Chapter Seven Atomic Structure and Periodicity Arrangement of




![Lanthanides & Actinides f-block elements: These elements have the configuration [core] nsx (n - Lanthanides & Actinides f-block elements: These elements have the configuration [core] nsx (n -](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-26.jpg)
![Some Anomalies For instance, the electron configuration for copper is [Ar] 4 s 1 Some Anomalies For instance, the electron configuration for copper is [Ar] 4 s 1](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-28.jpg)

![Who are they? 1. 1 S 22 P 63 S 2 2. [KR]5 S Who are they? 1. 1 S 22 P 63 S 2 2. [KR]5 S](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-31.jpg)
![Who are they? 1. 1 s 22 p 63 s 2 Magnesium 2. [Kr]5 Who are they? 1. 1 s 22 p 63 s 2 Magnesium 2. [Kr]5](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-32.jpg)
![What’s wrong? Mg: [Ar]3 s 2 Fe: 1 s 22 p 63 s 23 What’s wrong? Mg: [Ar]3 s 2 Fe: 1 s 22 p 63 s 23](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-33.jpg)
![Mistakes! Mg: [Ar]3 s 2 Fe: 1 s 22 p 63 s 23 p Mistakes! Mg: [Ar]3 s 2 Fe: 1 s 22 p 63 s 23 p](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-34.jpg)
![Corrections Mg: [Ne]3 s 2 Fe: 1 s 22 p 63 s 23 p Corrections Mg: [Ne]3 s 2 Fe: 1 s 22 p 63 s 23 p](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-35.jpg)











![Write the noble gas notation for the following ions: Mg 2+ [He]2 s Write the noble gas notation for the following ions: Mg 2+ [He]2 s](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-64.jpg)





- Slides: 78

Chapter Seven: Atomic Structure and Periodicity

Arrangement of Electrons in Atoms Reminder: electrons in atoms are arranged as… SHELLS (n) SUBSHELLS (l) ORBITALS (ml)

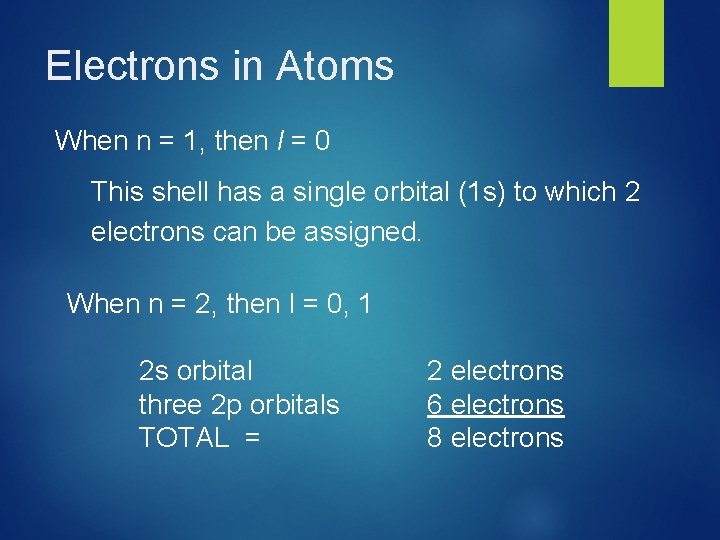
Electrons in Atoms When n = 1, then l = 0 This shell has a single orbital (1 s) to which 2 electrons can be assigned. When n = 2, then l = 0, 1 2 s orbital three 2 p orbitals TOTAL = 2 electrons 6 electrons 8 electrons

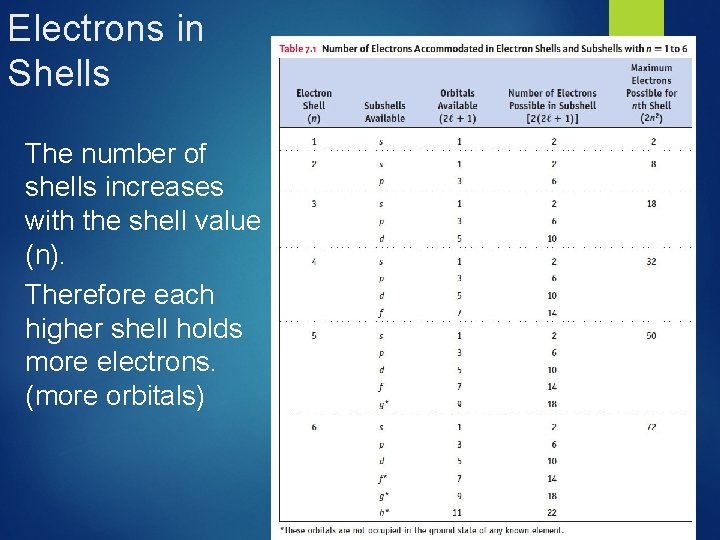
Electrons in Shells The number of shells increases with the shell value (n). Therefore each higher shell holds more electrons. (more orbitals)

Quantum Numbers Electrons in an atom are arranged by: (quantum number) Principle energy levels (shells) n angular energy levels (sub-shells) l oriented energy levels (orbitals) ml Within an individual orbital, there may only be two electrons differentiated by their spin. Spin states (electrons) ms

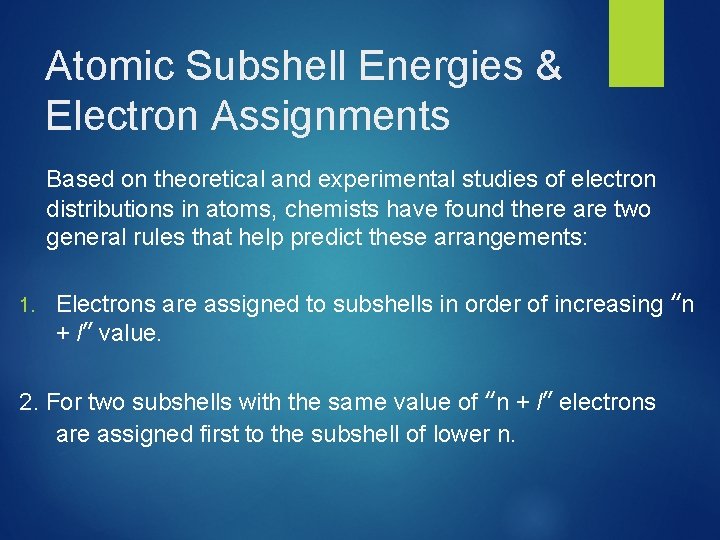
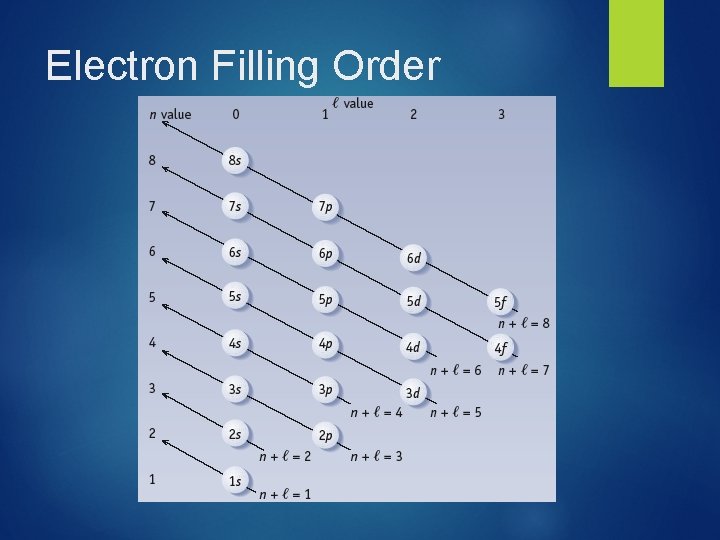
Atomic Subshell Energies & Electron Assignments Based on theoretical and experimental studies of electron distributions in atoms, chemists have found there are two general rules that help predict these arrangements: 1. Electrons are assigned to subshells in order of increasing “n + l” value. 2. For two subshells with the same value of “n + l” electrons are assigned first to the subshell of lower n.

Single-Electron Atom Energy Levels Energy 4 s 3 s 3 p 2 s 2 p 3 d In a Hydrogen atom (1–electron) the orbitals of a subshell are equal in energy (degenerate) 1 s

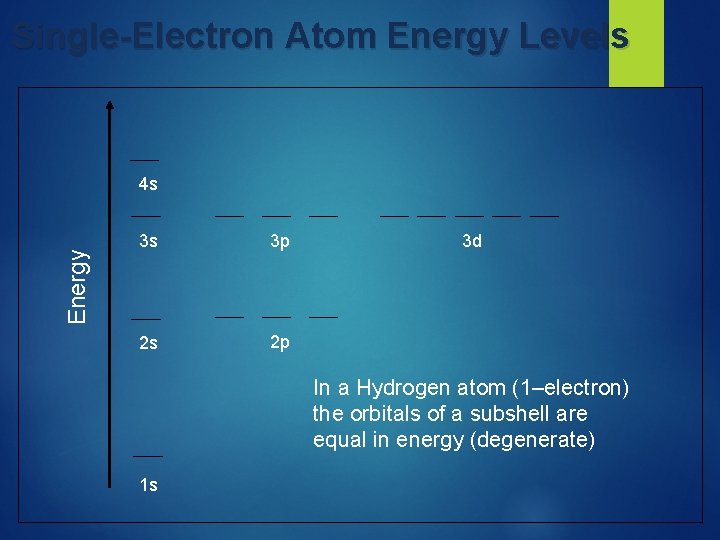
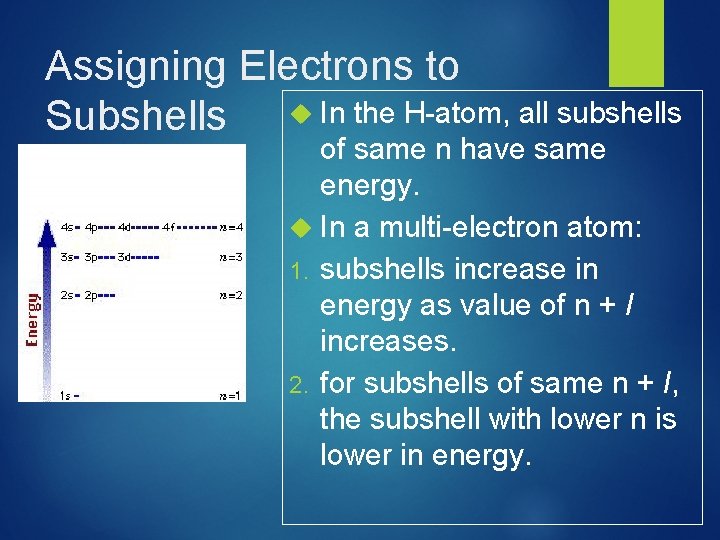
Multi-Electron Atom Energy Levels 3 d 4 s 3 p E=n+l 3 s 2 p 2 s For multi-electron atoms, screening results in the 4 sorbital having a lower energy that of the 3 d-orbital. 4 s: n + l = 4 + 0 = 4 vs. 3 d: n + l = 3 + 2 = 5 1 s

