Chapter 7 Atomic Structure and Periodicity Electromagnetic Radiation





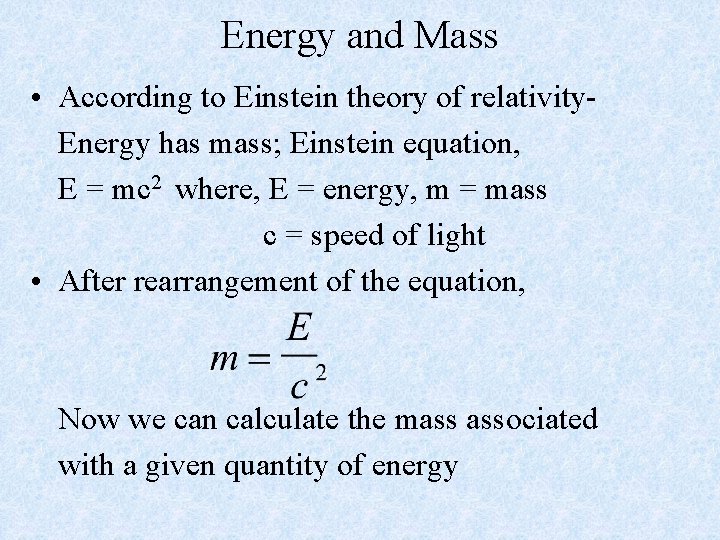
- Slides: 23

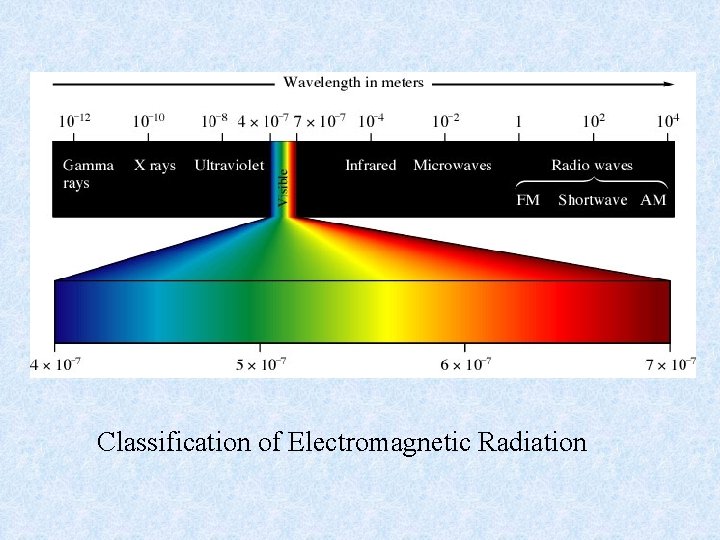
Chapter 7 Atomic Structure and Periodicity • Electromagnetic Radiation Radiant energy that exhibits wavelength-like behavior and travels through space at the speed of light in a vacuum. • Example: The sun light, energy used in microwave oven, the x-rays used by doctors.

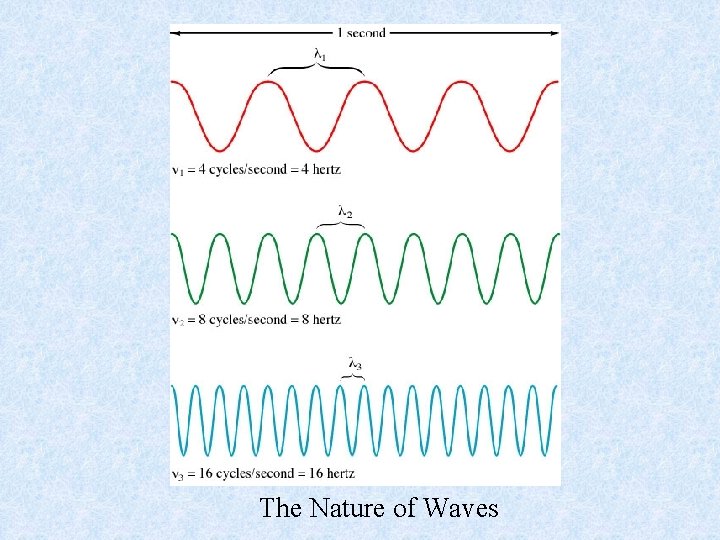
Waves have 3 primary characteristics: 1. Wavelength ( ): distance between two consecutive peaks in a wave. 2. Frequency ( ): number of waves (cycles) per second that pass a given point in space. 3. Speed: speed of light is 2. 9979 108 m/s. We will use 3. 00 x 108 m/s.

