ATOMIC ELECTRON CONFIGURATIONS AND PERIODICITY 1 Arrangement of



![Transition Metals Table 8. 4 All 4 th period elements have the configuration [argon] Transition Metals Table 8. 4 All 4 th period elements have the configuration [argon]](https://slidetodoc.com/presentation_image_h2/4deada5f3d3515d269ad47a1e29bcbec/image-18.jpg)
![Lanthanides and Actinides 20 All these elements have the configuration [core] nsx (n - Lanthanides and Actinides 20 All these elements have the configuration [core] nsx (n -](https://slidetodoc.com/presentation_image_h2/4deada5f3d3515d269ad47a1e29bcbec/image-20.jpg)

- Slides: 29

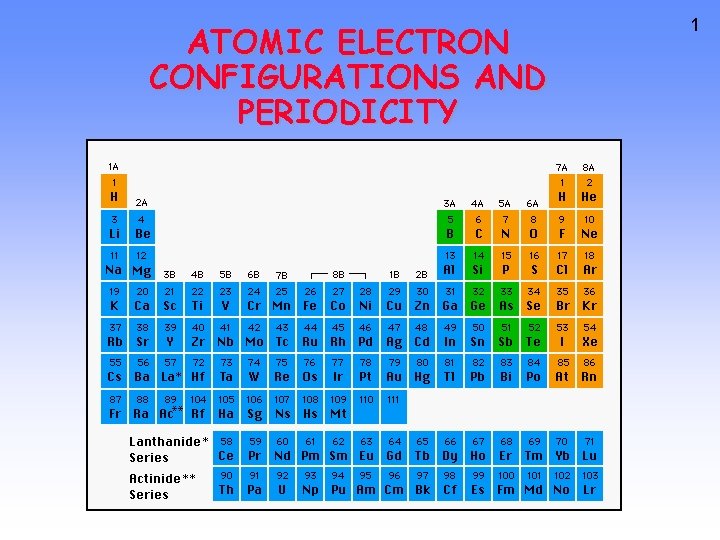
ATOMIC ELECTRON CONFIGURATIONS AND PERIODICITY 1

Arrangement of Electrons in Atoms Each orbital can be assigned no more than 2 electrons! This is tied to the existence of a 4 th quantum number, the electron spin quantum number, ms. 2

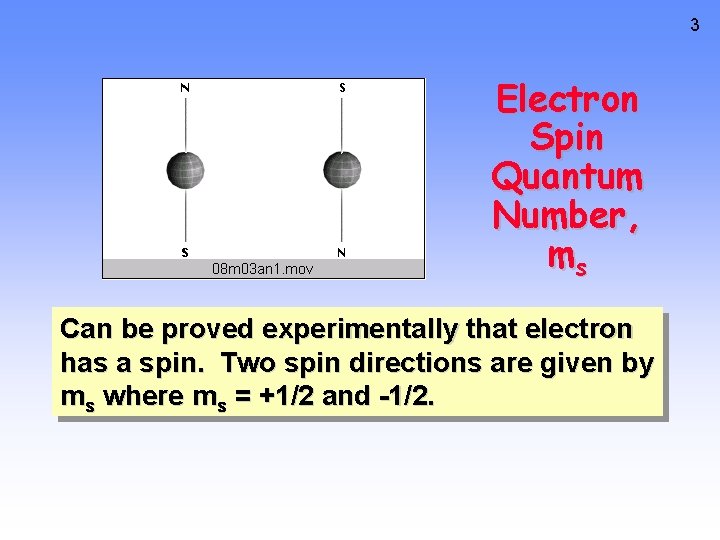
3 Electron Spin Quantum Number, ms Can be proved experimentally that electron has a spin. Two spin directions are given by ms where ms = +1/2 and -1/2.