Effective Nuclear Charge, Z* Z* is the net charge experienced by a particular electron in a multi-electron atom resulting from a balance of the attractive force of the nucleus and the repulsive forces of other electrons. Z* increases across a period owing to incomplete screening by inner electrons This explains why E(4 s electron) < E(3 p electron) Z* [ Z (no. inner electrons) ] Charge felt by 2 s electron in: Li. Z* = 3 2 = 1 Be Z* = 4 2 = 2 B Z* = 5 2 = 3 and so on!

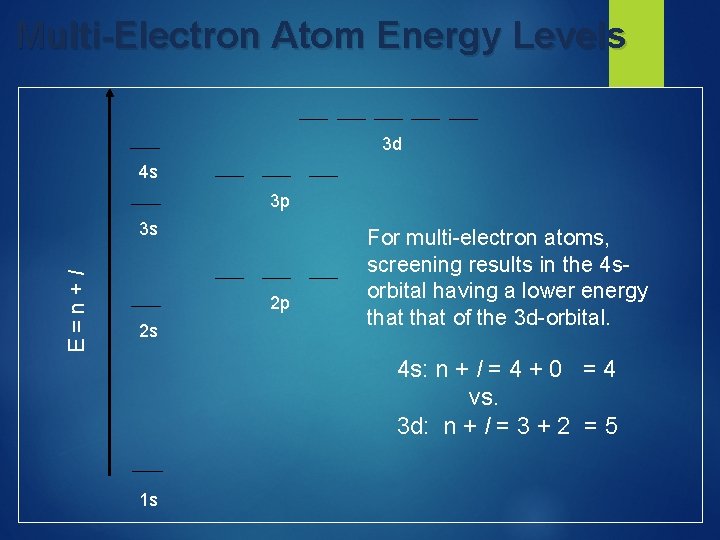
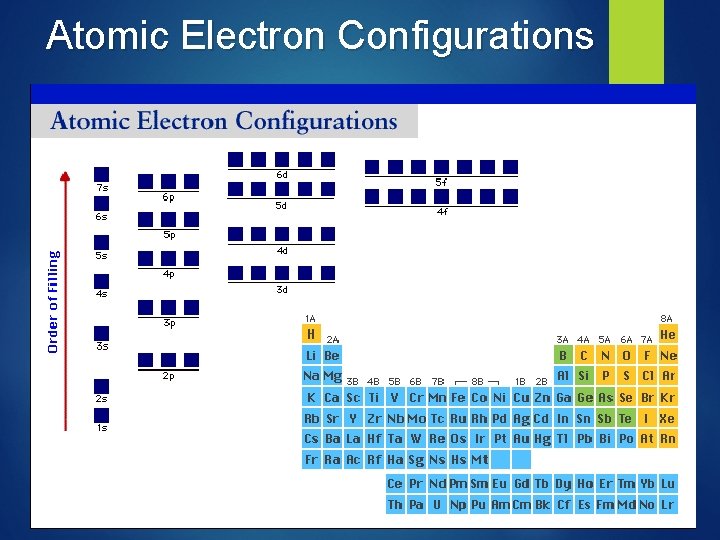
Electron Configurations & the Periodic Table

Electron Filling Order

Assigning Electrons to Subshells In the H-atom, all subshells of same n have same energy. In a multi-electron atom: 1. subshells increase in energy as value of n + l increases. 2. for subshells of same n + I, the subshell with lower n is lower in energy.

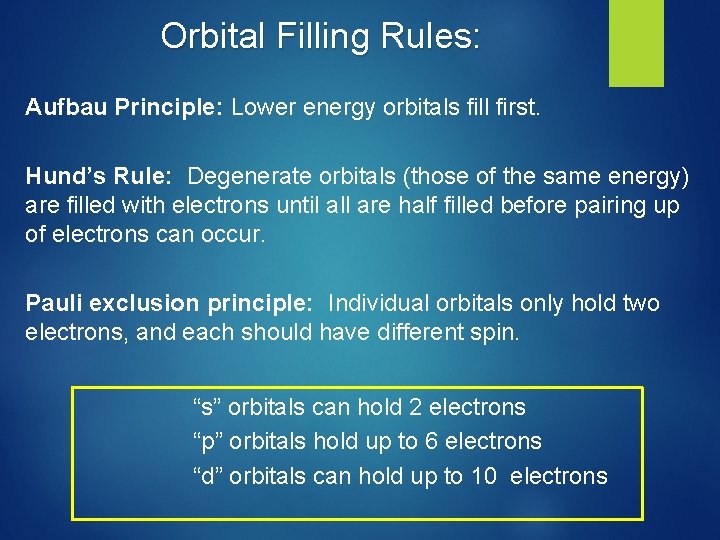
Orbital Filling Rules: Aufbau Principle: Lower energy orbitals fill first. Hund’s Rule: Degenerate orbitals (those of the same energy) are filled with electrons until all are half filled before pairing up of electrons can occur. Pauli exclusion principle: Individual orbitals only hold two electrons, and each should have different spin. “s” orbitals can hold 2 electrons “p” orbitals hold up to 6 electrons “d” orbitals can hold up to 10 electrons

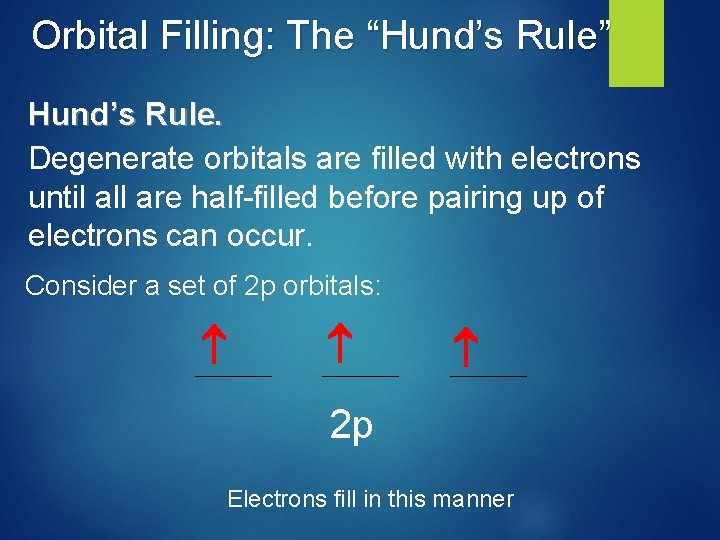
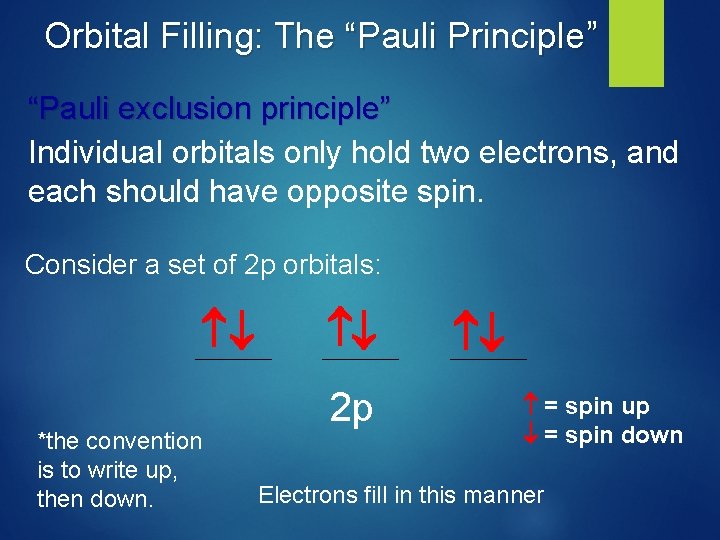
Orbital Filling: The “Hund’s Rule” Hund’s Rule. Degenerate orbitals are filled with electrons until all are half-filled before pairing up of electrons can occur. Consider a set of 2 p orbitals: 2 p Electrons fill in this manner

Orbital Filling: The “Pauli Principle” “Pauli exclusion principle” Individual orbitals only hold two electrons, and each should have opposite spin. Consider a set of 2 p orbitals: *the convention is to write up, then down. 2 p = spin up = spin down Electrons fill in this manner

