Electron Configurations and Periodicity 8 1 Electron Spin

![Aufbau Principle • Here a few examples. – Using the abbreviation [He] for 1 Aufbau Principle • Here a few examples. – Using the abbreviation [He] for 1](https://slidetodoc.com/presentation_image_h2/22a159a6ca7ec6afbdfcd6213b2a5345/image-14.jpg)










- Slides: 51

Electron Configurations and Periodicity 8. 1 Electron Spin and the Pauli Exclusion Principle 8. 2 Building-up Principle and the Periodic Table 8. 3 Writing Electron Configurations using the Periodic Table

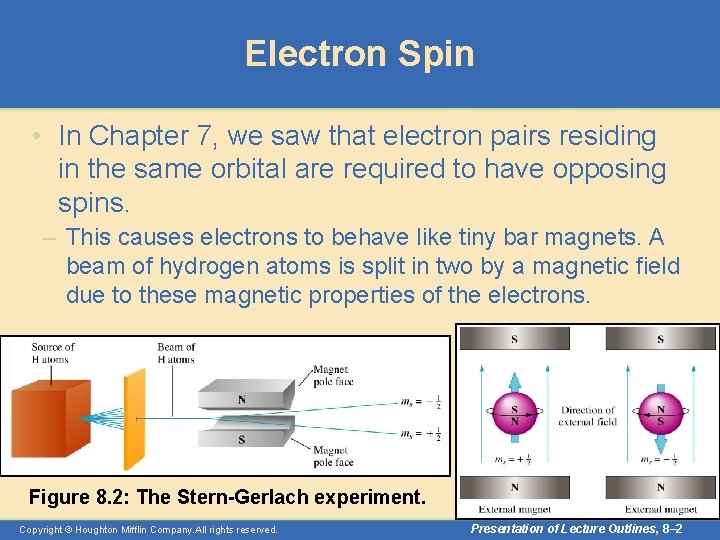
Electron Spin • In Chapter 7, we saw that electron pairs residing in the same orbital are required to have opposing spins. – This causes electrons to behave like tiny bar magnets. A beam of hydrogen atoms is split in two by a magnetic field due to these magnetic properties of the electrons. Figure 8. 2: The Stern-Gerlach experiment. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 2

Electron Configuration • An “electron configuration” of an atom is a particular distribution of electrons among available sub shells. – The notation for a configuration lists the sub-shell symbols sequentially with a superscript indicating the number of electrons occupying that sub shell. – For example, lithium (atomic number 3) has two electrons in the “ 1 s” sub shell and one electron in the “ 2 s” sub shell 1 s 2 2 s 1. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 3

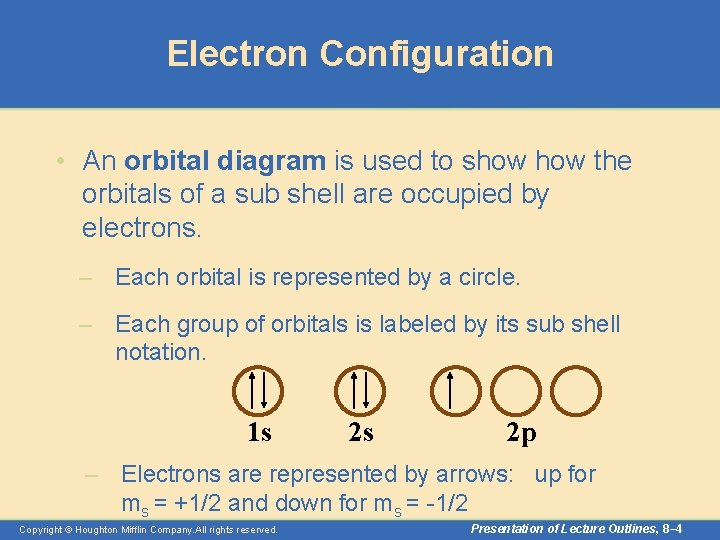
Electron Configuration • An orbital diagram is used to show the orbitals of a sub shell are occupied by electrons. – Each orbital is represented by a circle. – Each group of orbitals is labeled by its sub shell notation. 1 s 2 s 2 p – Electrons are represented by arrows: up for ms = +1/2 and down for ms = -1/2 Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 4

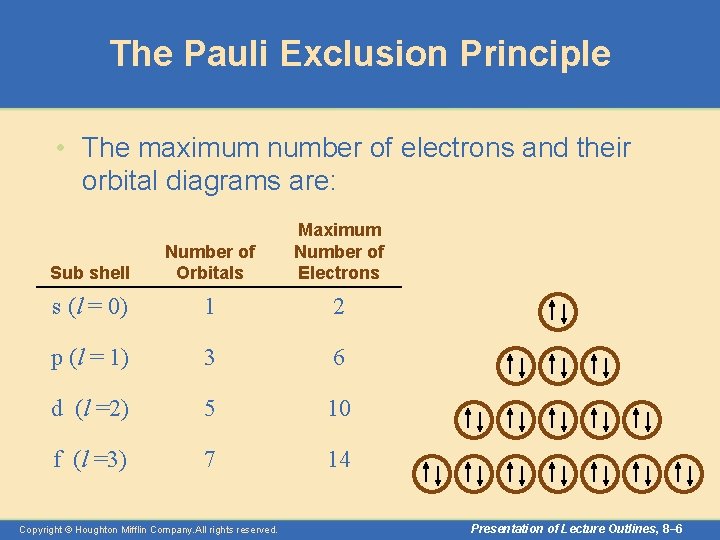
The Pauli Exclusion Principle • The Pauli exclusion principle, which summarizes experimental observations, states that no two electrons can have the same four quantum numbers. – In other words, an orbital can hold at most two electrons, and then only if the electrons have opposite spins. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 5

The Pauli Exclusion Principle • The maximum number of electrons and their orbital diagrams are: Sub shell Number of Orbitals Maximum Number of Electrons s (l = 0) 1 2 p (l = 1) 3 6 d (l =2) 5 10 f (l =3) 7 14 Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 6