Electron Spin Quantum Number Diamagnetic: NOT attracted to a magnetic field Paramagnetic: substance is attracted to a magnetic field. Substance has unpaired electrons. 4

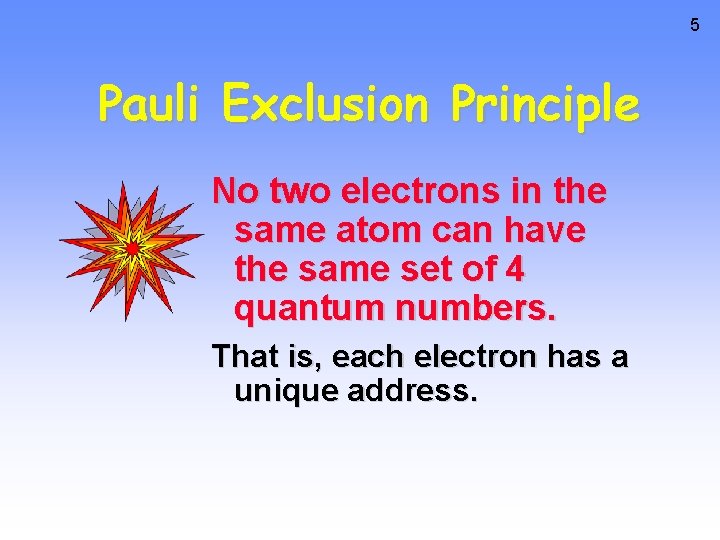
5 Pauli Exclusion Principle No two electrons in the same atom can have the same set of 4 quantum numbers. That is, each electron has a unique address.

6

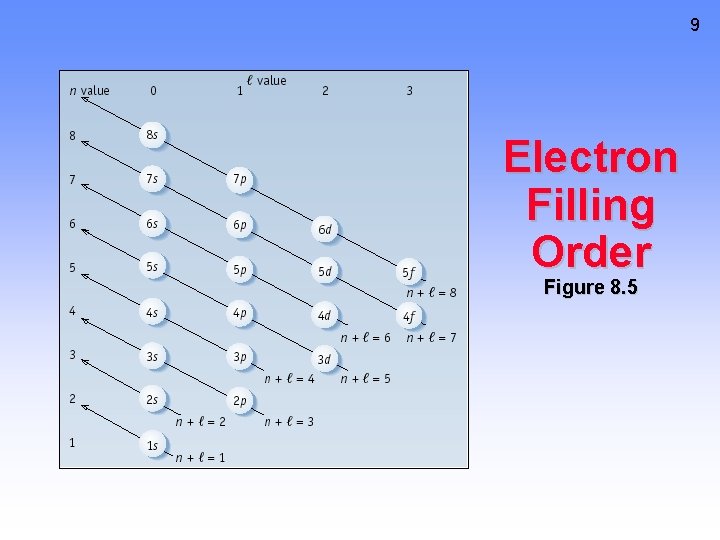
7 Assigning Electrons to Atoms • Electrons generally assigned to orbitals of successively higher energy. • For H atoms, E = - C(1/n 2). E depends only on n. • For many-electron atoms, energy depends on both n and l. • See Figure 8. 5, page 295 and Screen 8. 7.

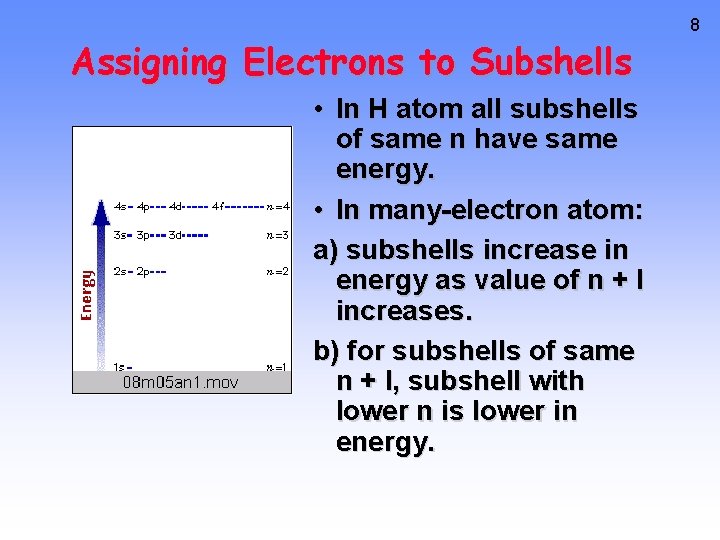
Assigning Electrons to Subshells • In H atom all subshells of same n have same energy. • In many-electron atom: a) subshells increase in energy as value of n + l increases. b) for subshells of same n + l, subshell with lower n is lower in energy. 8