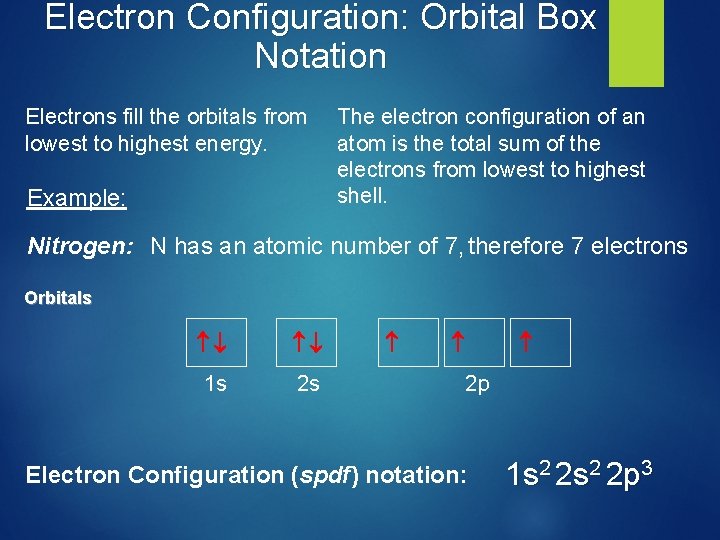
Electron Configuration: Orbital Box Notation Electrons fill the orbitals from lowest to highest energy. The electron configuration of an atom is the total sum of the electrons from lowest to highest shell. Example: Nitrogen: N has an atomic number of 7, therefore 7 electrons Orbitals 1 s 2 s 2 p Electron Configuration (spdf) notation: 1 s 2 2 p 3

Atomic Electron Configurations

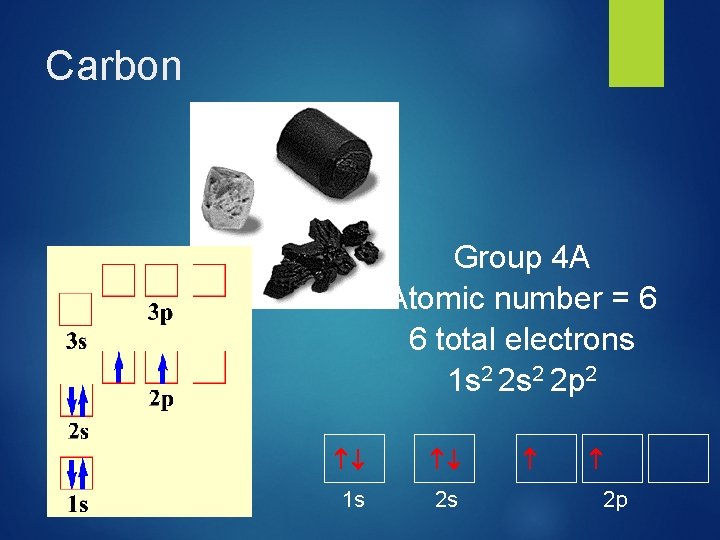
Carbon Group 4 A Atomic number = 6 6 total electrons 1 s 2 2 p 2 1 s 2 p

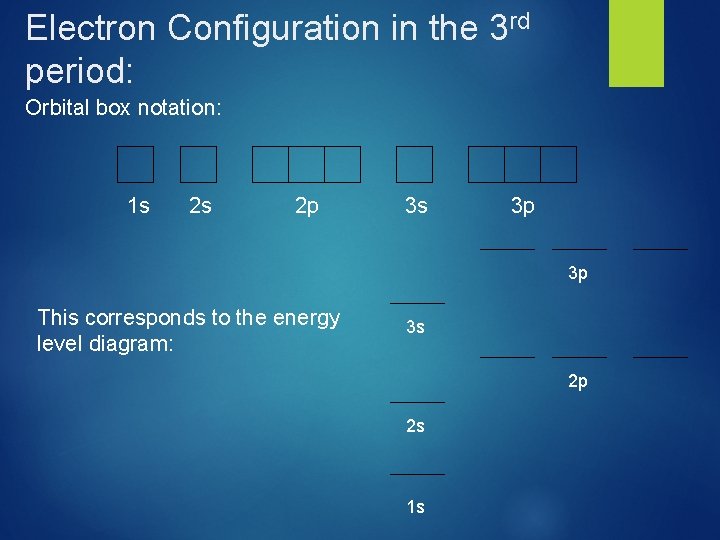
Electron Configuration in the 3 rd period: Orbital box notation: 1 s 2 s 2 p 3 s 3 p 3 p This corresponds to the energy level diagram: 3 s 2 p 2 s 1 s

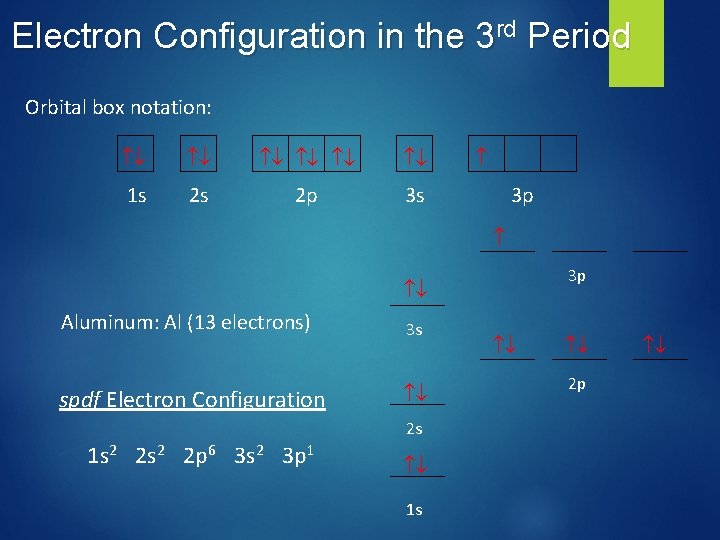
Electron Configuration in the 3 rd Period Orbital box notation: 1 s 2 s 2 p 3 s 3 p Aluminum: Al (13 electrons) 3 s spdf Electron Configuration 2 s 1 s 2 2 p 6 3 s 2 3 p 1 1 s 2 p

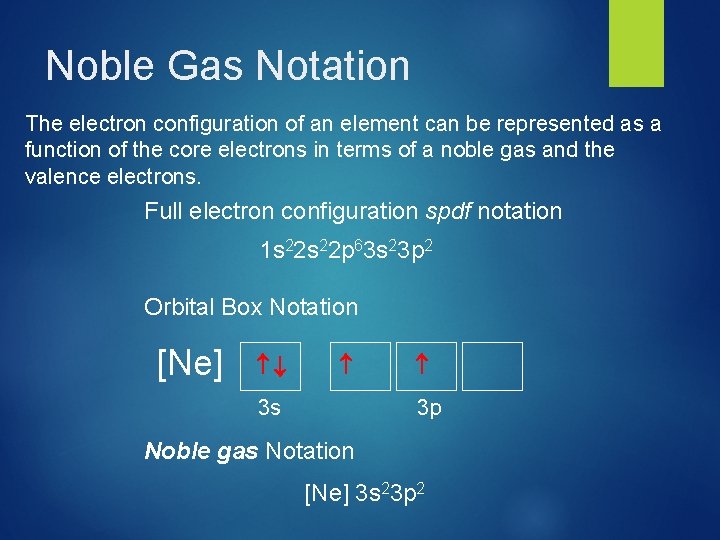
Noble Gas Notation The electron configuration of an element can be represented as a function of the core electrons in terms of a noble gas and the valence electrons. Full electron configuration spdf notation 1 s 22 p 63 s 23 p 2 Orbital Box Notation [Ne] 3 s 3 p Noble gas Notation [Ne] 3 s 23 p 2

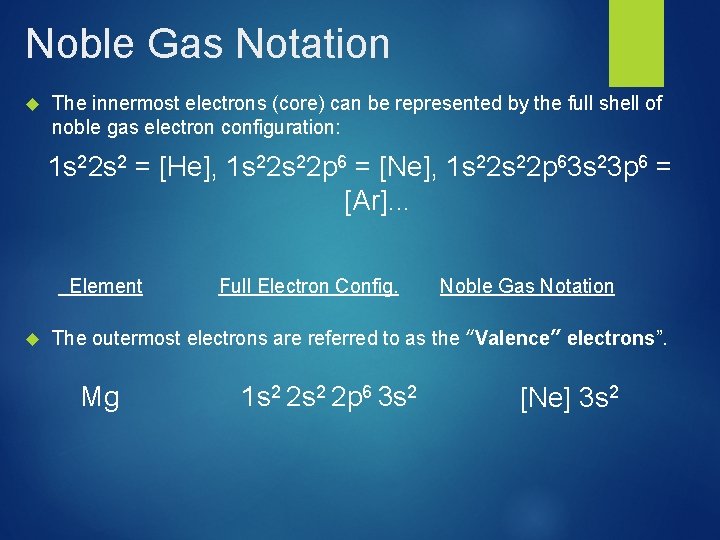
Noble Gas Notation The innermost electrons (core) can be represented by the full shell of noble gas electron configuration: 1 s 22 s 2 = [He], 1 s 22 p 6 = [Ne], 1 s 22 p 63 s 23 p 6 = [Ar]. . . Element Full Electron Config. Noble Gas Notation The outermost electrons are referred to as the “Valence” electrons”. Mg 1 s 2 2 p 6 3 s 2 [Ne] 3 s 2

Transition Metal All 4 th period and beyond d-block elements have the electron configuration [Ar] nsx (n - 1)dy Where n is the period and x, y are particular to the element. Chromium Iron Copper

Transition Elements Electron Configurations are written by shell even though the electrons fill by the periodic table: Ni: last electron to fill: 3 d 8 electron configuration by filling: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8 electron configuration by shell: (write this way) 1 s 2 2 p 6 3 s 2 3 p 6 3 d 8 4 s 2