The Nature of Waves

Wavelength and frequency can be interconverted and they have an inverse relationship = c/ = frequency (s 1) = wavelength (m) c = speed of light (m s 1) • Wavelength is also given in nm (1 nm = 10 -9 m) and Angstroms (Å) (1 Å = 10 -10 m). • The frequency value of s 1 or 1/s is also called “hertz (Hz)” like KHz on the radio.

Classification of Electromagnetic Radiation

Example: When green light is emitted from an oxygen atom it has a wavelength of 558 nm. What is the frequency? We know, = c/ where, c = speed of light = 3. 00 x 108 m/s = wavelength = 558 nm (need to convert in m)

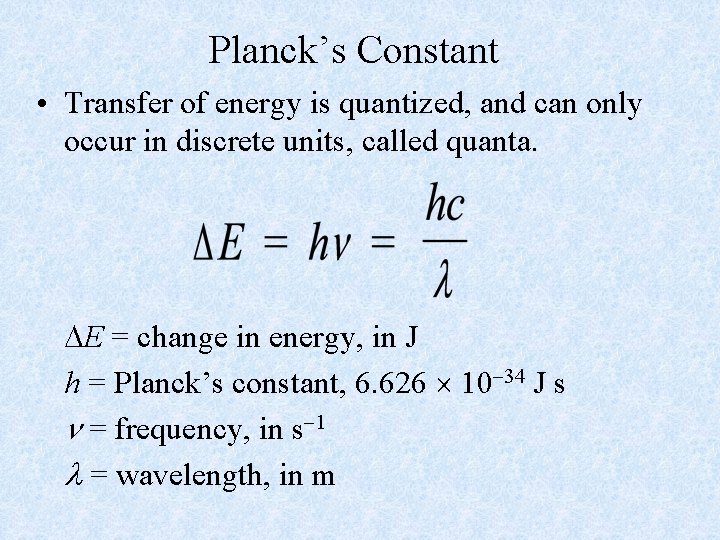
Planck’s Constant • Transfer of energy is quantized, and can only occur in discrete units, called quanta. E = change in energy, in J h = Planck’s constant, 6. 626 10 34 J s = frequency, in s 1 = wavelength, in m

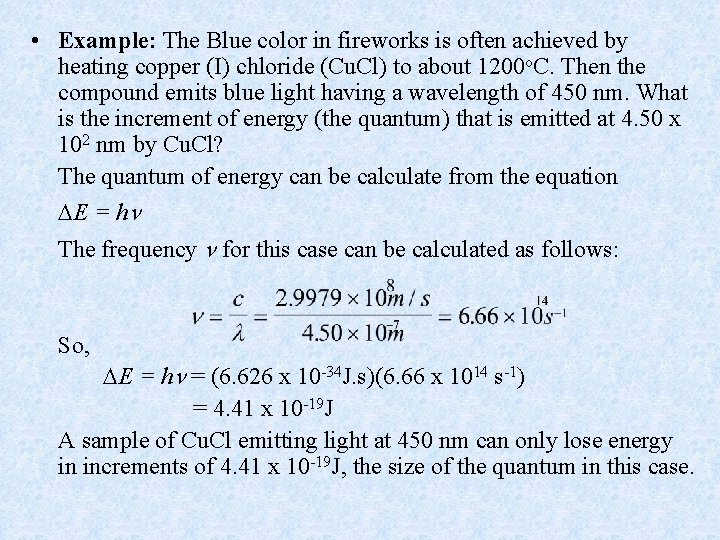
• Example: The Blue color in fireworks is often achieved by heating copper (I) chloride (Cu. Cl) to about 1200 o. C. Then the compound emits blue light having a wavelength of 450 nm. What is the increment of energy (the quantum) that is emitted at 4. 50 x 102 nm by Cu. Cl? The quantum of energy can be calculate from the equation E = h The frequency for this case can be calculated as follows: So, E = h = (6. 626 x 10 -34 J. s)(6. 66 x 1014 s-1) = 4. 41 x 10 -19 J A sample of Cu. Cl emitting light at 450 nm can only lose energy in increments of 4. 41 x 10 -19 J, the size of the quantum in this case.