9 Electron Filling Order Figure 8. 5

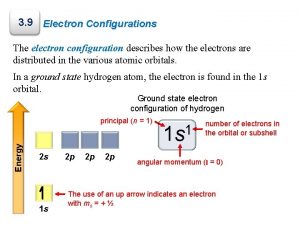
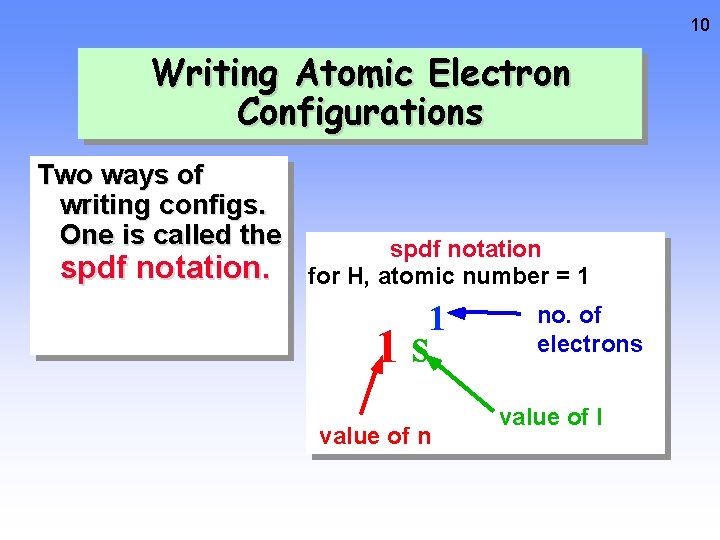
10 Writing Atomic Electron Configurations Two ways of writing configs. One is called the spdf notation for H, atomic number = 1 1 1 s value of n no. of electrons value of l

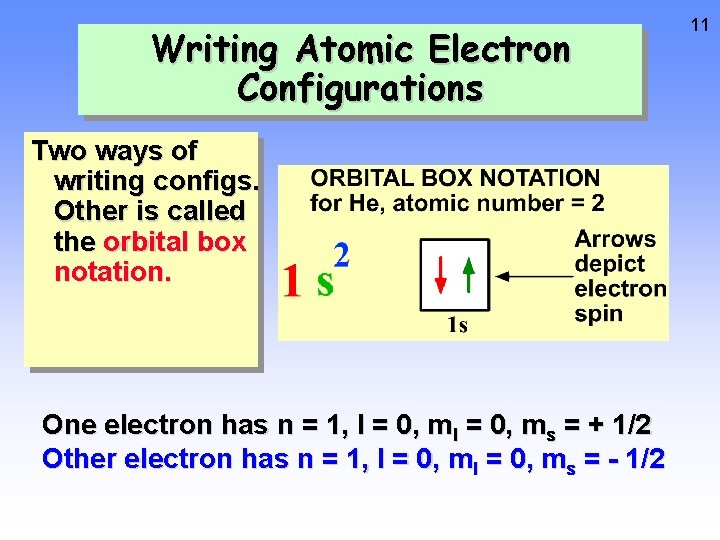
Writing Atomic Electron Configurations Two ways of writing configs. Other is called the orbital box notation. One electron has n = 1, l = 0, ms = + 1/2 Other electron has n = 1, l = 0, ms = - 1/2 11

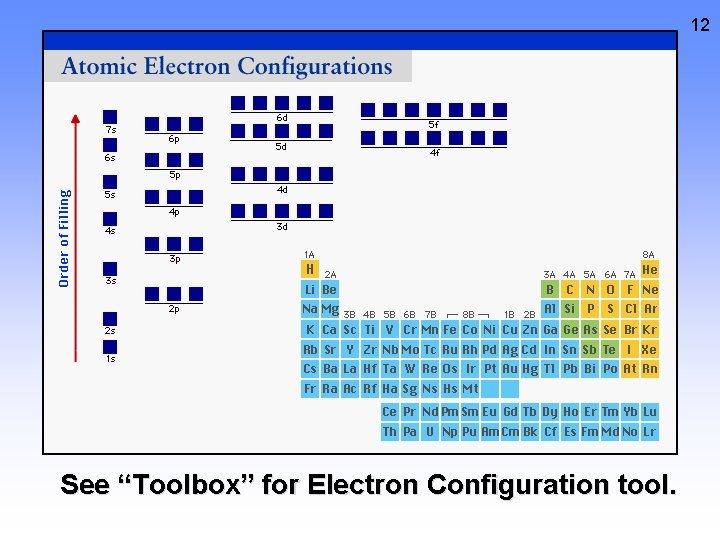
12 See “Toolbox” for Electron Configuration tool.