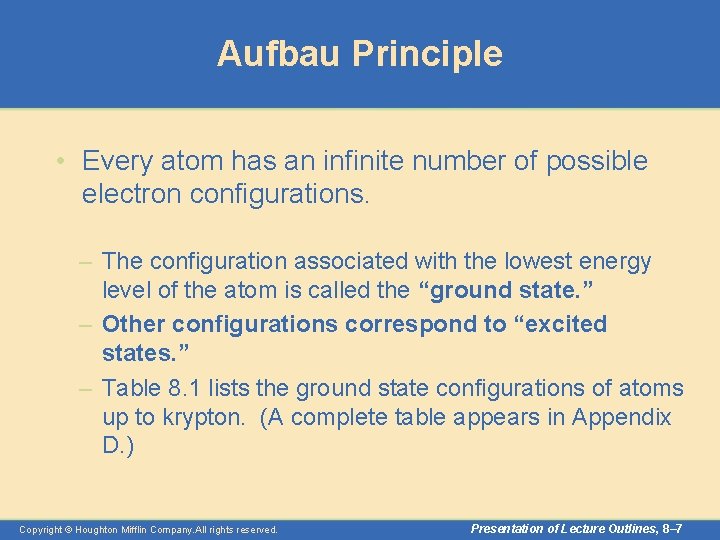
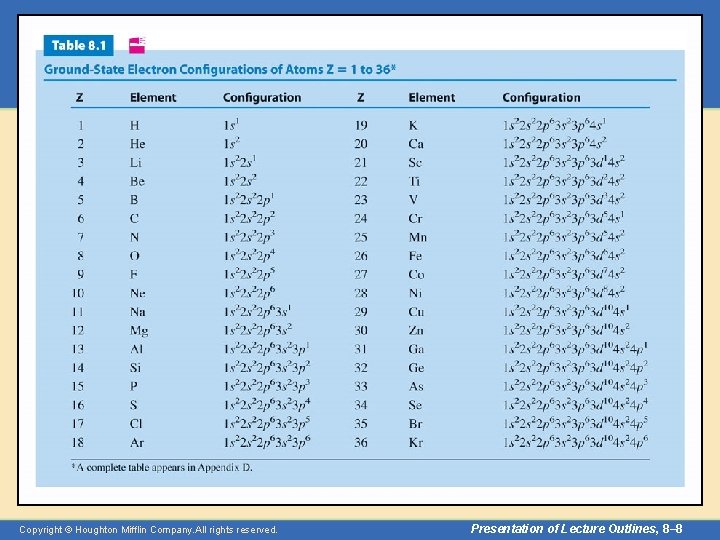
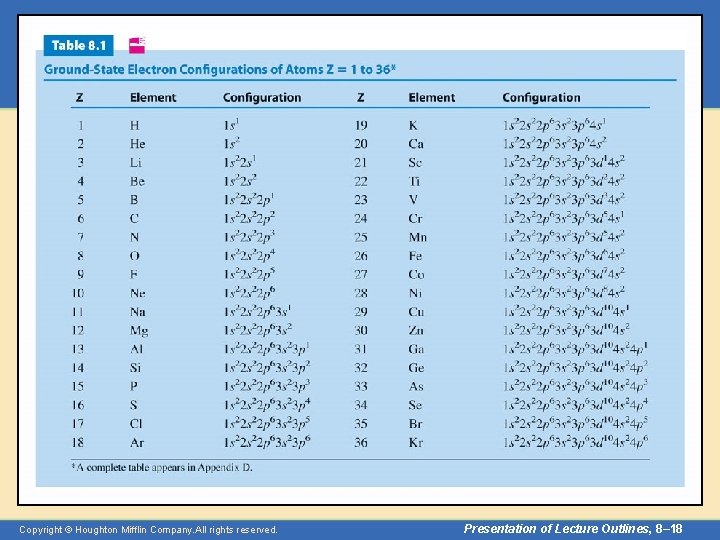
Aufbau Principle • Every atom has an infinite number of possible electron configurations. – The configuration associated with the lowest energy level of the atom is called the “ground state. ” – Other configurations correspond to “excited states. ” – Table 8. 1 lists the ground state configurations of atoms up to krypton. (A complete table appears in Appendix D. ) Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 7

Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 8

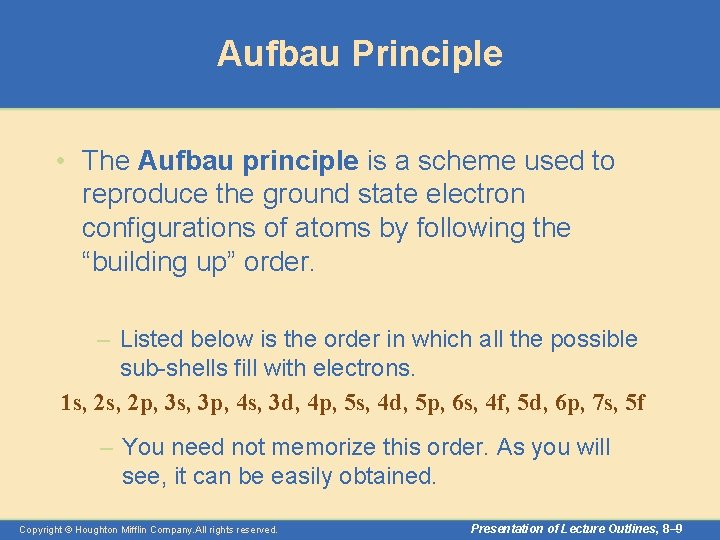
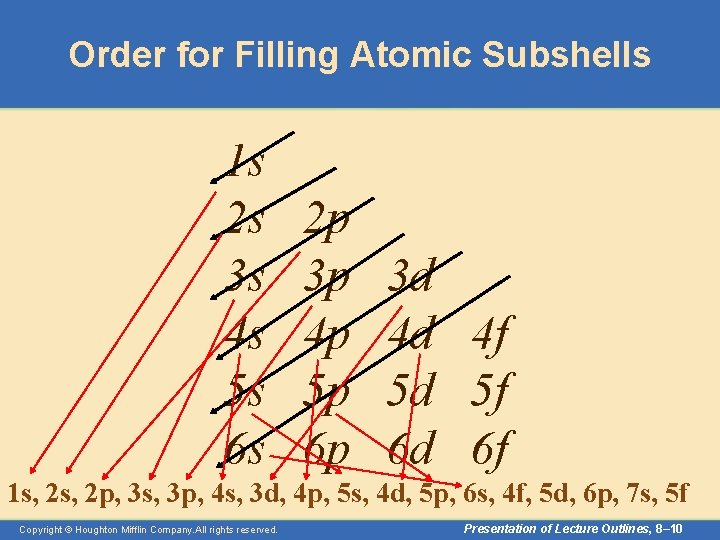
Aufbau Principle • The Aufbau principle is a scheme used to reproduce the ground state electron configurations of atoms by following the “building up” order. – Listed below is the order in which all the possible sub-shells fill with electrons. 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p, 6 s, 4 f, 5 d, 6 p, 7 s, 5 f – You need not memorize this order. As you will see, it can be easily obtained. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 9

