ELECTRONIC CONFIGURATION Electron Configuration What do I mean




- Slides: 12

ELECTRONIC CONFIGURATION

Electron Configuration • What do I mean by “electron configuration? ” • The electron configuration is the specific way in which the atomic orbitals are filled. • Think of it as being similar to your address. The electron configuration tells me where all the electrons “live. ”




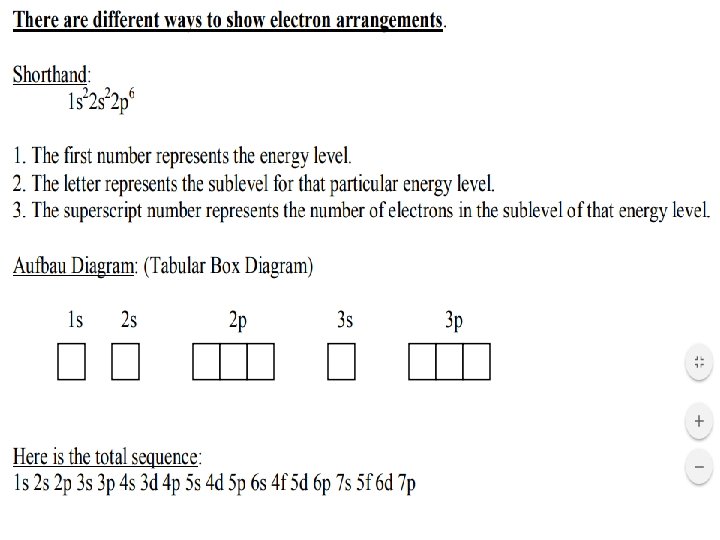
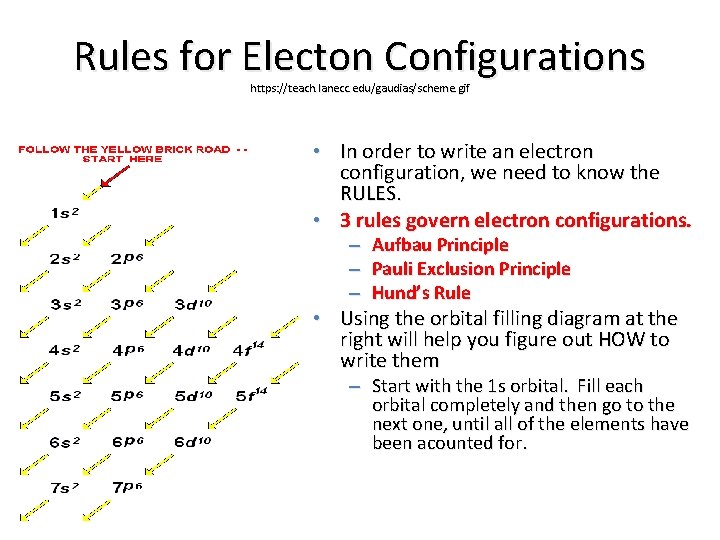
Rules for Electon Configurations https: //teach. lanecc. edu/gaudias/scheme. gif • In order to write an electron configuration, we need to know the RULES. • 3 rules govern electron configurations. – Aufbau Principle – Pauli Exclusion Principle – Hund’s Rule • Using the orbital filling diagram at the right will help you figure out HOW to write them – Start with the 1 s orbital. Fill each orbital completely and then go to the next one, until all of the elements have been acounted for.

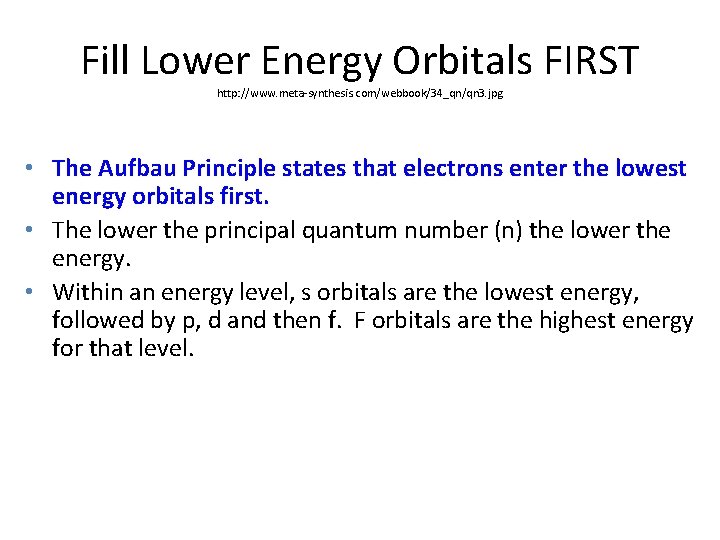
Fill Lower Energy Orbitals FIRST http: //www. meta-synthesis. com/webbook/34_qn/qn 3. jpg • The Aufbau Principle states that electrons enter the lowest energy orbitals first. • The lower the principal quantum number (n) the lower the energy. • Within an energy level, s orbitals are the lowest energy, followed by p, d and then f. F orbitals are the highest energy for that level.