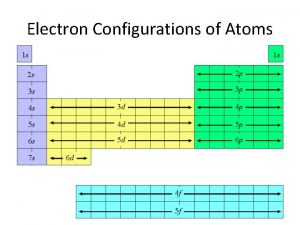
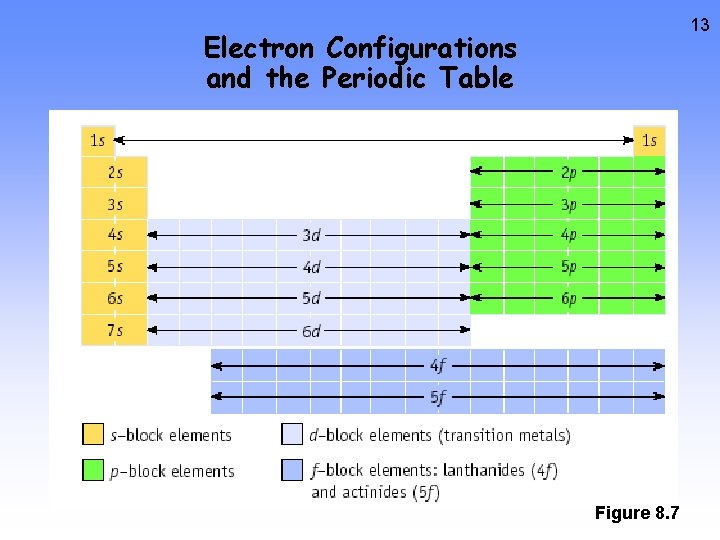
13 Electron Configurations and the Periodic Table Figure 8. 7

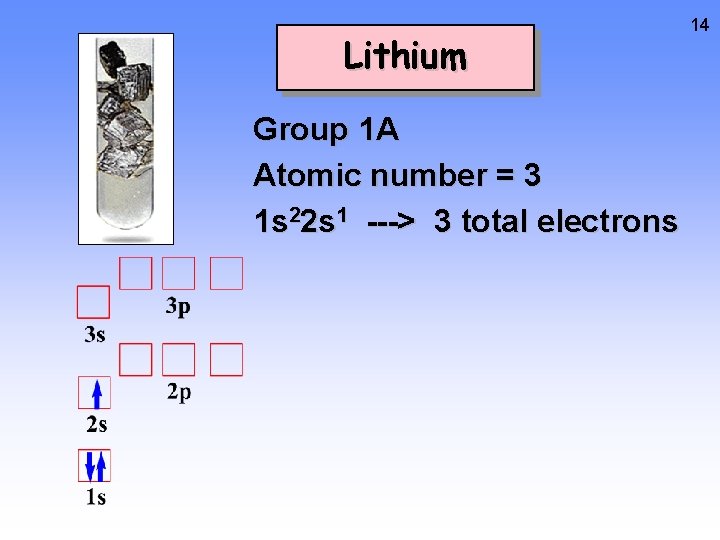
Lithium Group 1 A Atomic number = 3 1 s 22 s 1 ---> 3 total electrons 14

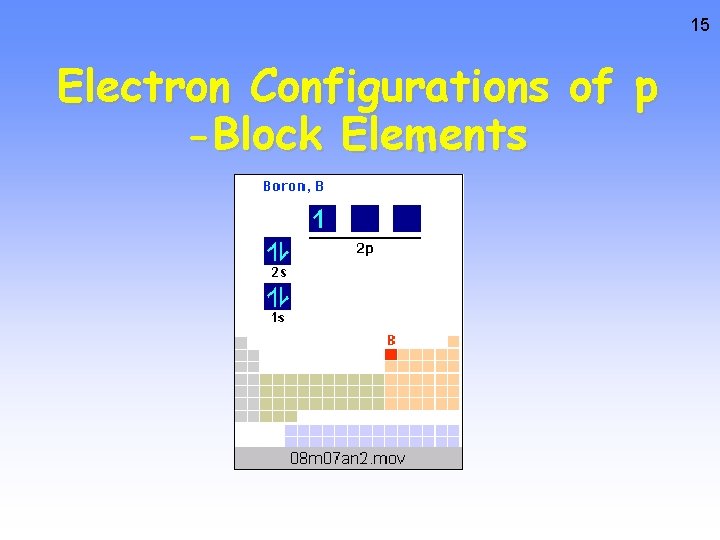
15 Electron Configurations of p -Block Elements