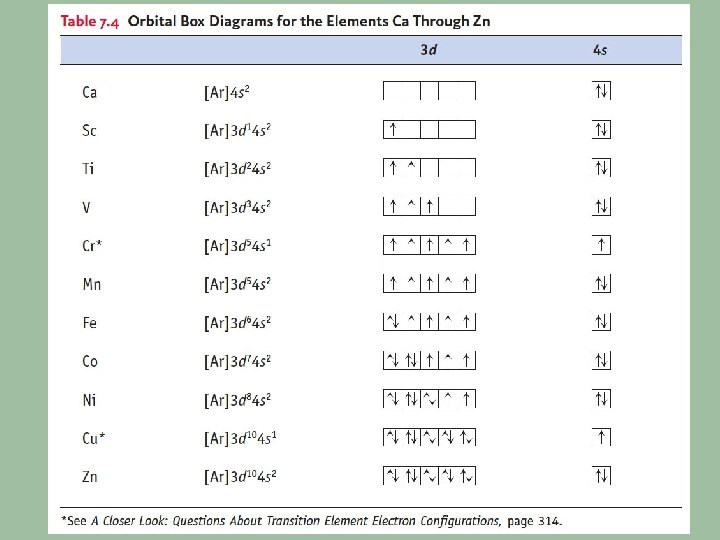
![Lanthanides Actinides fblock elements These elements have the configuration core nsx n Lanthanides & Actinides f-block elements: These elements have the configuration [core] nsx (n -](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-26.jpg)
Lanthanides & Actinides f-block elements: These elements have the configuration [core] nsx (n - 1)dy (n - 2)fz Where n is the period and x, y & z are particular to the element. Cerium: [Xe] 6 s 2 5 d 1 4 f 1 Uranium: [Rn] 7 s 2 6 d 1 5 f 3

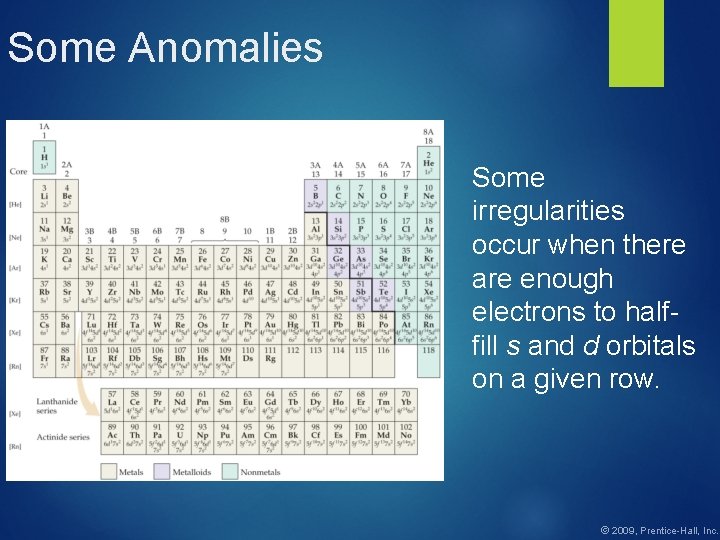
Some Anomalies Some irregularities occur when there are enough electrons to halffill s and d orbitals on a given row. © 2009, Prentice-Hall, Inc.
![Some Anomalies For instance the electron configuration for copper is Ar 4 s 1 Some Anomalies For instance, the electron configuration for copper is [Ar] 4 s 1](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-28.jpg)
Some Anomalies For instance, the electron configuration for copper is [Ar] 4 s 1 3 d 10 rather than the expected [Ar] 4 s 2 3 d 9. © 2009, Prentice-Hall, Inc.

Some Anomalies This occurs because the 4 s and 3 d orbitals are very close in energy. These anomalies occur in f-block atoms, as well. © 2009, Prentice-Hall, Inc.

Filling Rules Aufbau Principle: Electrons fill the lowest energy levels first. Pauli Exclusion Principle: Electrons can fill two/orbital, providing they have opposite spins ( ) Hund’s Rule: Orbitals of the same energy level (p, d, f) distribute electrons 1/orbital before adding the second.
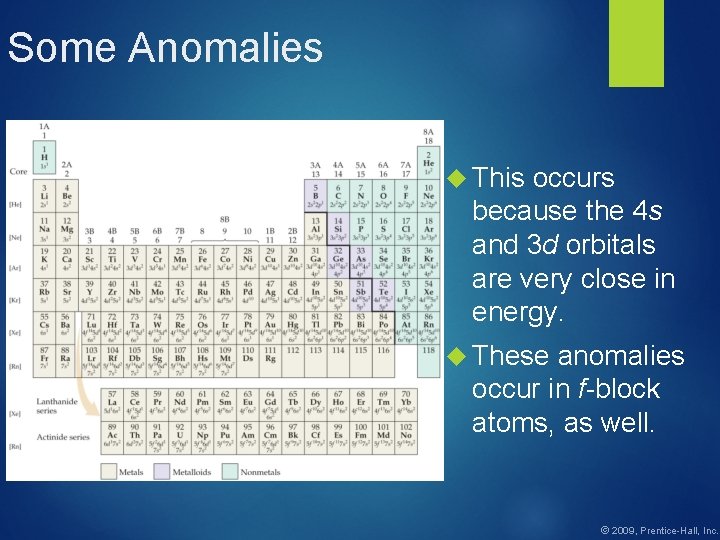
![Who are they 1 1 S 22 P 63 S 2 2 KR5 S Who are they? 1. 1 S 22 P 63 S 2 2. [KR]5 S](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-31.jpg)
Who are they? 1. 1 S 22 P 63 S 2 2. [KR]5 S 24 D 105 P 5 3. 1 S 22 P 3 4. [RN]7 S 1 5. [AR]4 S 23 D 10
![Who are they 1 1 s 22 p 63 s 2 Magnesium 2 Kr5 Who are they? 1. 1 s 22 p 63 s 2 Magnesium 2. [Kr]5](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-32.jpg)
Who are they? 1. 1 s 22 p 63 s 2 Magnesium 2. [Kr]5 s 24 d 105 p 5 Iodine 3. 1 s 22 p 3 Nitrogen 4. [Rn]7 s 1 Francium 5. [Ar]4 s 23 d 10 Zinc
![Whats wrong Mg Ar3 s 2 Fe 1 s 22 p 63 s 23 What’s wrong? Mg: [Ar]3 s 2 Fe: 1 s 22 p 63 s 23](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-33.jpg)
What’s wrong? Mg: [Ar]3 s 2 Fe: 1 s 22 p 63 s 23 p 64 s 24 d 6 Al: [Ne] 3 s 3
![Mistakes Mg Ar3 s 2 Fe 1 s 22 p 63 s 23 p Mistakes! Mg: [Ar]3 s 2 Fe: 1 s 22 p 63 s 23 p](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-34.jpg)
Mistakes! Mg: [Ar]3 s 2 Fe: 1 s 22 p 63 s 23 p 64 s 24 d 6 Al: [Ne] 3 s 3
![Corrections Mg Ne3 s 2 Fe 1 s 22 p 63 s 23 p Corrections Mg: [Ne]3 s 2 Fe: 1 s 22 p 63 s 23 p](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-35.jpg)
Corrections Mg: [Ne]3 s 2 Fe: 1 s 22 p 63 s 23 p 64 s 23 d 6 Al: [Ne] 3 s 23 p 1

Valence Electrons: Electrons at the highest energy level. Electrons available to be gained/lost/shared in a chemical reaction Valence electron configuration: nsxpx (total of 8 valence electrons) Representation of valence electrons in Lewis dot notation:

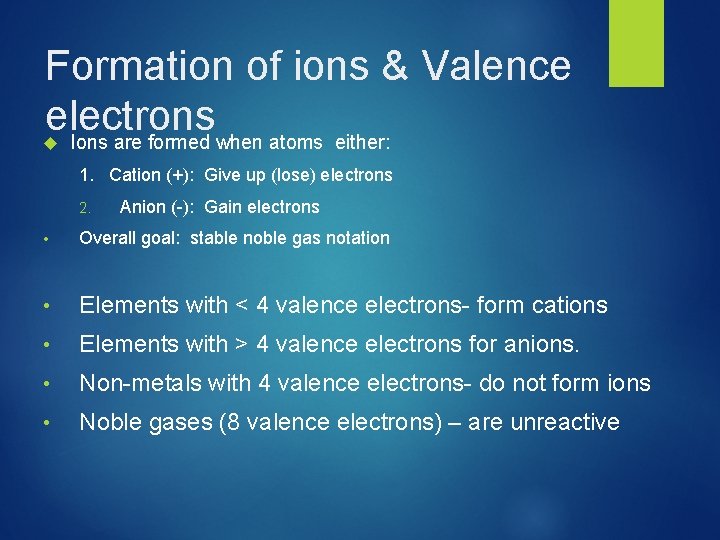
Formation of ions & Valence electrons Ions are formed when atoms either: 1. Cation (+): Give up (lose) electrons 2. Anion (-): Gain electrons • Overall goal: stable noble gas notation • Elements with < 4 valence electrons- form cations • Elements with > 4 valence electrons for anions. • Non-metals with 4 valence electrons- do not form ions • Noble gases (8 valence electrons) – are unreactive

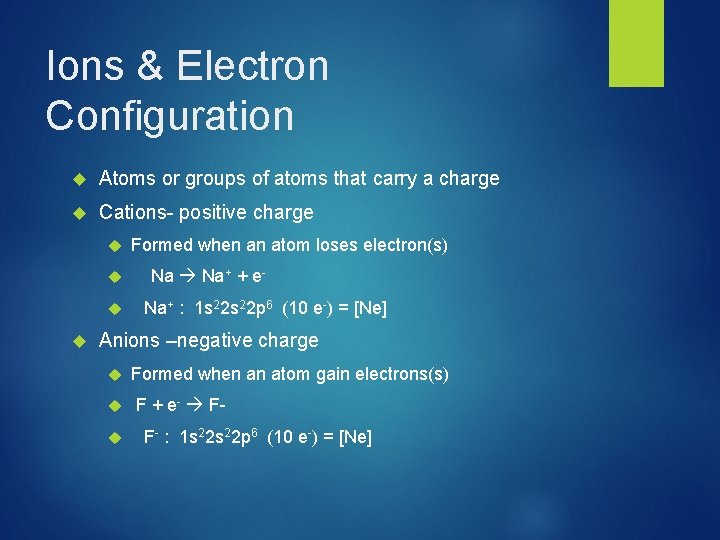
Ions & Electron Configuration Atoms or groups of atoms that carry a charge Cations- positive charge Formed when an atom loses electron(s) Na Na+ + e. Na+ : 1 s 22 p 6 (10 e-) = [Ne] Anions –negative charge Formed when an atom gain electrons(s) F + e- FF- : 1 s 22 p 6 (10 e-) = [Ne]