Mr. Ahmad is going to derive Einstein’s equation for a moving object on the board taking time dilation into account.
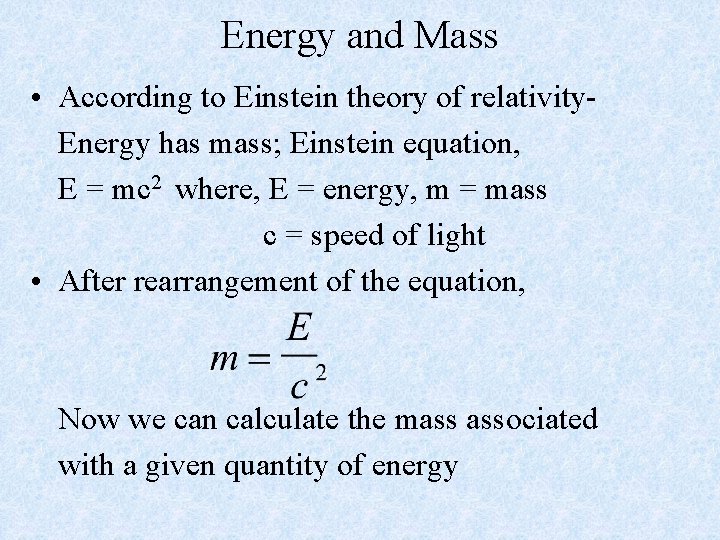
Energy and Mass • According to Einstein theory of relativity. Energy has mass; Einstein equation, E = mc 2 where, E = energy, m = mass c = speed of light • After rearrangement of the equation, Now we can calculate the mass associated with a given quantity of energy

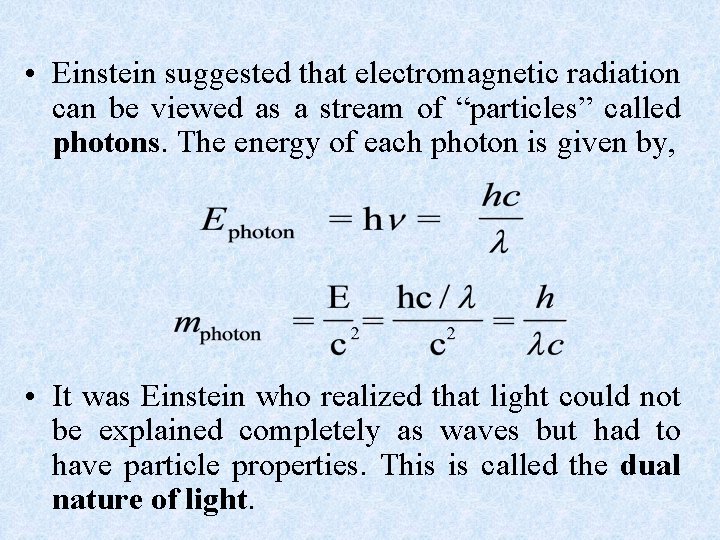
• Einstein suggested that electromagnetic radiation can be viewed as a stream of “particles” called photons. The energy of each photon is given by, • It was Einstein who realized that light could not be explained completely as waves but had to have particle properties. This is called the dual nature of light.