Sodium Group 1 A Atomic number = 11 1 s 2 2 p 6 3 s 1 or “neon core” + 3 s 1 [Ne] 3 s 1 (uses rare gas notation) Note that we have begun a new period. All Group 1 A elements have [core]ns 1 configurations. 16

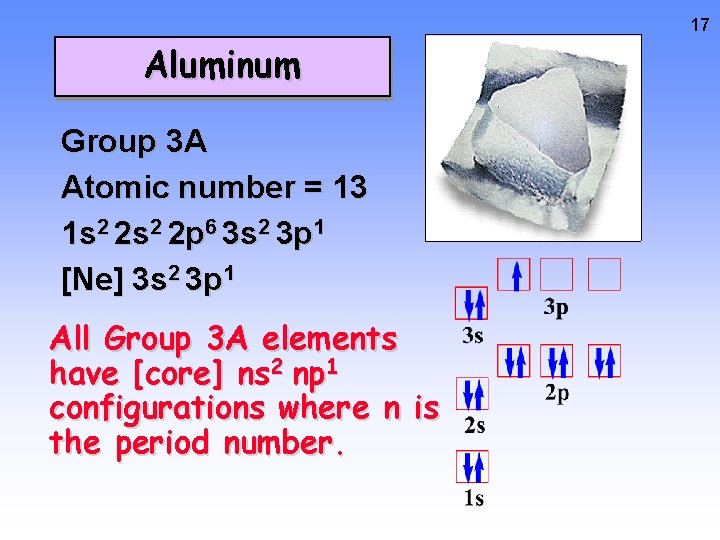
17 Aluminum Group 3 A Atomic number = 13 1 s 2 2 p 6 3 s 2 3 p 1 [Ne] 3 s 2 3 p 1 All Group 3 A elements have [core] ns 2 np 1 configurations where n is the period number.
![Transition Metals Table 8 4 All 4 th period elements have the configuration argon Transition Metals Table 8. 4 All 4 th period elements have the configuration [argon]](https://slidetodoc.com/presentation_image_h2/4deada5f3d3515d269ad47a1e29bcbec/image-18.jpg)
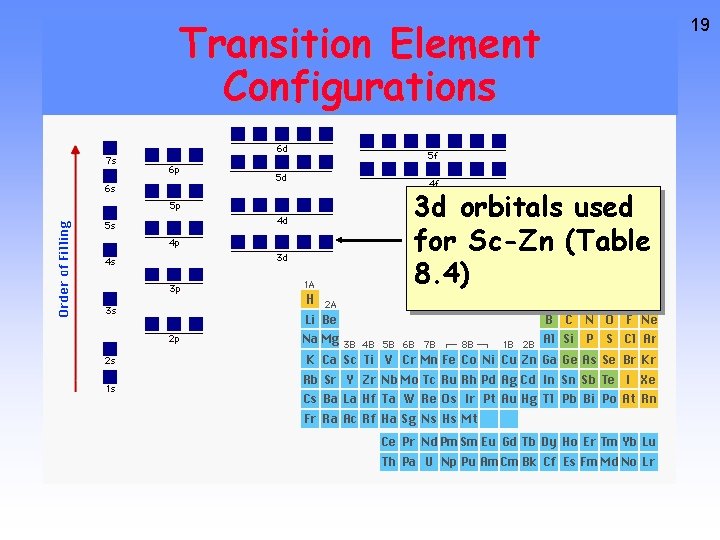
Transition Metals Table 8. 4 All 4 th period elements have the configuration [argon] nsx (n - 1)dy and so are “d-block” elements. Chromium Iron Copper 18