Isoelectronic series: Ions that contain the same number of electrons. Example: Al 3+, Mg 2+, Na+, F-, O 2 -, N 3 -

Transition Metals & Ions Transition metals: multi-valent ions (more than one possible charge) Electrons are removed from the highest quantum number first: Example: Cu+ & Cu 2+ Cu+: [Ar] 3 d 10 Cu 2+ : [Ar] 3 d 9

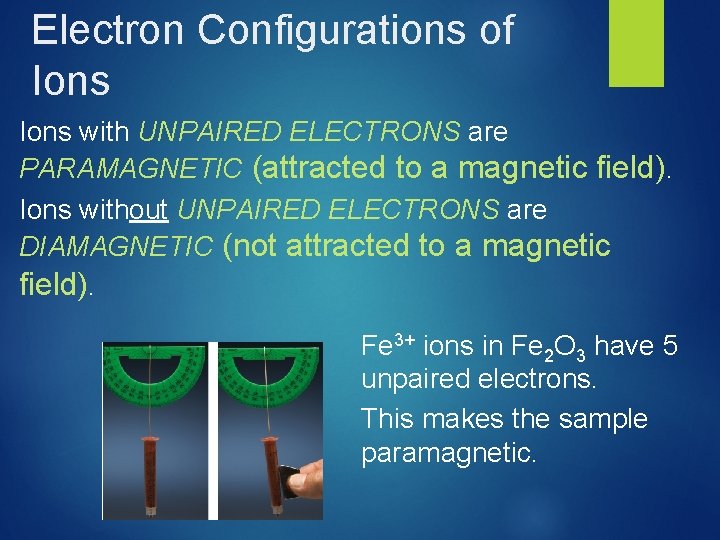
Electron Spin & Magnetism • Diamagnetic Substances: Substances Are NOT attracted to a magnetic field • Paramagnetic Substances: Substances ARE attracted to a magnetic field. • Substances with unpaired electrons are paramagnetic.

Electron Configurations of Ions with UNPAIRED ELECTRONS are PARAMAGNETIC (attracted to a magnetic field). Ions without UNPAIRED ELECTRONS are DIAMAGNETIC (not attracted to a magnetic field). Fe 3+ ions in Fe 2 O 3 have 5 unpaired electrons. This makes the sample paramagnetic.

Practice: Electron Configuration Write the following electron configurations 1. Silicon- orbital notation 2. Strontium – long (spdf) notation 3. Bismuth – noble gas notation 4. Silver – noble gas notation 5. O 2 - - orbital notation 6. Fe 2+ - noble gas notation

Practice #2: Electron Configuration Write the following electron configurations 1. Chromium- long (spdf) notation 2. Sulfur – orbital notation 3. Ag+ – noble gas notation 4. Americium – noble gas notation 5. Mg 2+ - orbital notation 6. Krypton – noble gas notation

Development of Periodic Table Elements in the same group generally have similar chemical properties. Physical properties are not necessarily similar, however. © 2009, Prentice-Hall, Inc.

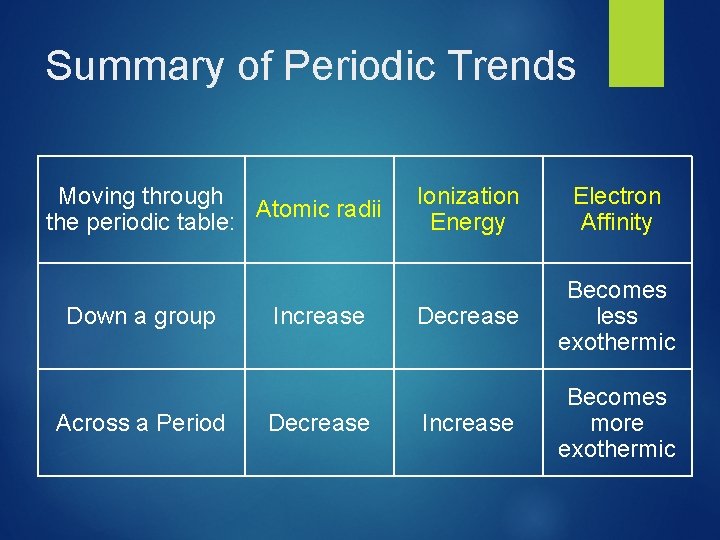
Periodic Trends this chapter, we will rationalize observed trends in: Sizes of atoms and ions. Ionization Electron energy. affinity. © 2009, Prentice-Hall, Inc. In

General Periodic Trends Atomic and ionic size Ionization energy Electron affinity Higher effective nuclear charge Electrons held more tightly Larger orbitals. Electrons held less tightly.

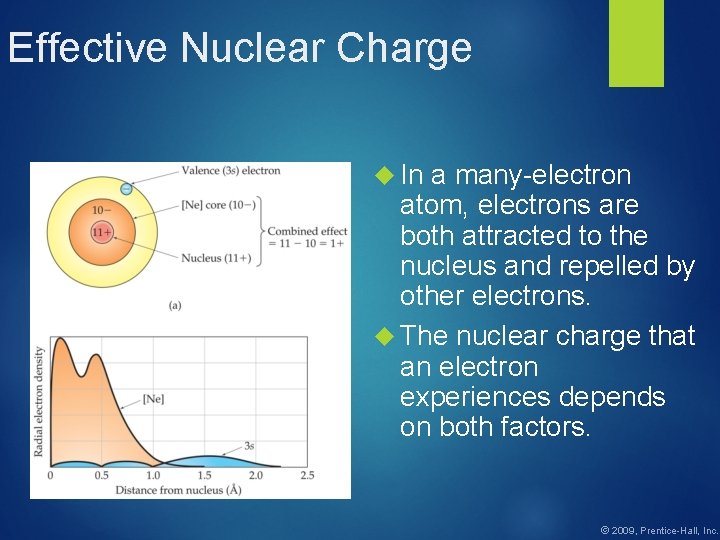
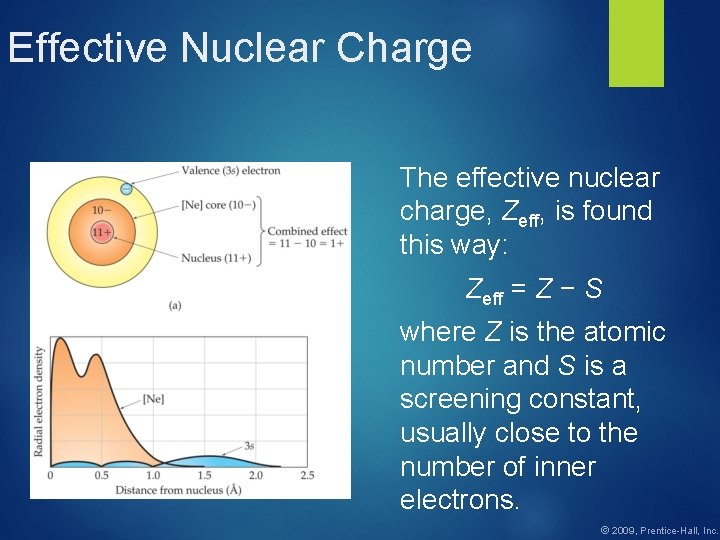
Effective Nuclear Charge In a many-electron atom, electrons are both attracted to the nucleus and repelled by other electrons. The nuclear charge that an electron experiences depends on both factors. © 2009, Prentice-Hall, Inc.

Effective Nuclear Charge The effective nuclear charge, Zeff, is found this way: Zeff = Z − S where Z is the atomic number and S is a screening constant, usually close to the number of inner electrons. © 2009, Prentice-Hall, Inc.

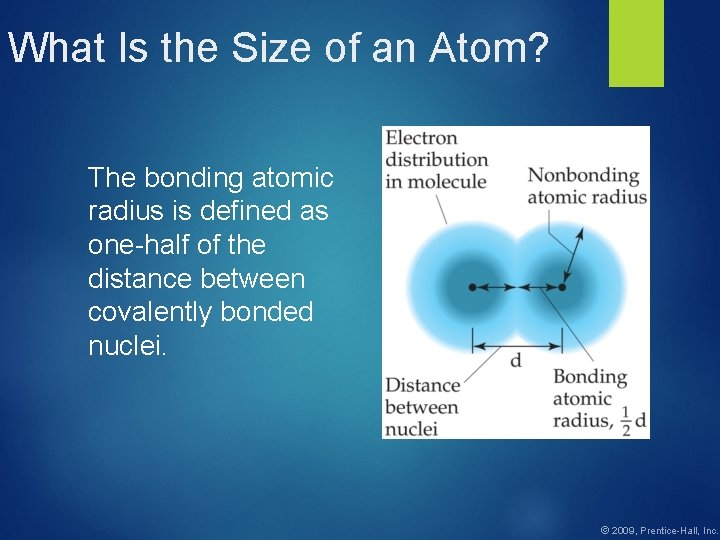
What Is the Size of an Atom? The bonding atomic radius is defined as one-half of the distance between covalently bonded nuclei. © 2009, Prentice-Hall, Inc.