Order for Filling Atomic Subshells 1 s 2 s 3 s 4 s 5 s 6 s 2 p 3 p 4 p 5 p 6 p 3 d 4 d 4 f 5 d 5 f 6 d 6 f 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p, 6 s, 4 f, 5 d, 6 p, 7 s, 5 f Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 10

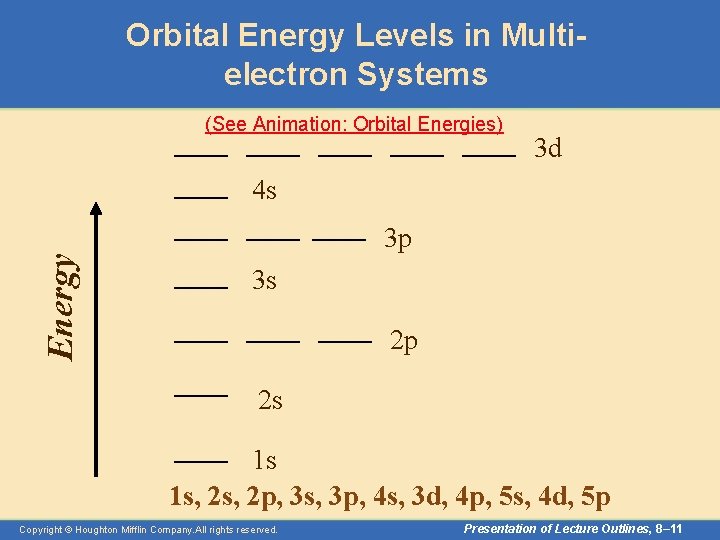
Orbital Energy Levels in Multielectron Systems (See Animation: Orbital Energies) 3 d Energy 4 s 3 p 3 s 2 p 2 s 1 s 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 11

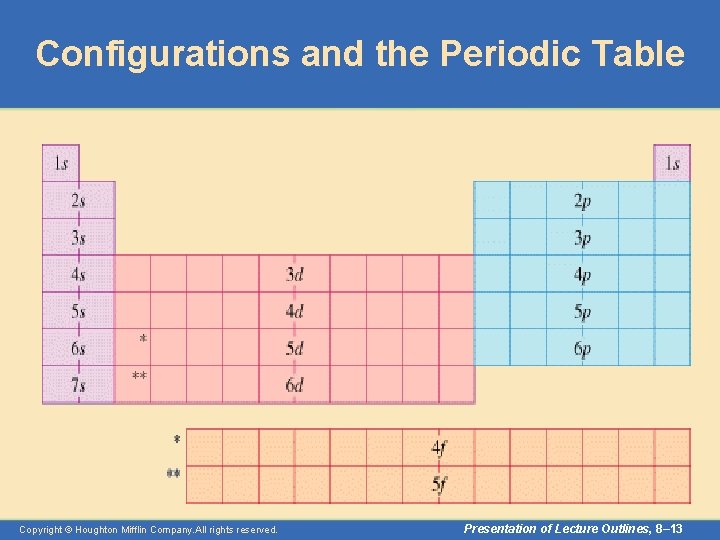
Configurations and the Periodic Table • The following slide illustrates how the periodic table provides a sound way to remember the Aufbau sequence. – In many cases you need only the configuration of the outer electrons. – You can determine this from their position on the periodic table. – The total number of valence electrons for an atom equals its group number. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 12

Configurations and the Periodic Table Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 13
![Aufbau Principle Here a few examples Using the abbreviation He for 1 Aufbau Principle • Here a few examples. – Using the abbreviation [He] for 1](https://slidetodoc.com/presentation_image_h2/22a159a6ca7ec6afbdfcd6213b2a5345/image-14.jpg)
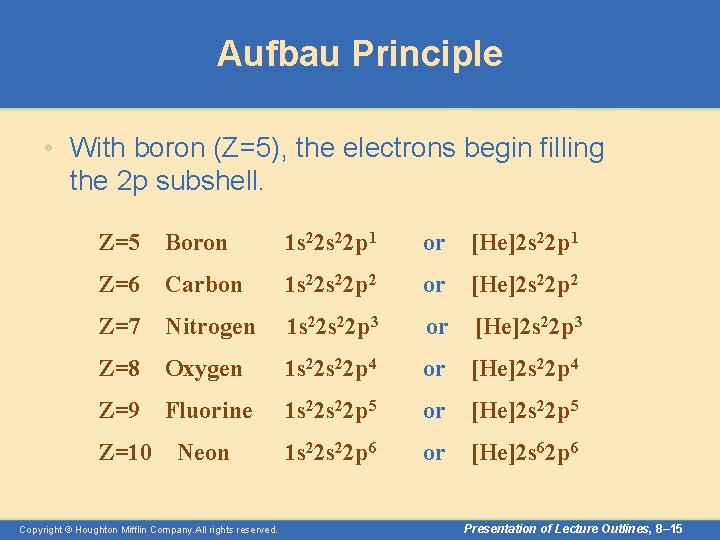
Aufbau Principle • Here a few examples. – Using the abbreviation [He] for 1 s 2, the configurations are Z=4 Beryllium 1 s 22 s 2 or [He]2 s 2 Z=3 Lithium 1 s 22 s 1 or [He]2 s 1 Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 14

Aufbau Principle • With boron (Z=5), the electrons begin filling the 2 p subshell. Z=5 Boron 1 s 22 p 1 or [He]2 s 22 p 1 Z=6 Carbon 1 s 22 p 2 or [He]2 s 22 p 2 Z=7 Nitrogen 1 s 22 p 3 or [He]2 s 22 p 3 Z=8 Oxygen 1 s 22 p 4 or [He]2 s 22 p 4 Z=9 Fluorine 1 s 22 p 5 or [He]2 s 22 p 5 Z=10 Neon 1 s 22 p 6 or [He]2 s 62 p 6 Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 15

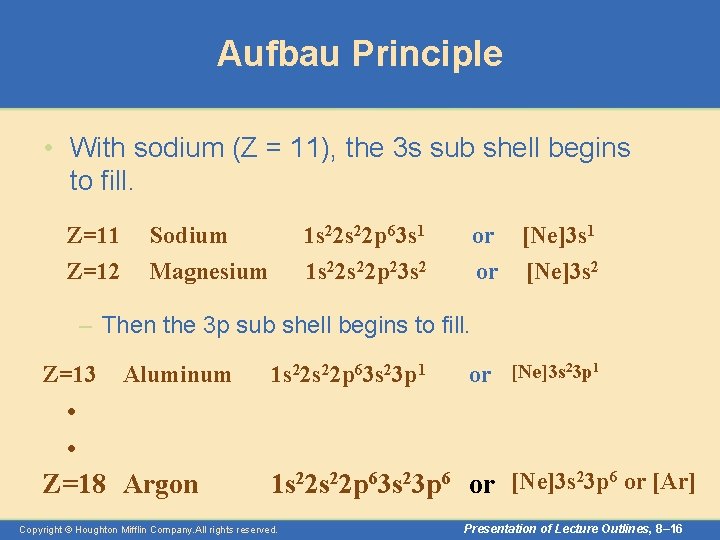
Aufbau Principle • With sodium (Z = 11), the 3 s sub shell begins to fill. Z=11 Z=12 Sodium Magnesium 1 s 22 p 63 s 1 1 s 22 p 23 s 2 or [Ne]3 s 1 or [Ne]3 s 2 – Then the 3 p sub shell begins to fill. Z=13 Aluminum • • Z=18 Argon 1 s 22 p 63 s 23 p 1 or [Ne]3 s 23 p 1 1 s 22 p 63 s 23 p 6 or [Ne]3 s 23 p 6 or [Ar] Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 16