Transition Element Configurations 3 d orbitals used for Sc-Zn (Table 8. 4) 19
![Lanthanides and Actinides 20 All these elements have the configuration core nsx n Lanthanides and Actinides 20 All these elements have the configuration [core] nsx (n -](https://slidetodoc.com/presentation_image_h2/4deada5f3d3515d269ad47a1e29bcbec/image-20.jpg)
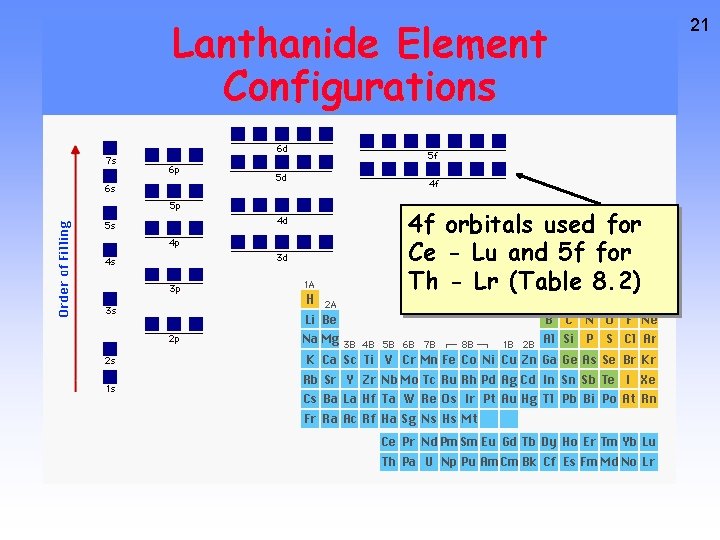
Lanthanides and Actinides 20 All these elements have the configuration [core] nsx (n - 1)dy (n - 2)fz or nsx (n - 2)fz and so are “f-block” elements. Cerium [Xe] 6 s 2 5 d 1 4 f 1 Uranium [Rn] 7 s 2 6 d 1 5 f 3 Only for Ce, Lu, and Gd!!! The rest are like Uranium

Lanthanide Element Configurations 4 f orbitals used for Ce - Lu and 5 f for Th - Lr (Table 8. 2) 21

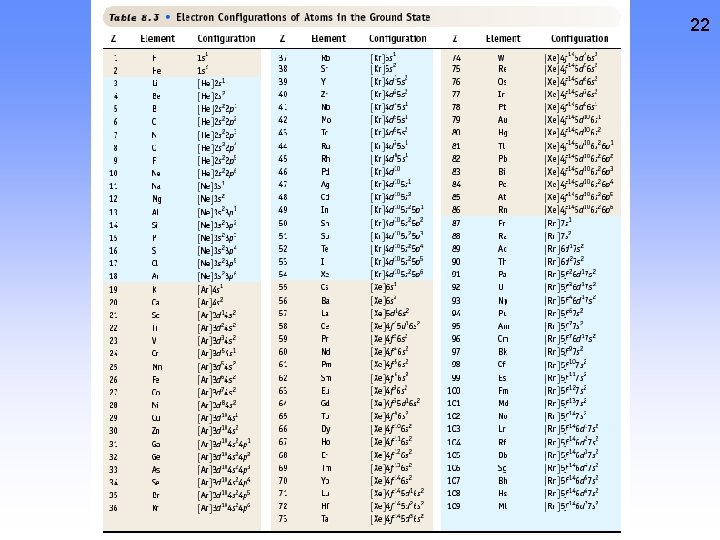
22

23 Transition Metals • Cr, Mo (d 4) – Lose 1 electron from “s” and bump to d 5 • Cu, Ag, Au (d 9) – Lose 1 electron from “s” and bump to d 10