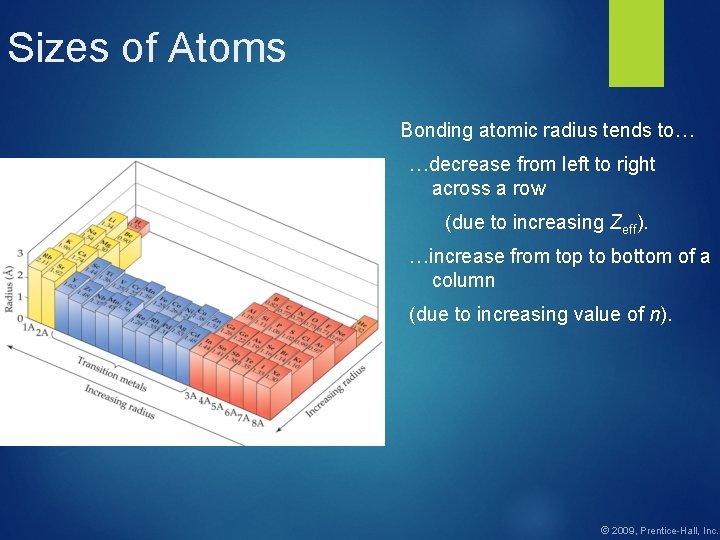
Sizes of Atoms Bonding atomic radius tends to… …decrease from left to right across a row (due to increasing Zeff). …increase from top to bottom of a column (due to increasing value of n). © 2009, Prentice-Hall, Inc.

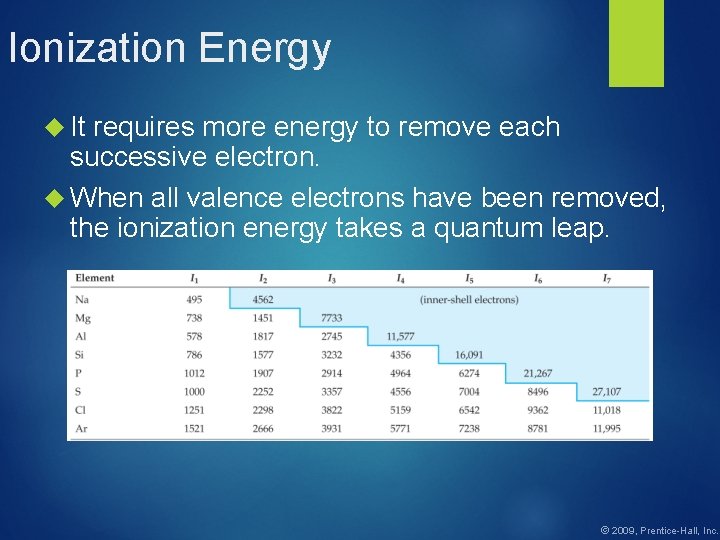
Ionization Energy ionization energy is the amount of energy required to remove an electron from the ground state of a gaseous atom or ion. The first ionization energy is that energy required to remove first electron. A(g) + energy A+ + e- The second ionization energy is that energy required to remove second electron, etc. A+(g) + energy A 2+ + e- © 2009, Prentice-Hall, Inc. The

Ionization Energy It requires more energy to remove each successive electron. When all valence electrons have been removed, the ionization energy takes a quantum leap. © 2009, Prentice-Hall, Inc.

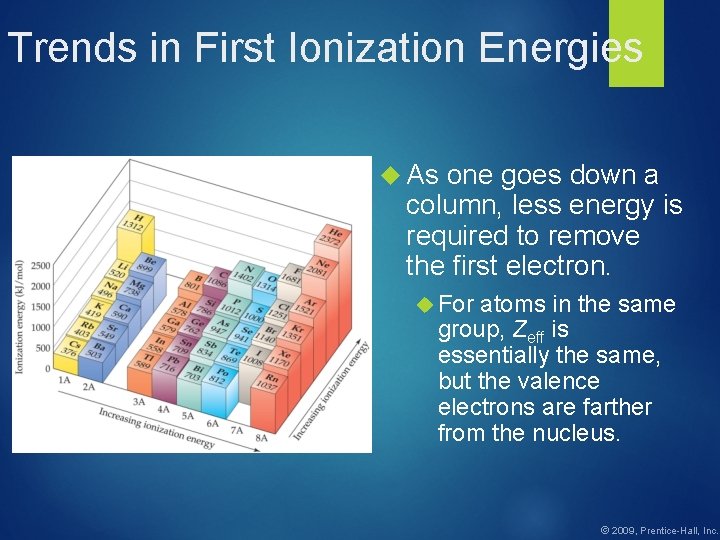
Trends in First Ionization Energies As one goes down a column, less energy is required to remove the first electron. For atoms in the same group, Zeff is essentially the same, but the valence electrons are farther from the nucleus. © 2009, Prentice-Hall, Inc.

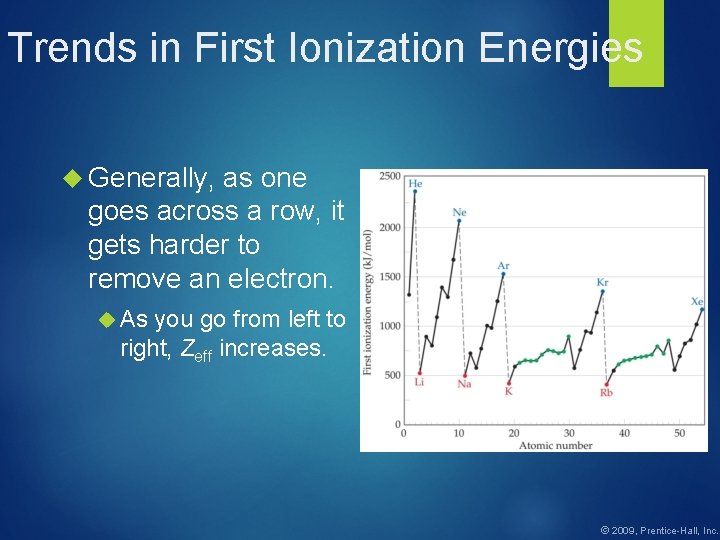
Trends in First Ionization Energies Generally, as one goes across a row, it gets harder to remove an electron. As you go from left to right, Zeff increases. © 2009, Prentice-Hall, Inc.

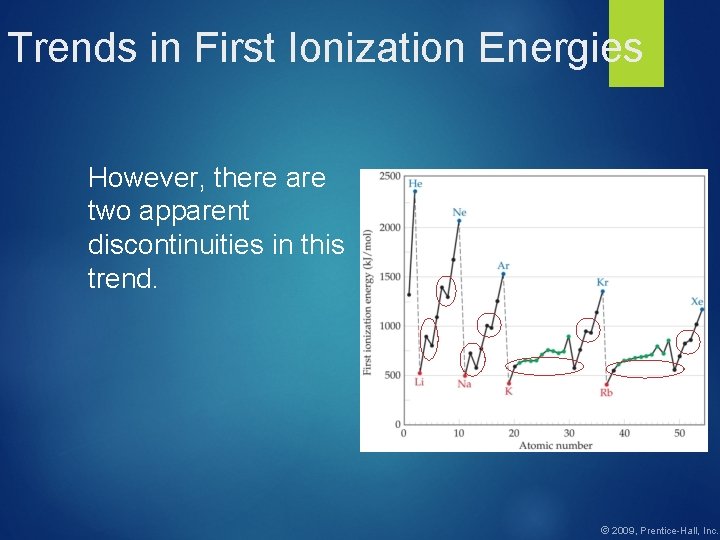
Trends in First Ionization Energies However, there are two apparent discontinuities in this trend. © 2009, Prentice-Hall, Inc.

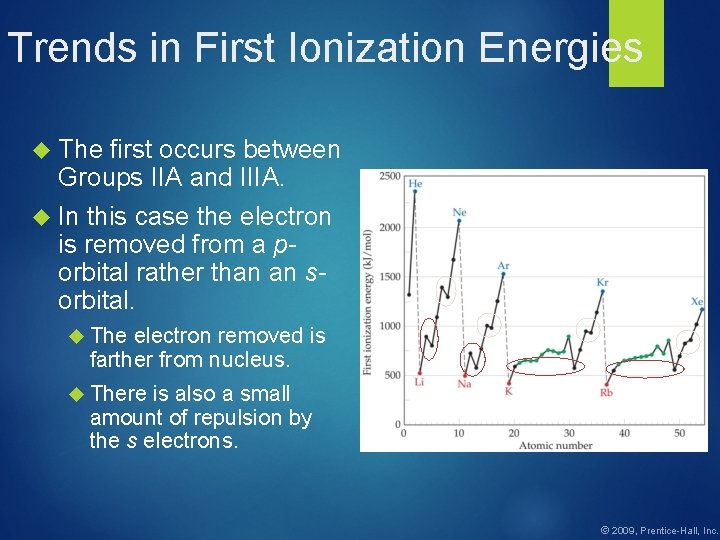
Trends in First Ionization Energies The first occurs between Groups IIA and IIIA. In this case the electron is removed from a porbital rather than an sorbital. The electron removed is farther from nucleus. There is also a small amount of repulsion by the s electrons. © 2009, Prentice-Hall, Inc.

Electron Affinity ( EAH) Cl(g) + e− Cl− © 2009, Prentice-Hall, Inc. Electron affinity is the energy change accompanying the addition of an electron to a gaseous atom:

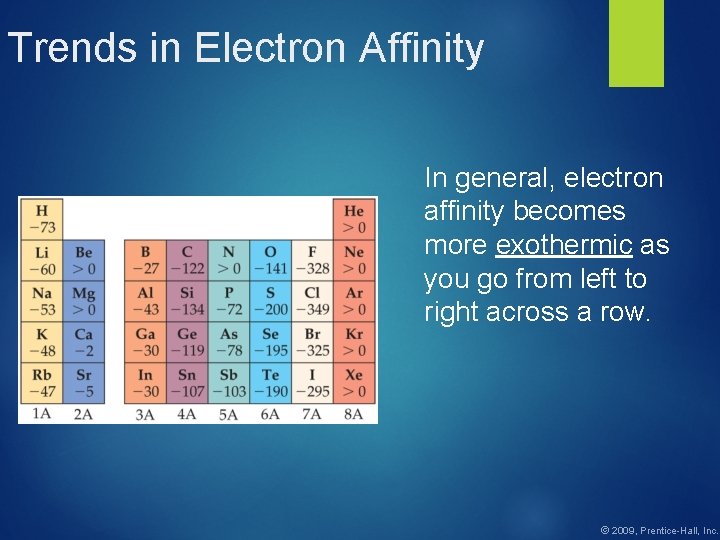
Trends in Electron Affinity In general, electron affinity becomes more exothermic as you go from left to right across a row. © 2009, Prentice-Hall, Inc.