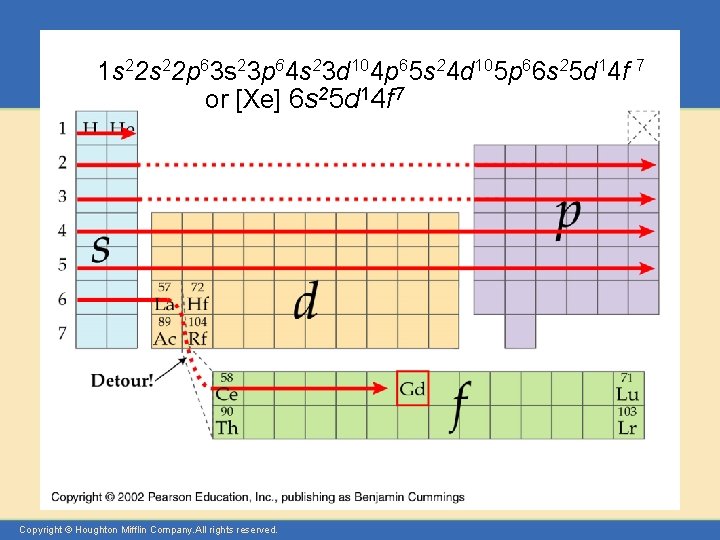
Let’s Practice Silicon 1 s 22 p 63 s 23 p 2 Sulfur 1 s 22 p 63 s 23 p 4 or [Ne] 3 s 23 p 4 Arsenic Iron Gadmium Copyright © Houghton Mifflin Company. All rights reserved. 1 s 22 p 63 s 23 p 64 s 23 d 104 p 3 1 s 22 p 63 s 23 p 64 s 23 d 6 or [Ar] 4 s 23 d 6 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 5 s 24 d 105 p 66 s 25 d 14 f 7 or [Xe] 6 s 25 d 14 f 7 Presentation of Lecture Outlines, 8– 17

Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 18

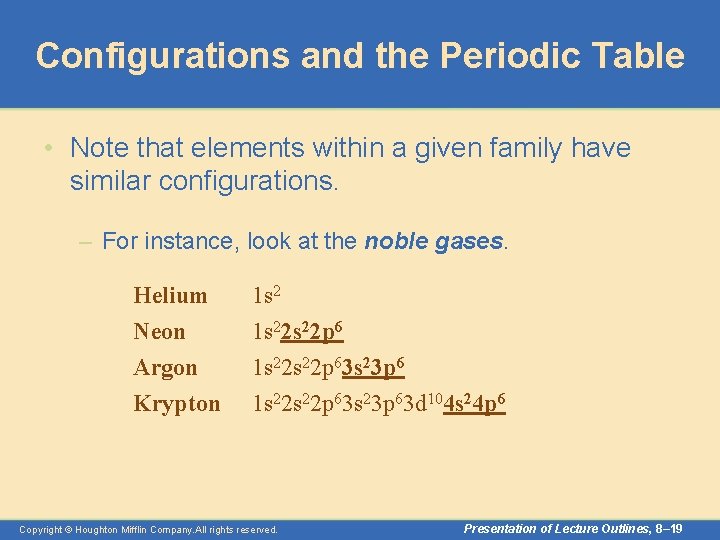
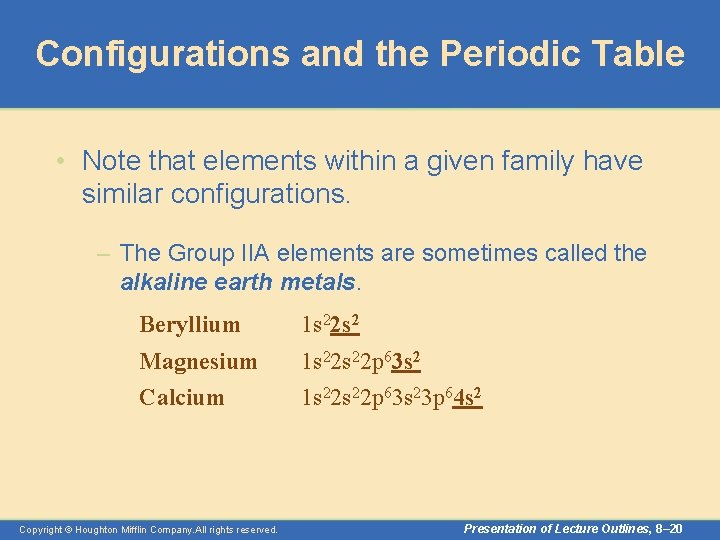
Configurations and the Periodic Table • Note that elements within a given family have similar configurations. – For instance, look at the noble gases. Helium Neon Argon Krypton 1 s 22 s 22 p 63 s 23 p 63 d 104 s 24 p 6 Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 19

Configurations and the Periodic Table • Note that elements within a given family have similar configurations. – The Group IIA elements are sometimes called the alkaline earth metals. Beryllium 1 s 22 s 2 Magnesium Calcium 1 s 22 s 22 p 63 s 23 p 64 s 2 Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 20