No more than 2 Electrons in Any Orbital…ever. http: //www. fnal. gov/pub/inquiring/timeline/images/pauli. jpg • The next rule is the Pauli Exclusion Principal. • The Pauli Exclusion Principle states that an atomic orbital may have up to 2 electrons and then it is full. • The spins have to be paired. • We usually represent this with an up arrow and a down arrow. • Since there is only 1 s orbital per energy level, only 2 electrons fill that orbital. Quantum numbers describe an electrons position, and no 2 electrons can have the exact same quantum numbers. Because of that, electrons must have opposite spins from each other in order to “share” the same orbital.

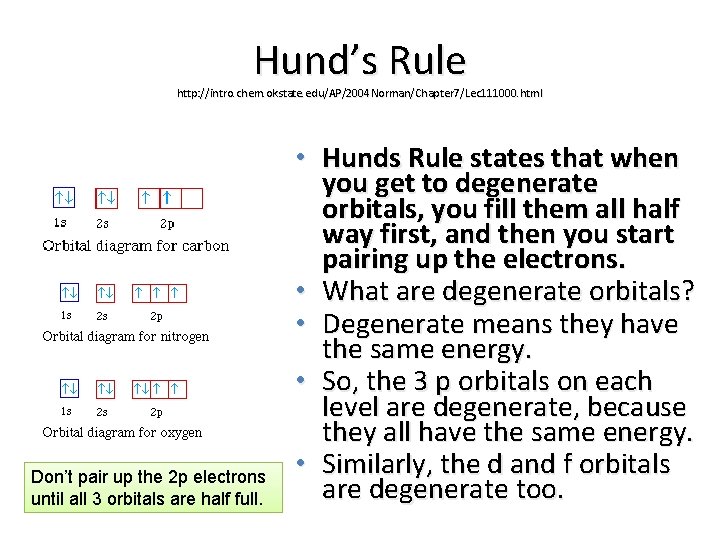
Hund’s Rule http: //intro. chem. okstate. edu/AP/2004 Norman/Chapter 7/Lec 111000. html Don’t pair up the 2 p electrons until all 3 orbitals are half full. • Hunds Rule states that when you get to degenerate orbitals, you fill them all half way first, and then you start pairing up the electrons. • What are degenerate orbitals? • Degenerate means they have the same energy. • So, the 3 p orbitals on each level are degenerate, because they all have the same energy. • Similarly, the d and f orbitals are degenerate too.