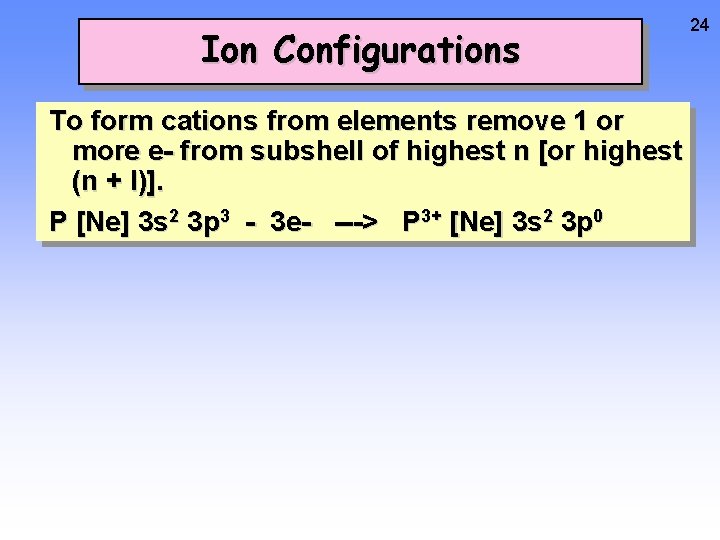
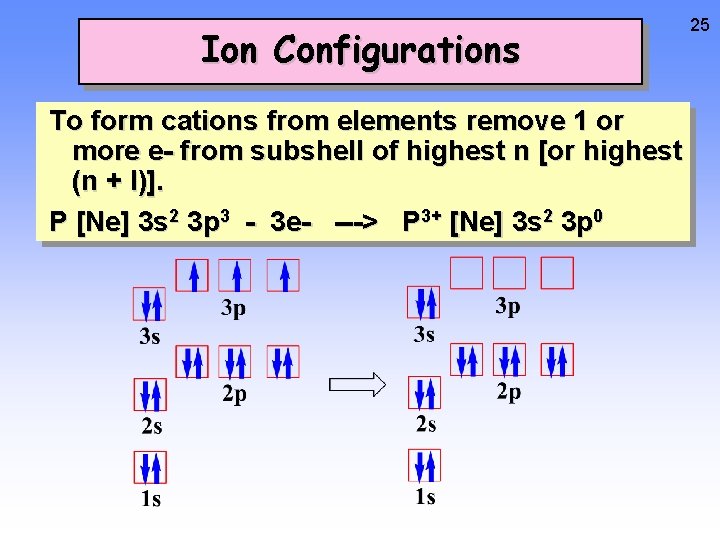
Ion Configurations To form cations from elements remove 1 or more e- from subshell of highest n [or highest (n + l)]. P [Ne] 3 s 2 3 p 3 - 3 e- ---> P 3+ [Ne] 3 s 2 3 p 0 24

Ion Configurations To form cations from elements remove 1 or more e- from subshell of highest n [or highest (n + l)]. P [Ne] 3 s 2 3 p 3 - 3 e- ---> P 3+ [Ne] 3 s 2 3 p 0 25

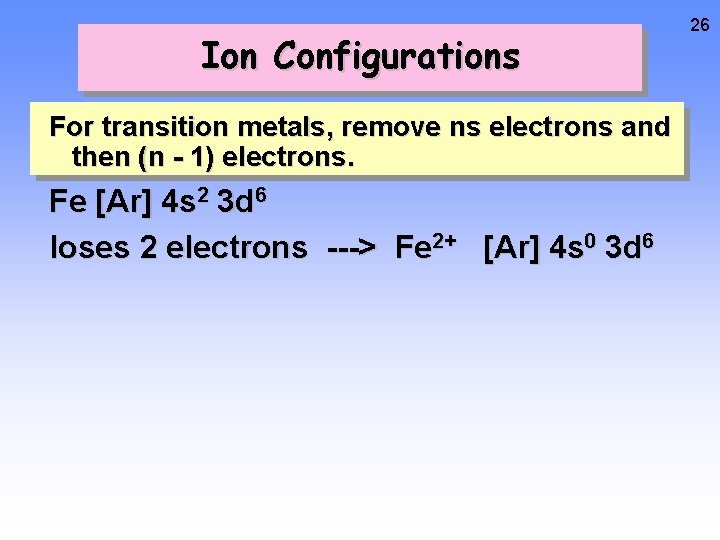
Ion Configurations For transition metals, remove ns electrons and then (n - 1) electrons. Fe [Ar] 4 s 2 3 d 6 loses 2 electrons ---> Fe 2+ [Ar] 4 s 0 3 d 6 26