Electromagnetic Radiation

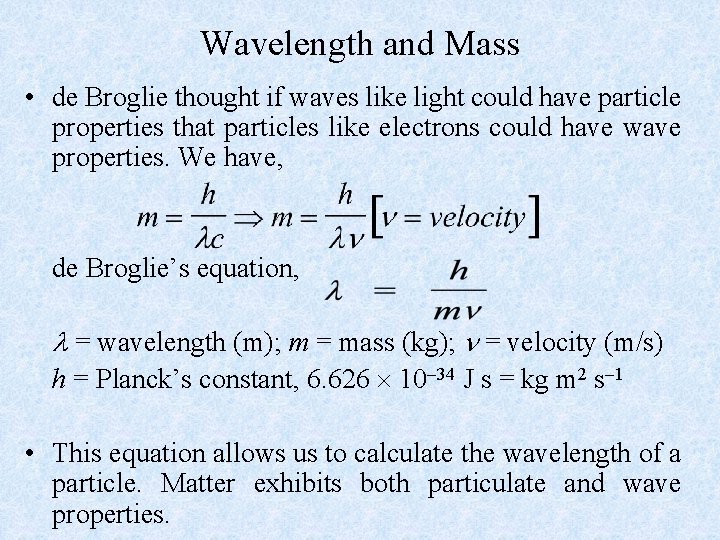
Wavelength and Mass • de Broglie thought if waves like light could have particle properties that particles like electrons could have wave properties. We have, de Broglie’s equation, = wavelength (m); m = mass (kg); = velocity (m/s) h = Planck’s constant, 6. 626 10 34 J s = kg m 2 s 1 • This equation allows us to calculate the wavelength of a particle. Matter exhibits both particulate and wave properties.

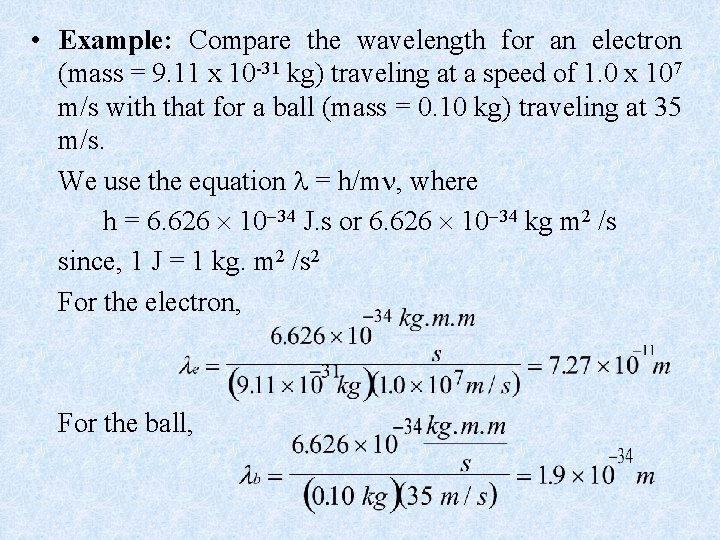
• Example: Compare the wavelength for an electron (mass = 9. 11 x 10 -31 kg) traveling at a speed of 1. 0 x 107 m/s with that for a ball (mass = 0. 10 kg) traveling at 35 m/s. We use the equation = h/m , where h = 6. 626 10 34 J. s or 6. 626 10 34 kg m 2 /s since, 1 J = 1 kg. m 2 /s 2 For the electron, For the ball,

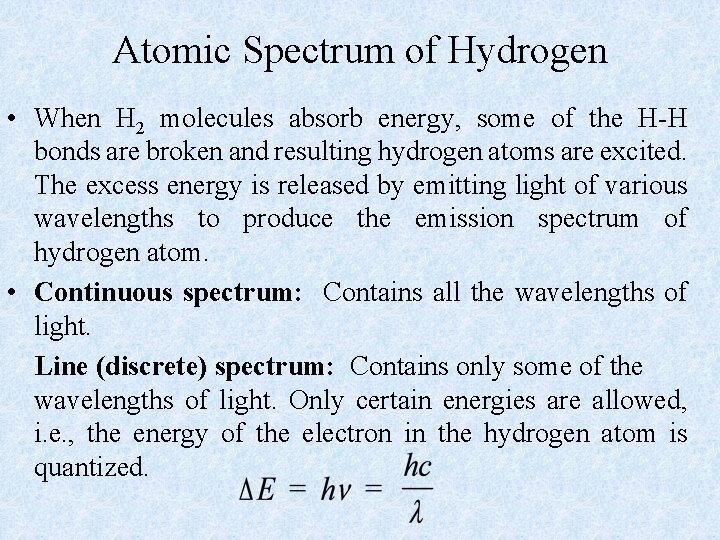
Atomic Spectrum of Hydrogen • When H 2 molecules absorb energy, some of the H-H bonds are broken and resulting hydrogen atoms are excited. The excess energy is released by emitting light of various wavelengths to produce the emission spectrum of hydrogen atom. • Continuous spectrum: Contains all the wavelengths of light. Line (discrete) spectrum: Contains only some of the wavelengths of light. Only certain energies are allowed, i. e. , the energy of the electron in the hydrogen atom is quantized.