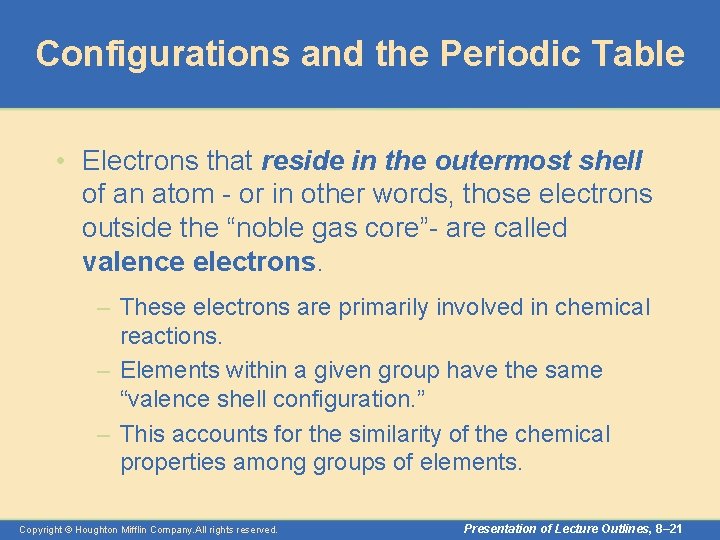
Configurations and the Periodic Table • Electrons that reside in the outermost shell of an atom - or in other words, those electrons outside the “noble gas core”- are called valence electrons. – These electrons are primarily involved in chemical reactions. – Elements within a given group have the same “valence shell configuration. ” – This accounts for the similarity of the chemical properties among groups of elements. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 21

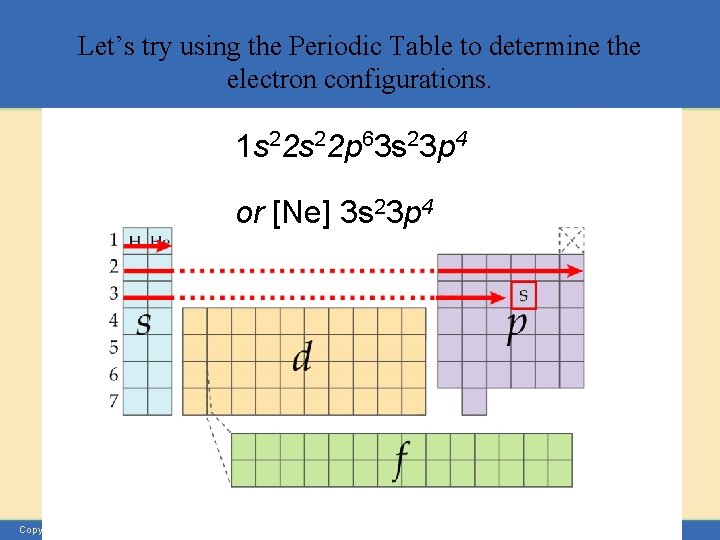
Let’s try using the Periodic Table to determine the electron configurations. 1 s 22 p 63 s 23 p 4 or [Ne] 3 s 23 p 4 Copyright © Houghton Mifflin Company. All rights reserved.

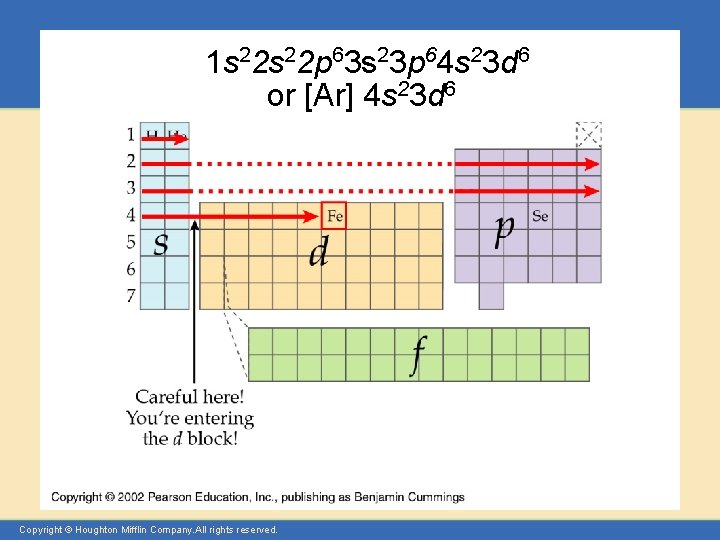
1 s 22 p 63 s 23 p 64 s 23 d 6 or [Ar] 4 s 23 d 6 Copyright © Houghton Mifflin Company. All rights reserved.

1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 25 d 14 f 7 or [Xe] 6 s 25 d 14 f 7 Copyright © Houghton Mifflin Company. All rights reserved.

Electron Configurations and Periodicity 8. 4 Orbital Diagrams of Atoms; Hund’s Rule 8. 5 Mendeleev’s Predictions from the Periodic Table 8. 6 Some Periodic Properties 8. 7 Periodicity in the Main-Group Elements

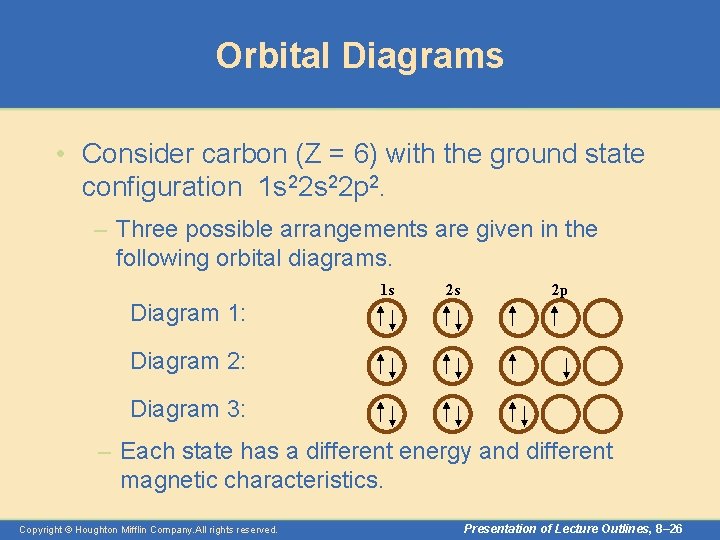
Orbital Diagrams • Consider carbon (Z = 6) with the ground state configuration 1 s 22 p 2. – Three possible arrangements are given in the following orbital diagrams. 1 s 2 s 2 p Diagram 1: Diagram 2: Diagram 3: – Each state has a different energy and different magnetic characteristics. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 26