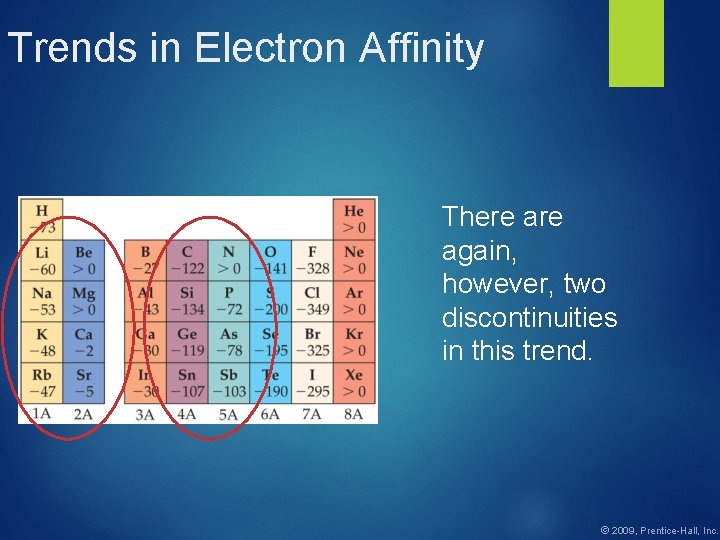
Trends in Electron Affinity There again, however, two discontinuities in this trend. © 2009, Prentice-Hall, Inc.

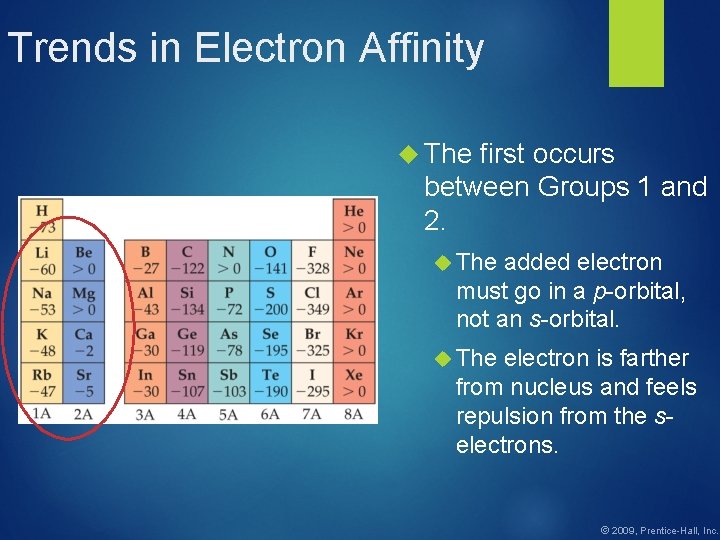
Trends in Electron Affinity The first occurs between Groups 1 and 2. The added electron must go in a p-orbital, not an s-orbital. The electron is farther from nucleus and feels repulsion from the selectrons. © 2009, Prentice-Hall, Inc.

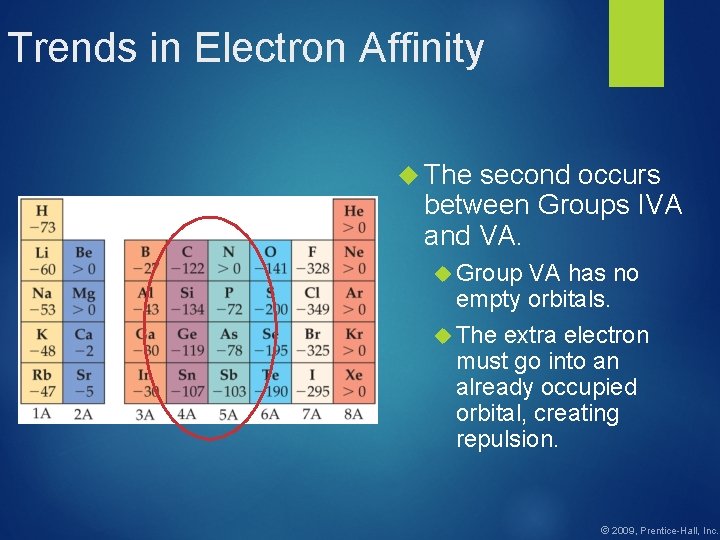
Trends in Electron Affinity The second occurs between Groups IVA and VA. Group VA has no empty orbitals. The extra electron must go into an already occupied orbital, creating repulsion. © 2009, Prentice-Hall, Inc.

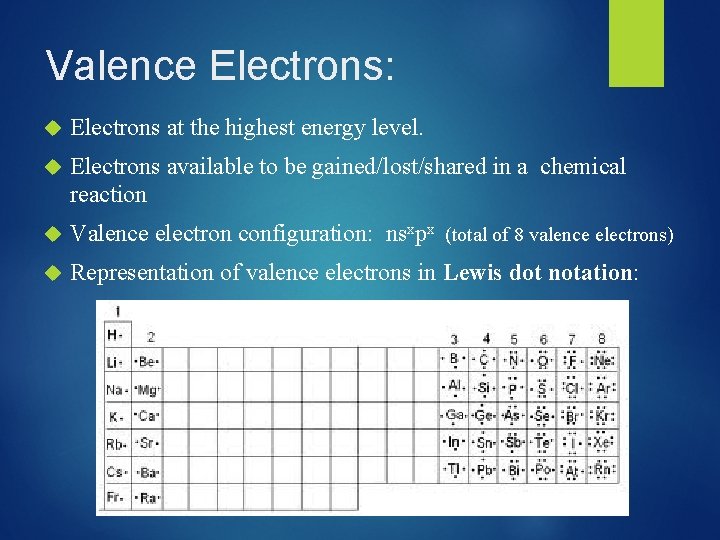
Ions: Write the noble gas notation for the following ions: Mg 2+ P 3 Fe 3+
![Write the noble gas notation for the following ions Mg 2 He2 s Write the noble gas notation for the following ions: Mg 2+ [He]2 s](https://slidetodoc.com/presentation_image/a4b52a72c732e60dec184bc74a058c79/image-64.jpg)
Write the noble gas notation for the following ions: Mg 2+ [He]2 s 22 p 6 P 3 - [Ne] 3 s 23 p 6 Fe 3+ [Ar] 3 d 5

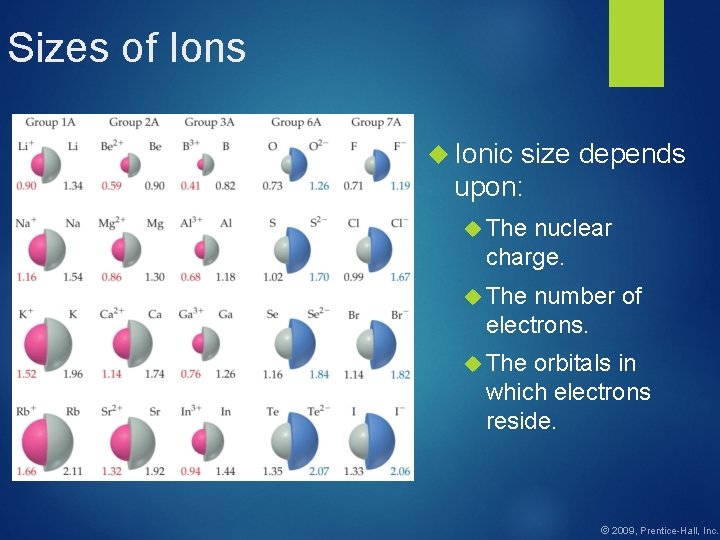
Sizes of Ions Ionic size depends upon: The nuclear charge. The number of electrons. The orbitals in which electrons reside. © 2009, Prentice-Hall, Inc.

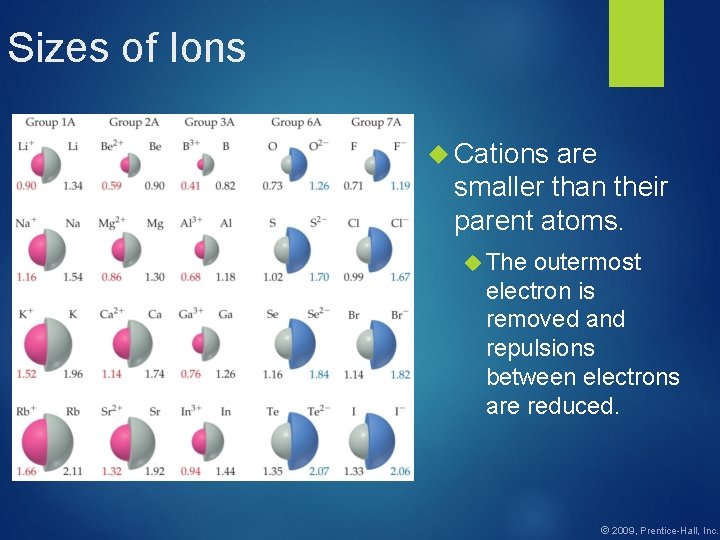
Sizes of Ions Cations are smaller than their parent atoms. The outermost electron is removed and repulsions between electrons are reduced. © 2009, Prentice-Hall, Inc.

