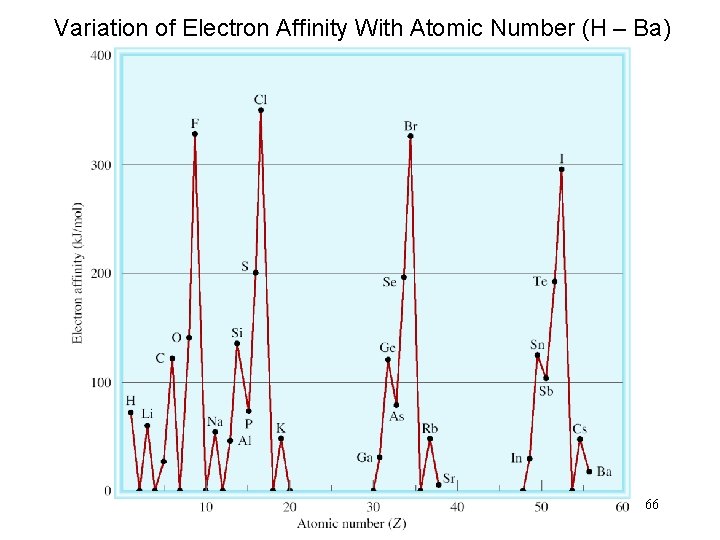
Variation of Electron Affinity With Atomic Number (H – Ba) 66