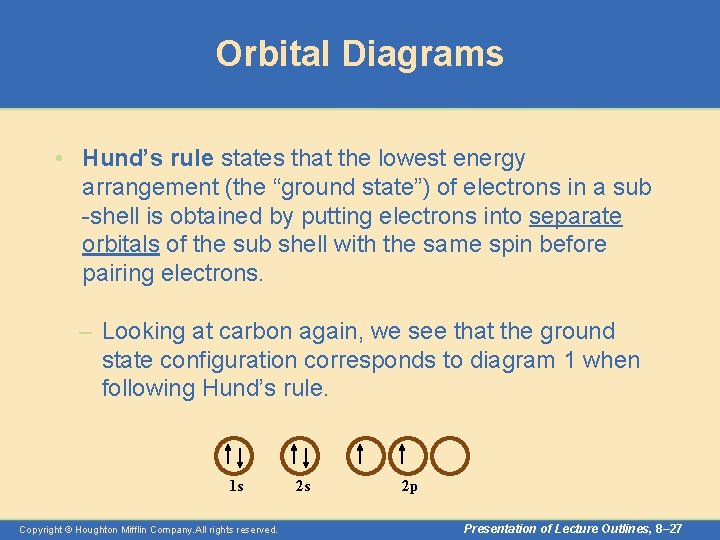
Orbital Diagrams • Hund’s rule states that the lowest energy arrangement (the “ground state”) of electrons in a sub -shell is obtained by putting electrons into separate orbitals of the sub shell with the same spin before pairing electrons. – Looking at carbon again, we see that the ground state configuration corresponds to diagram 1 when following Hund’s rule. 1 s Copyright © Houghton Mifflin Company. All rights reserved. 2 s 2 p Presentation of Lecture Outlines, 8– 27

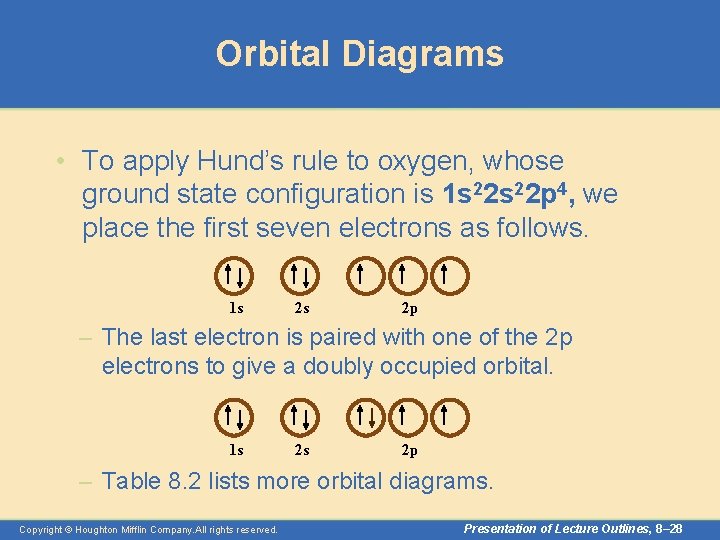
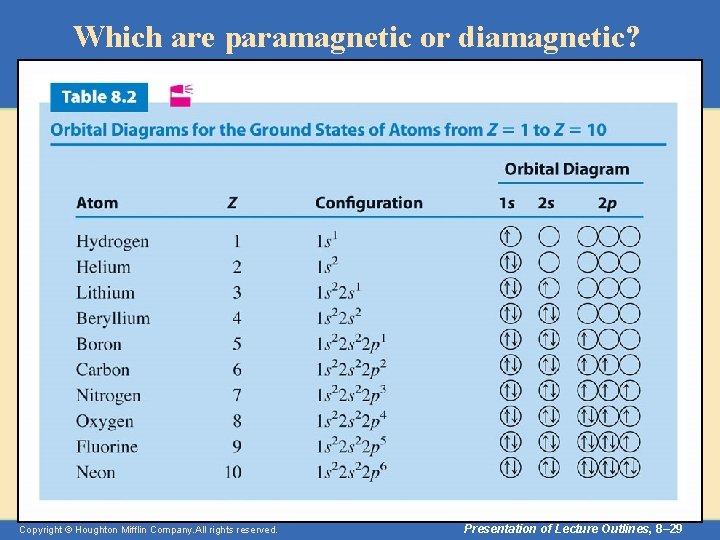
Orbital Diagrams • To apply Hund’s rule to oxygen, whose ground state configuration is 1 s 22 p 4, we place the first seven electrons as follows. 1 s 2 s 2 p – The last electron is paired with one of the 2 p electrons to give a doubly occupied orbital. 1 s 2 s 2 p – Table 8. 2 lists more orbital diagrams. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 28

Which are paramagnetic or diamagnetic? Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 29

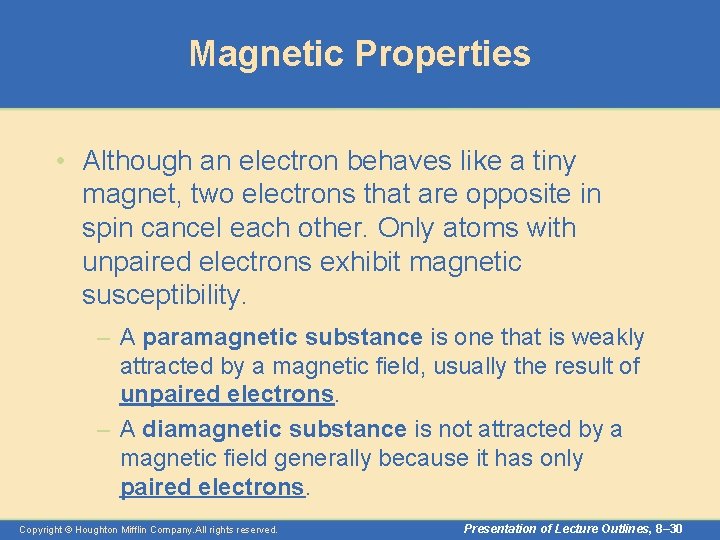
Magnetic Properties • Although an electron behaves like a tiny magnet, two electrons that are opposite in spin cancel each other. Only atoms with unpaired electrons exhibit magnetic susceptibility. – A paramagnetic substance is one that is weakly attracted by a magnetic field, usually the result of unpaired electrons. – A diamagnetic substance is not attracted by a magnetic field generally because it has only paired electrons. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 30

Periodic Properties • The periodic law states that when the elements are arranged by atomic number, their physical and chemical properties vary periodically. • We will look at three periodic properties: – Atomic radius – Ionization energy – Electron affinity Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 31