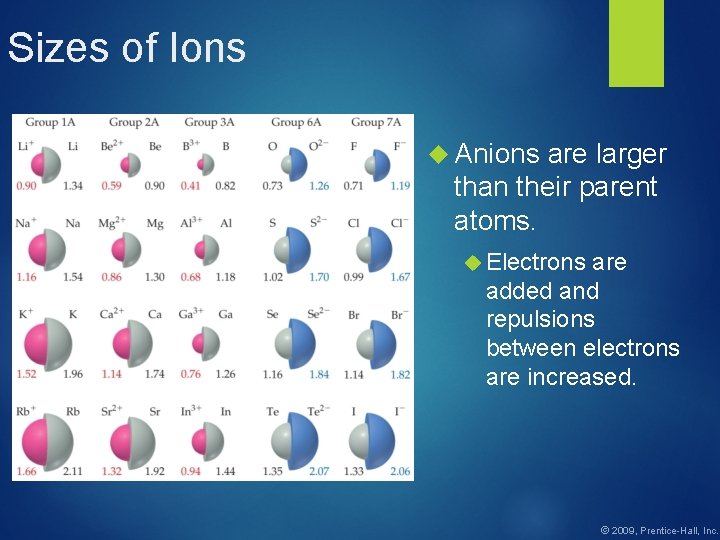
Sizes of Ions Anions are larger than their parent atoms. Electrons are added and repulsions between electrons are increased. © 2009, Prentice-Hall, Inc.

Sizes of Ions increase in size as you go down a column. This is due to increasing value of n. © 2009, Prentice-Hall, Inc.

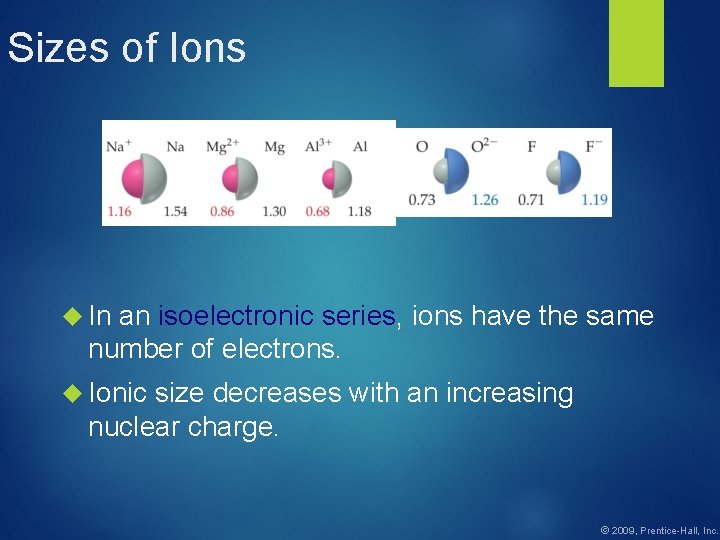
Sizes of Ions In an isoelectronic series, ions have the same number of electrons. Ionic size decreases with an increasing nuclear charge. © 2009, Prentice-Hall, Inc.

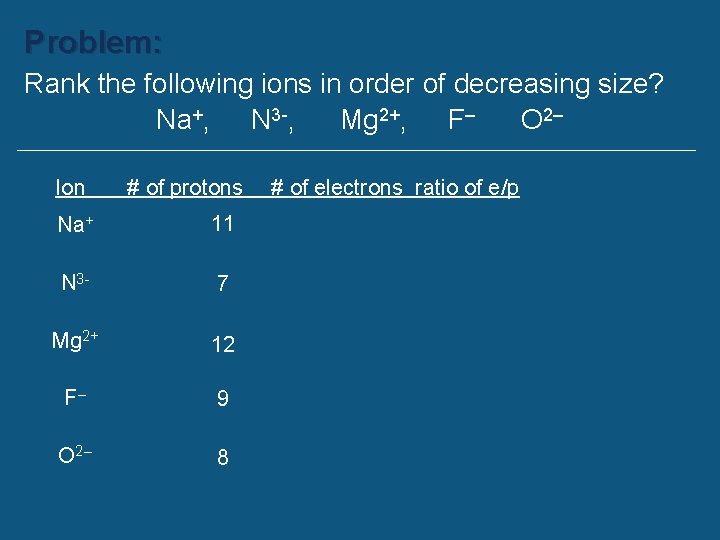
Problem: Rank the following ions in order of decreasing size? Na+, N 3 -, Mg 2+, F– O 2–

Problem: Rank the following ions in order of decreasing size? Na+, N 3 -, Mg 2+, F– O 2– Ion Na+ N 3 Mg 2+ F– O 2– # of protons # of electrons ratio of e/p

Problem: Rank the following ions in order of decreasing size? Na+, N 3 -, Mg 2+, F– O 2– Ion # of protons Na+ 11 N 3 - 7 Mg 2+ 12 F– 9 O 2– 8 # of electrons ratio of e/p

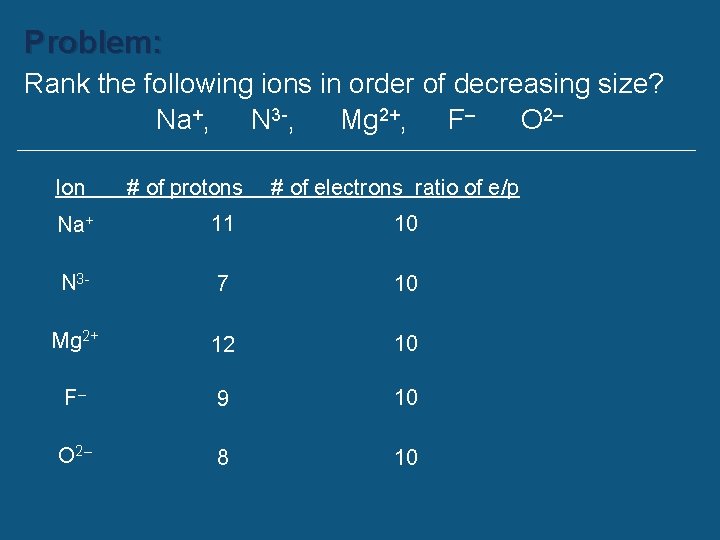
Problem: Rank the following ions in order of decreasing size? Na+, N 3 -, Mg 2+, F– O 2– Ion # of protons # of electrons ratio of e/p Na+ 11 10 N 3 - 7 10 Mg 2+ 12 10 F– 9 10 O 2– 8 10

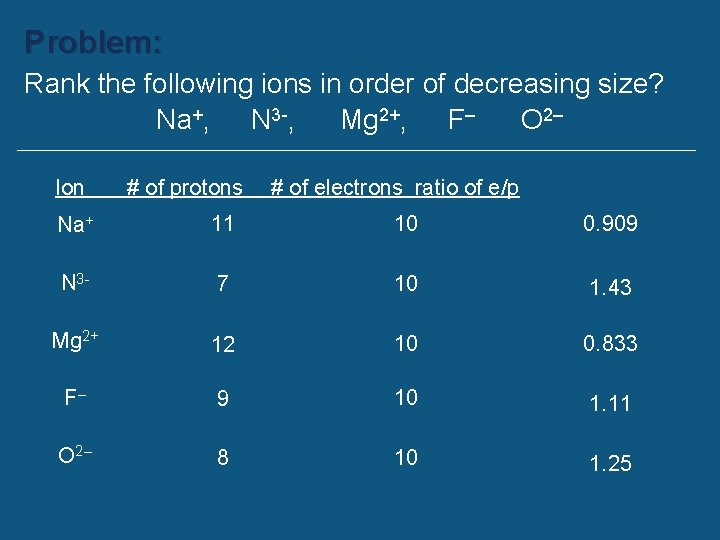
Problem: Rank the following ions in order of decreasing size? Na+, N 3 -, Mg 2+, F– O 2– Ion # of protons # of electrons ratio of e/p Na+ 11 10 0. 909 N 3 - 7 10 1. 43 Mg 2+ 12 10 0. 833 F– 9 10 1. 11 O 2– 8 10 1. 25

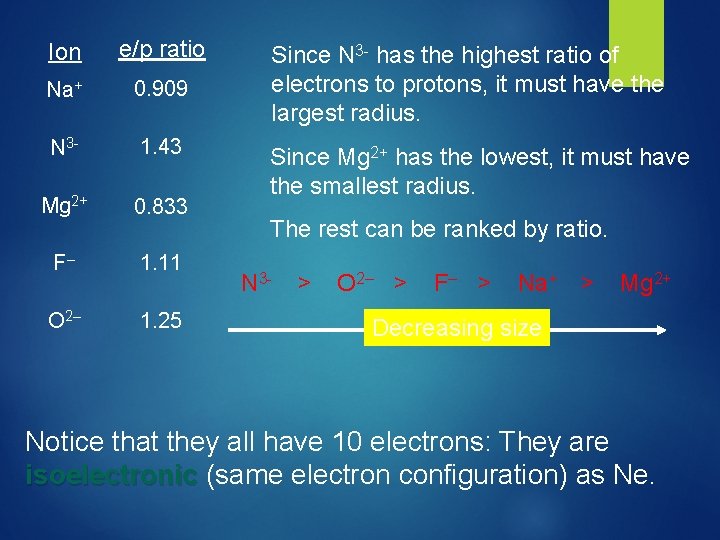
Ion e/p ratio Na+ 0. 909 N 3 - 1. 43 Mg 2+ 0. 833 F– 1. 11 O 2– 1. 25 Since N 3 - has the highest ratio of electrons to protons, it must have the largest radius.

Ion e/p ratio Na+ 0. 909 N 3 - 1. 43 Mg 2+ 0. 833 F– 1. 11 O 2– 1. 25 Since N 3 - has the highest ratio of electrons to protons, it must have the largest radius. Since Mg 2+ has the lowest, it must have the smallest radius. The rest can be ranked by ratio.

Ion e/p ratio Na+ 0. 909 N 3 - 1. 43 Mg 2+ 0. 833 F– 1. 11 O 2– 1. 25 Since N 3 - has the highest ratio of electrons to protons, it must have the largest radius. Since Mg 2+ has the lowest, it must have the smallest radius. The rest can be ranked by ratio. N 3 - > O 2– > F– > Na+ > Mg 2+ Decreasing size Notice that they all have 10 electrons: They are isoelectronic (same electron configuration) as Ne.

Summary of Periodic Trends Moving through Atomic radii the periodic table: Down a group Across a Period Increase Decrease Ionization Energy Electron Affinity Decrease Becomes less exothermic Increase Becomes more exothermic