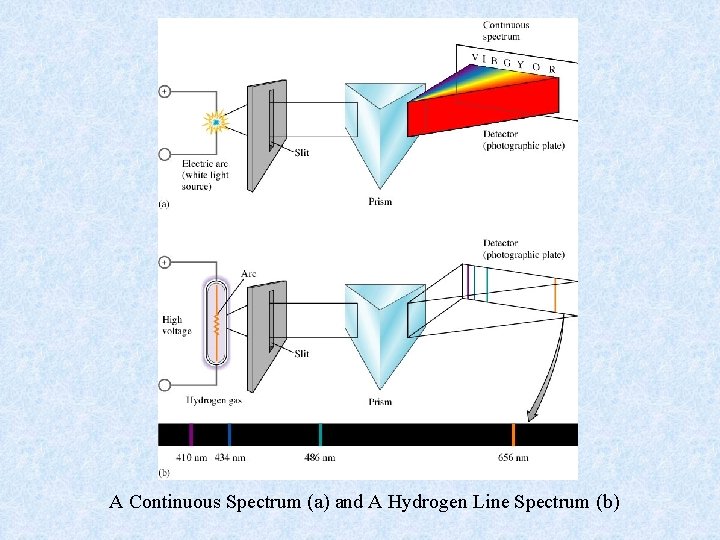
A Continuous Spectrum (a) and A Hydrogen Line Spectrum (b)

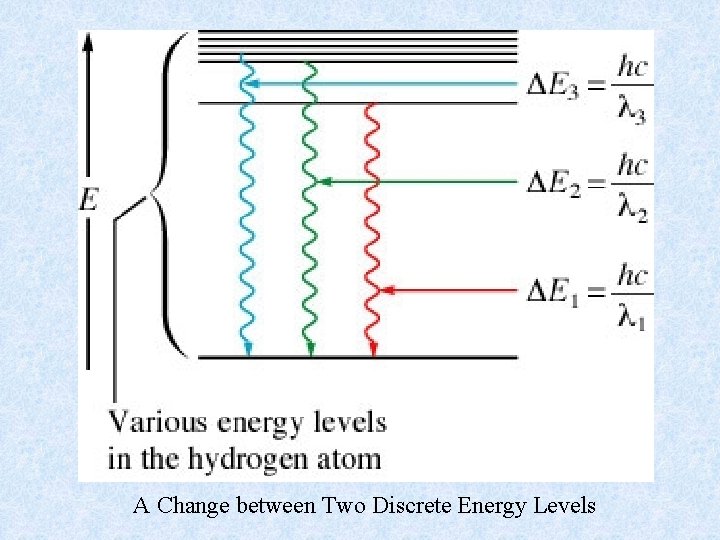
A Change between Two Discrete Energy Levels

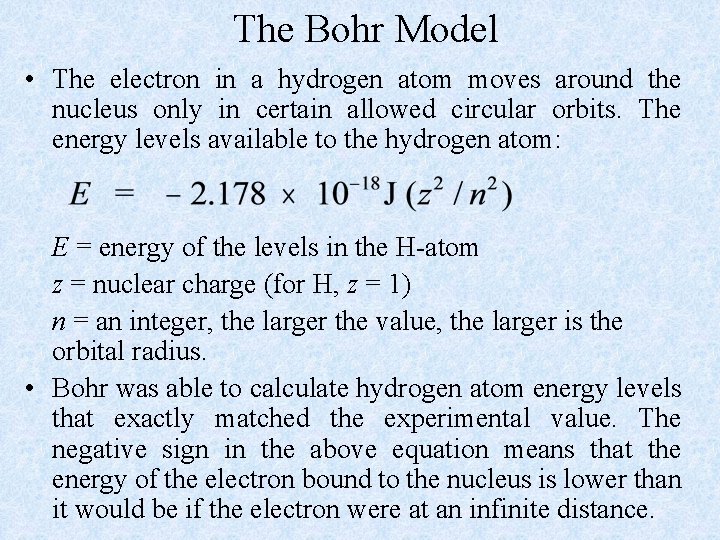
The Bohr Model • The electron in a hydrogen atom moves around the nucleus only in certain allowed circular orbits. The energy levels available to the hydrogen atom: E = energy of the levels in the H-atom z = nuclear charge (for H, z = 1) n = an integer, the larger the value, the larger is the orbital radius. • Bohr was able to calculate hydrogen atom energy levels that exactly matched the experimental value. The negative sign in the above equation means that the energy of the electron bound to the nucleus is lower than it would be if the electron were at an infinite distance.