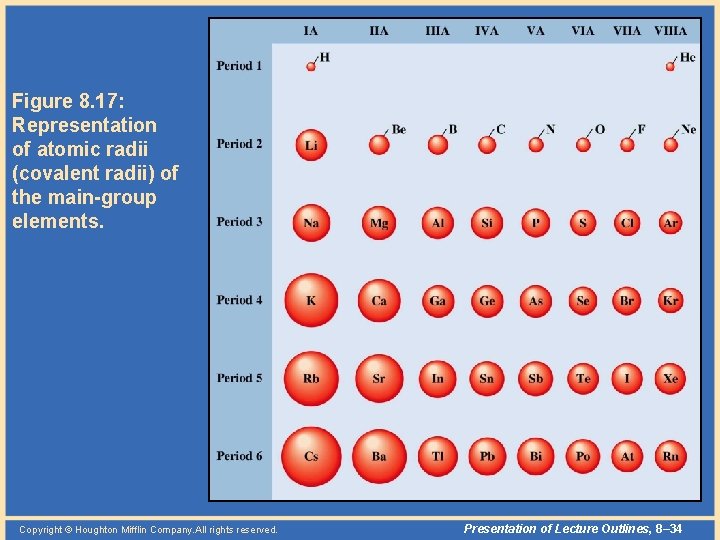
Periodic Properties • Atomic radius – Within each period (horizontal row), the atomic radius tends to decrease with increasing atomic number (nuclear charge). – Within each group (vertical column), the atomic radius tends to increase with the period number. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 32

Periodic Properties • Two factors determine the size of an atom. – One factor is the principal quantum number, n. The larger is “n”, the larger the size of the orbital. – The other factor is the effective nuclear charge, which is the positive charge an electron experiences from the nucleus minus any “shielding effects” from intervening electrons. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 33

Figure 8. 17: Representation of atomic radii (covalent radii) of the main-group elements. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 34

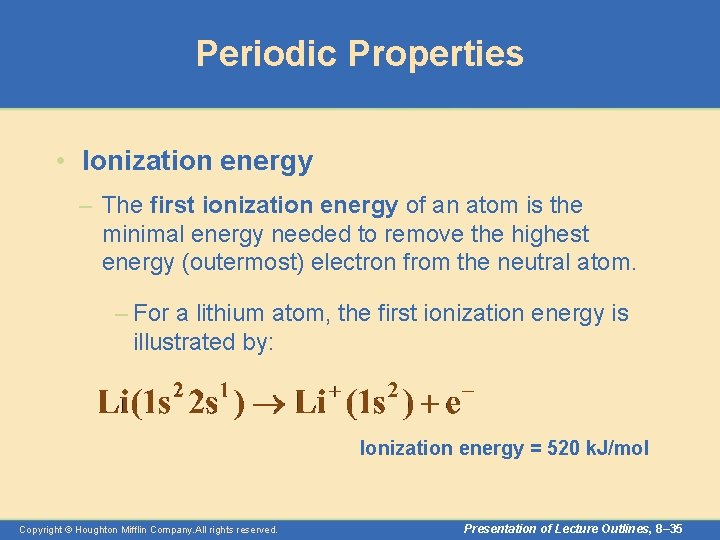
Periodic Properties • Ionization energy – The first ionization energy of an atom is the minimal energy needed to remove the highest energy (outermost) electron from the neutral atom. – For a lithium atom, the first ionization energy is illustrated by: Ionization energy = 520 k. J/mol Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 35

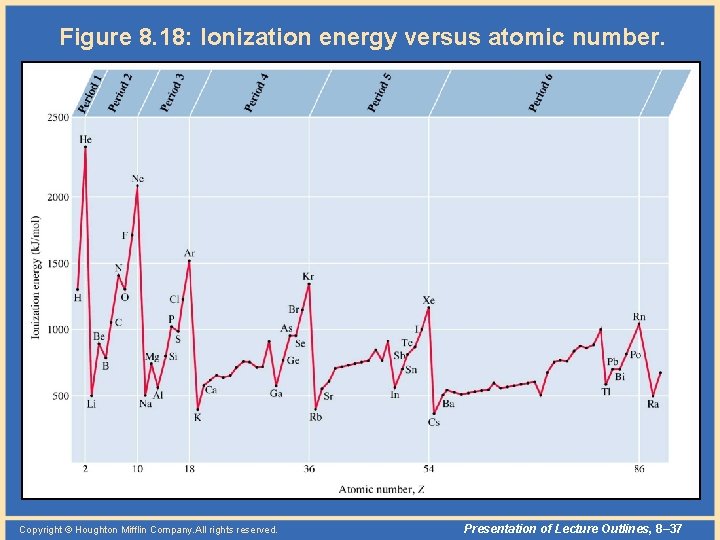
Periodic Properties • Ionization energy – There is a general trend that ionization energies increase with atomic number within a given period. – This follows the trend in size, as it is more difficult to remove an electron that is closer to the nucleus. – For the same reason, we find that ionization energies, again following the trend in size, decrease as we descend a column of elements. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 36

Figure 8. 18: Ionization energy versus atomic number. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 37

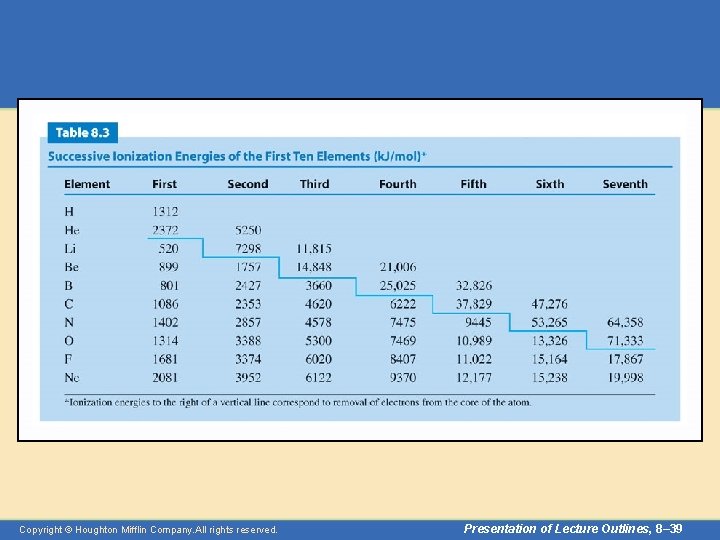
Periodic Properties • Ionization energy – The electrons of an atom can be removed successively. • The energies required at each step are known as the first ionization energy, the second ionization energy, and so forth. • Table 8. 3 lists the successive ionization energies of the first ten elements. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 38

Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 39

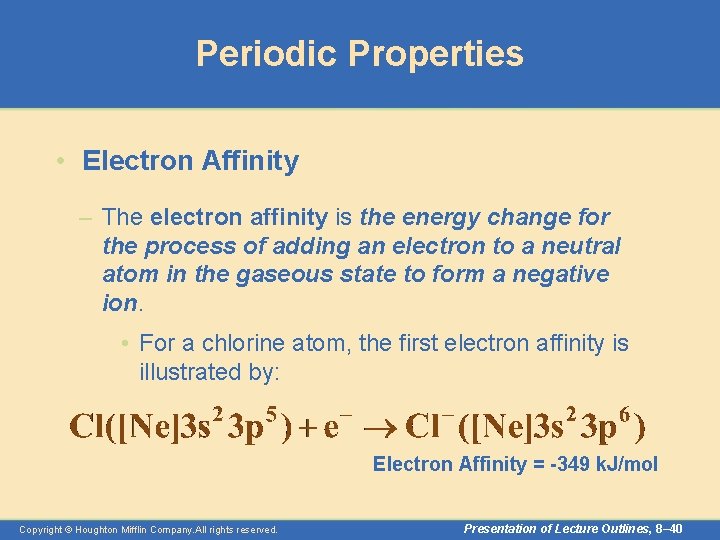
Periodic Properties • Electron Affinity – The electron affinity is the energy change for the process of adding an electron to a neutral atom in the gaseous state to form a negative ion. • For a chlorine atom, the first electron affinity is illustrated by: Electron Affinity = -349 k. J/mol Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 40

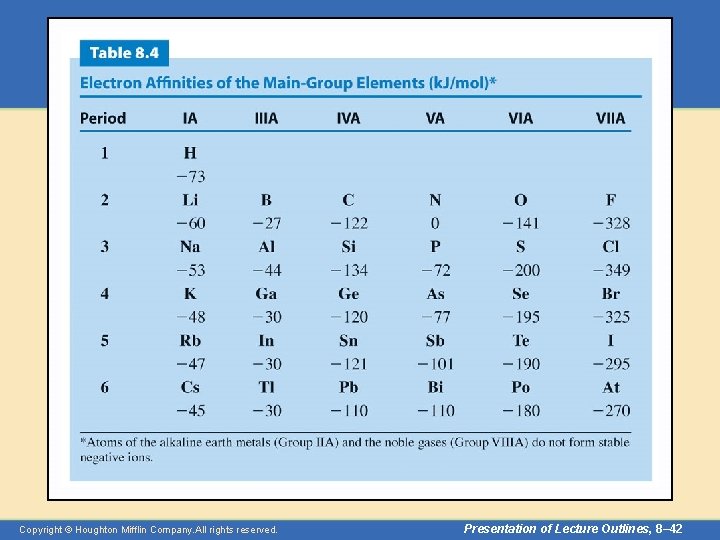
Periodic Properties • Electron Affinity – The more negative the electron affinity, the more stable the negative ion that is formed. – Broadly speaking, the general trend goes from lower left to upper right as electron affinities become more negative. – Table 8. 4 gives the electron affinities of the maingroup elements. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 41

Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 42

Group IA, Alkali Metals • • • Largest atomic radii React violently with water to form H 2 Readily ionized to 1+ Metallic character, oxidized in air R 2 O in most cases Sodium reacting with water. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 43

Group IIA, Alkali Earth Metals • • Readily ionized to 2+ React with water to form H 2 Closed s shell configuration Metallic Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 44

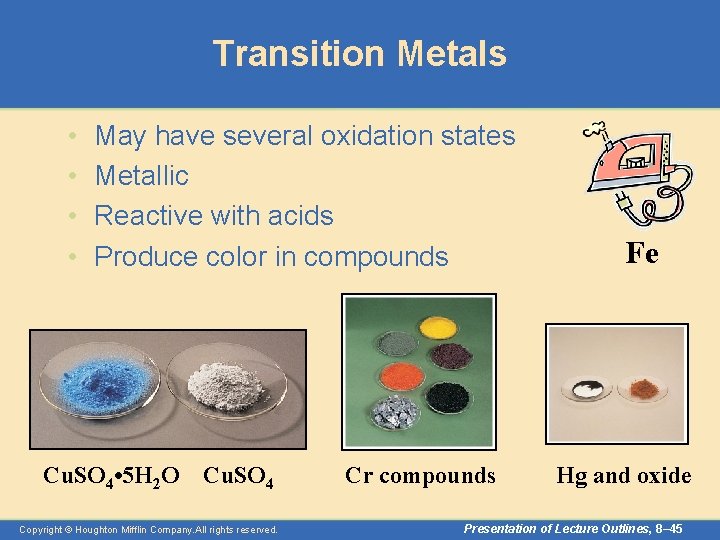
Transition Metals • • May have several oxidation states Metallic Reactive with acids Produce color in compounds Cu. SO 4 • 5 H 2 O Cu. SO 4 Copyright © Houghton Mifflin Company. All rights reserved. Cr compounds Fe Hg and oxide Presentation of Lecture Outlines, 8– 45

Group III A • Metals (except for boron) • Several oxidation states (commonly 3+) Gallium-melts in your hand. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 46

Group IV A • Form the most covalent compounds • Oxidation numbers vary between 4+ and 4 - Oxides from this group Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 47

Group V A • Form anions generally(1 -, 2 -, 3 -), though positive oxidation states are possible • Form metals, metalloids, and nonmetals I have no picture of Group V elements so this is one of my grandsons, Kaden. He’s in a group of his own. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 48

Group VI A • Form -2 anions generally, though positive oxidation states are possible • React vigorously with alkali and alkali earth metals • Nonmetals Burning of Sulfur Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 49

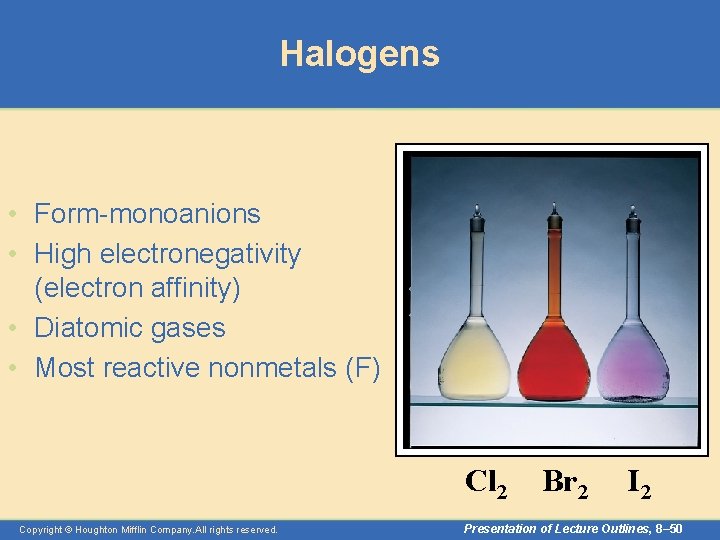
Halogens • Form-monoanions • High electronegativity (electron affinity) • Diatomic gases • Most reactive nonmetals (F) Cl 2 Copyright © Houghton Mifflin Company. All rights reserved. Br 2 I 2 Presentation of Lecture Outlines, 8– 50

Noble Gases • Minimal reactivity • Monatomic gases • Closed shell Noble Students Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 8– 51