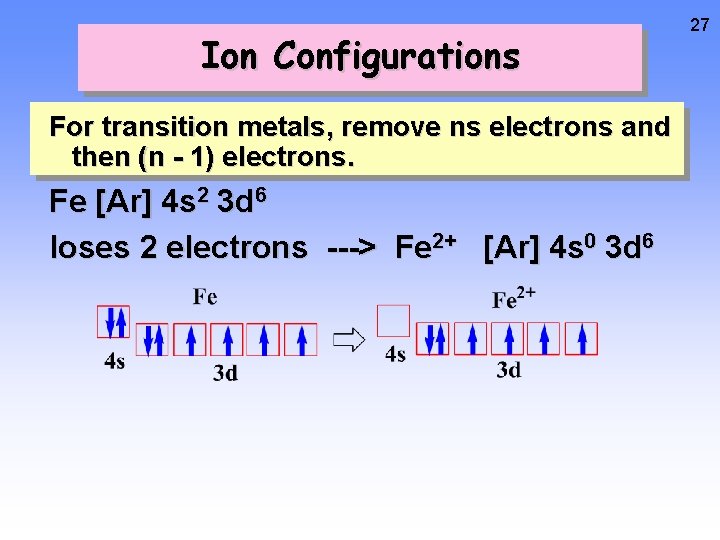
Ion Configurations For transition metals, remove ns electrons and then (n - 1) electrons. Fe [Ar] 4 s 2 3 d 6 loses 2 electrons ---> Fe 2+ [Ar] 4 s 0 3 d 6 27

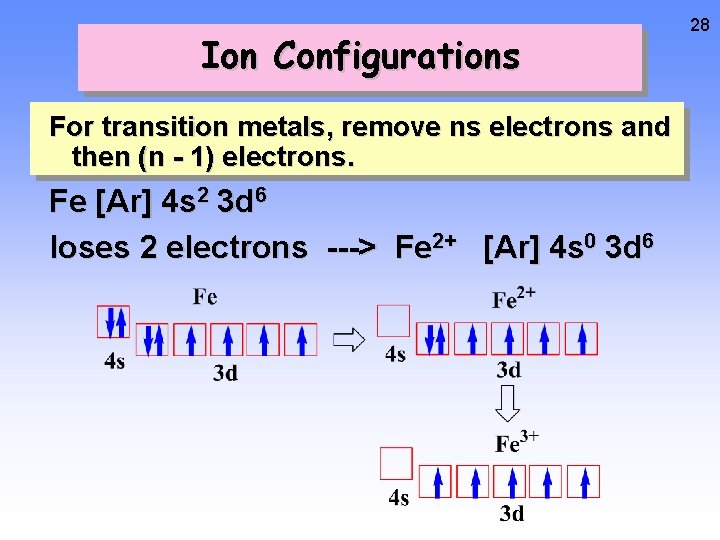
Ion Configurations For transition metals, remove ns electrons and then (n - 1) electrons. Fe [Ar] 4 s 2 3 d 6 loses 2 electrons ---> Fe 2+ [Ar] 4 s 0 3 d 6 28

29 Ion Configurations • Try and determine what charges the following metals will form…. • Ti (2 charges) – +2, +4 • Fe (2 charges) – +2, + 3 • Sn (2 charges) – +2, + 4 • Cu (2 charges) – +1, + 2
 1s 22 s22 p63 s2
1s 22 s22 p63 s2 Electronic configuration of mo (z=42)
Electronic configuration of mo (z=42) Ap chemistry chapter 7
Ap chemistry chapter 7 Oxygen periodic trends
Oxygen periodic trends Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity Electron configuration
Electron configuration Stable electron configurations are likely to contain
Stable electron configurations are likely to contain Ccechs
Ccechs An orbital is a region of space in an atom where there is
An orbital is a region of space in an atom where there is Dot
Dot Stable electron configurations
Stable electron configurations What is periodicity
What is periodicity Texas health steps quick reference guide
Texas health steps quick reference guide Cara memakan ikan
Cara memakan ikan Chemsheets periodicity
Chemsheets periodicity Aap bright futures periodicity schedule
Aap bright futures periodicity schedule Atomic radius electronegativity
Atomic radius electronegativity Filariasis
Filariasis Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Distinguish between mass number and atomic mass.
Distinguish between mass number and atomic mass. Stable electronic configuration
Stable electronic configuration Tetrachloromethane electron arrangement
Tetrachloromethane electron arrangement Orbital p
Orbital p Trends on the periodic table
Trends on the periodic table نصف القطر الذري
نصف القطر الذري Atomic number vs atomic radius
Atomic number vs atomic radius Rigid vs flexible pavement
Rigid vs flexible pavement Icq transistor
Icq transistor Electron configuration rule
Electron configuration rule