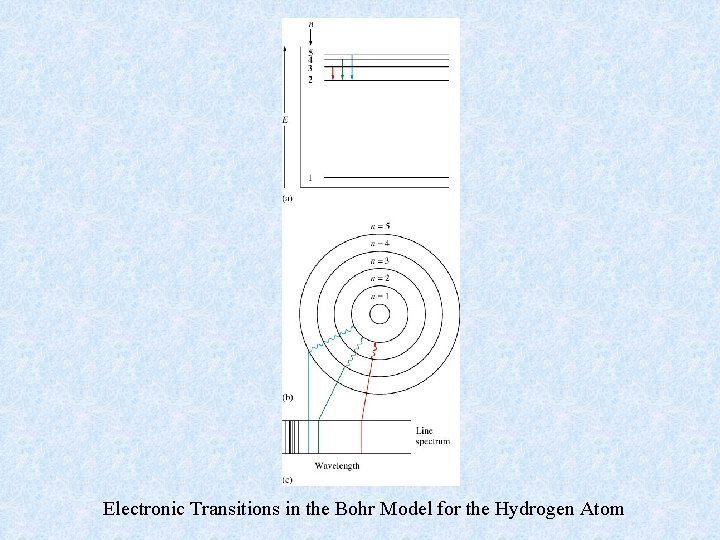
Electronic Transitions in the Bohr Model for the Hydrogen Atom

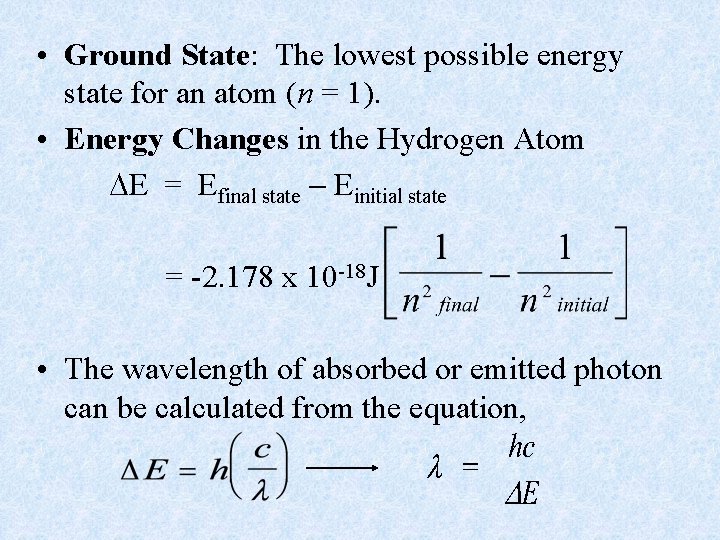
• Ground State: The lowest possible energy state for an atom (n = 1). • Energy Changes in the Hydrogen Atom E = Efinal state Einitial state = -2. 178 x 10 -18 J • The wavelength of absorbed or emitted photon can be calculated from the equation,