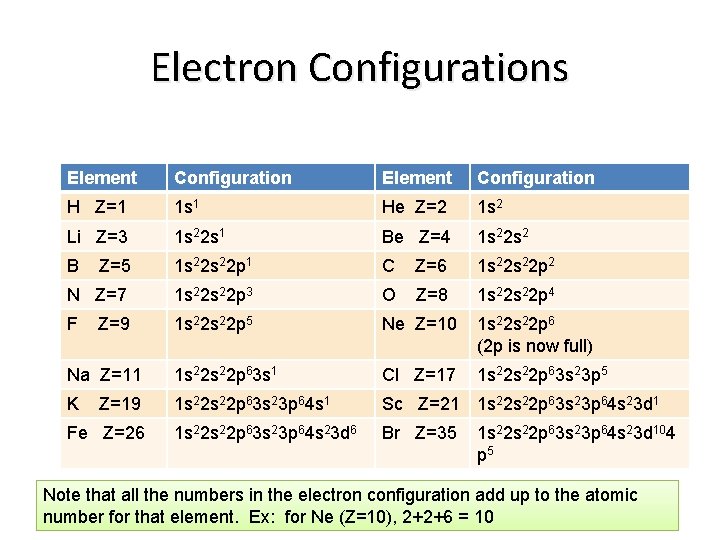
Electron Configurations Element Configuration H Z=1 1 s 1 He Z=2 1 s 2 Li Z=3 1 s 22 s 1 Be Z=4 1 s 22 s 2 B Z=5 1 s 22 p 1 C Z=6 1 s 22 p 2 N Z=7 1 s 22 p 3 O Z=8 1 s 22 p 4 F 1 s 22 p 5 Ne Z=10 1 s 22 p 6 (2 p is now full) Na Z=11 1 s 22 p 63 s 1 Cl Z=17 1 s 22 p 63 s 23 p 5 K 1 s 22 p 63 s 23 p 64 s 1 Sc Z=21 1 s 22 s 22 p 63 s 23 p 64 s 23 d 6 Br Z=35 Z=9 Z=19 Fe Z=26 1 s 22 p 63 s 23 p 64 s 23 d 104 p 5 Note that all the numbers in the electron configuration add up to the atomic number for that element. Ex: for Ne (Z=10), 2+2+6 = 10

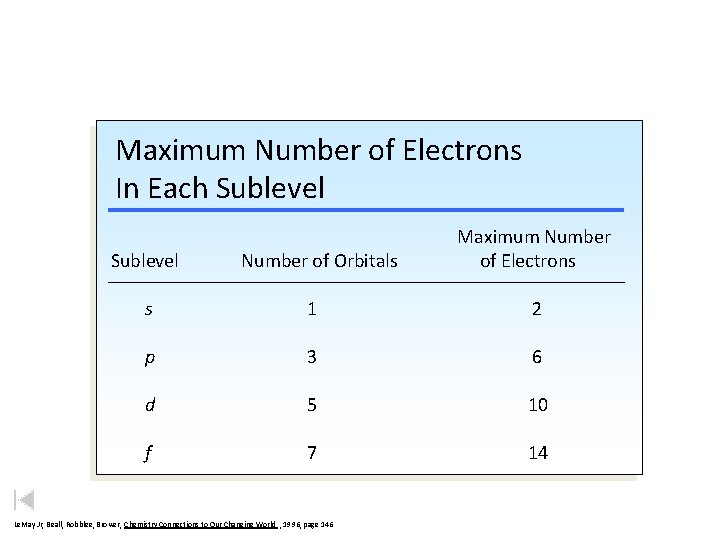
Maximum Number of Electrons In Each Sublevel Number of Orbitals Maximum Number of Electrons s 1 2 p 3 6 d 5 10 f 7 14 Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 146

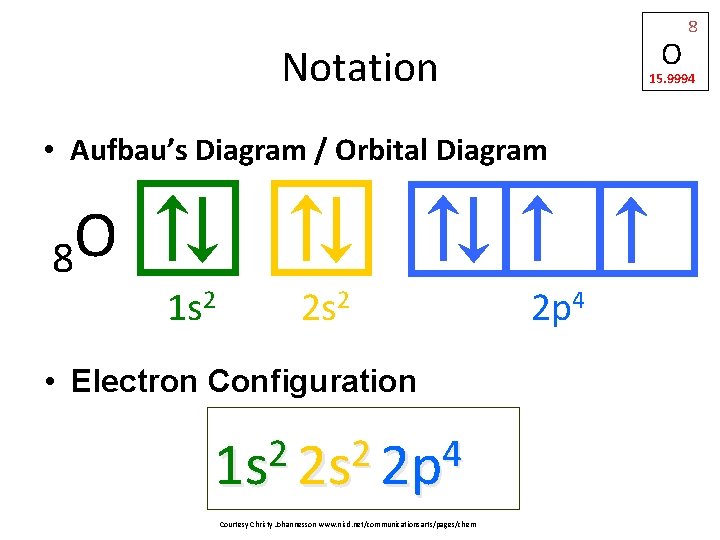
O Notation 15. 9994 • Aufbau’s Diagram / Orbital Diagram O 8 1 s 2 2 s 2 • Electron Configuration 2 2 4 1 s 2 s 2 p Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 8 2 p 4