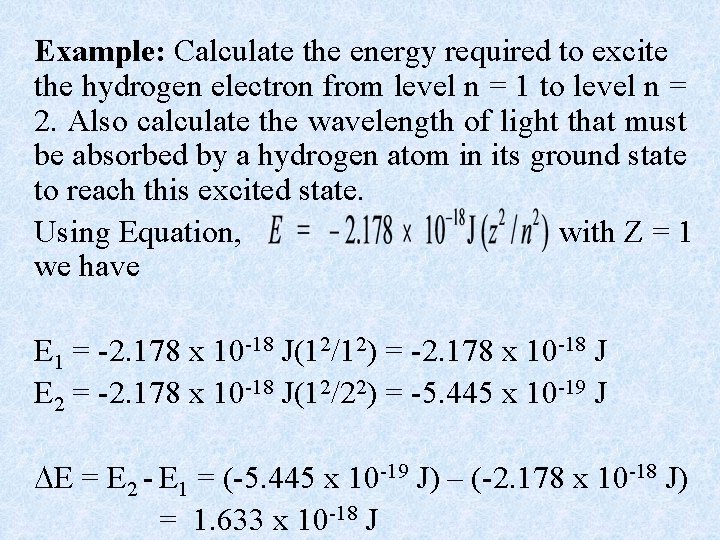
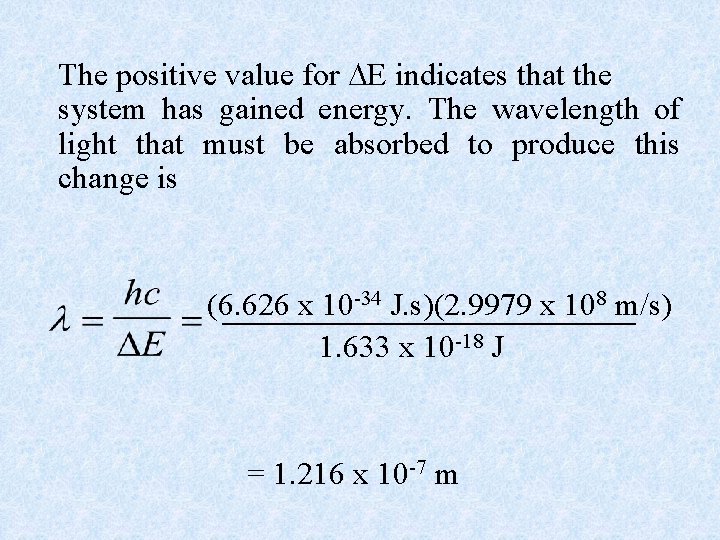
Example: Calculate the energy required to excite the hydrogen electron from level n = 1 to level n = 2. Also calculate the wavelength of light that must be absorbed by a hydrogen atom in its ground state to reach this excited state. Using Equation, with Z = 1 we have E 1 = -2. 178 x 10 -18 J(12/12) = -2. 178 x 10 -18 J E 2 = -2. 178 x 10 -18 J(12/22) = -5. 445 x 10 -19 J E = E 2 - E 1 = (-5. 445 x 10 -19 J) – (-2. 178 x 10 -18 J) = 1. 633 x 10 -18 J

The positive value for E indicates that the system has gained energy. The wavelength of light that must be absorbed to produce this change is (6. 626 x 10 -34 J. s)(2. 9979 x 108 m/s) 1. 633 x 10 -18 J = 1. 216 x 10 -7 m

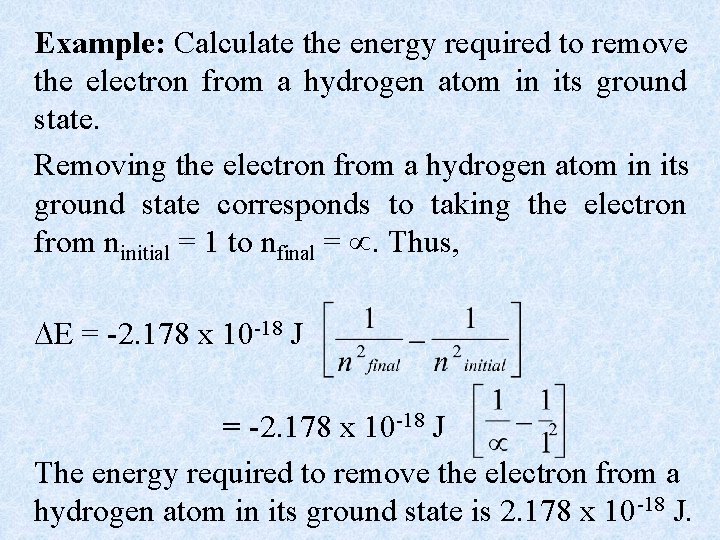
Example: Calculate the energy required to remove the electron from a hydrogen atom in its ground state. Removing the electron from a hydrogen atom in its ground state corresponds to taking the electron from ninitial = 1 to nfinal = . Thus, E = -2. 178 x 10 -18 J The energy required to remove the electron from a hydrogen atom in its ground state is 2. 178 x 10 -18 J.