SECTION 3 ELECTRON CONFIGURATIONS CHAPTER 9 ELECTRONS IN

















![Electron Configuration • Chromium: 1 5 [Ar]4 s 3 d • Copper: [Ar]4 s Electron Configuration • Chromium: 1 5 [Ar]4 s 3 d • Copper: [Ar]4 s](https://slidetodoc.com/presentation_image_h/91e9df93309af9dc382e8b12019a6b98/image-45.jpg)

- Slides: 47

SECTION 3: ELECTRON CONFIGURATIONS CHAPTER 9: ELECTRONS IN ATOMS AND THE PERIODIC TABLE

Learning Goals • Apply the Pauli exclusion principle, the aufbau principle, and Hund's rule to write electron configurations using orbital diagrams and electron configuration notation.

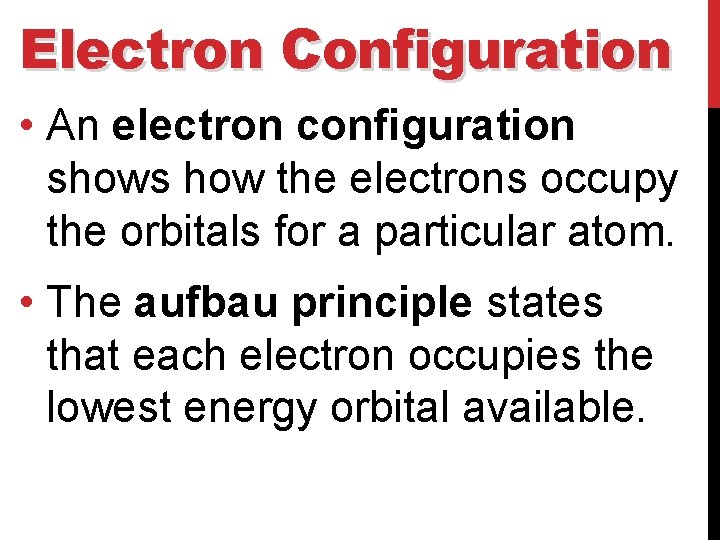
Electron Configuration • An electron configuration shows how the electrons occupy the orbitals for a particular atom. • The aufbau principle states that each electron occupies the lowest energy orbital available.

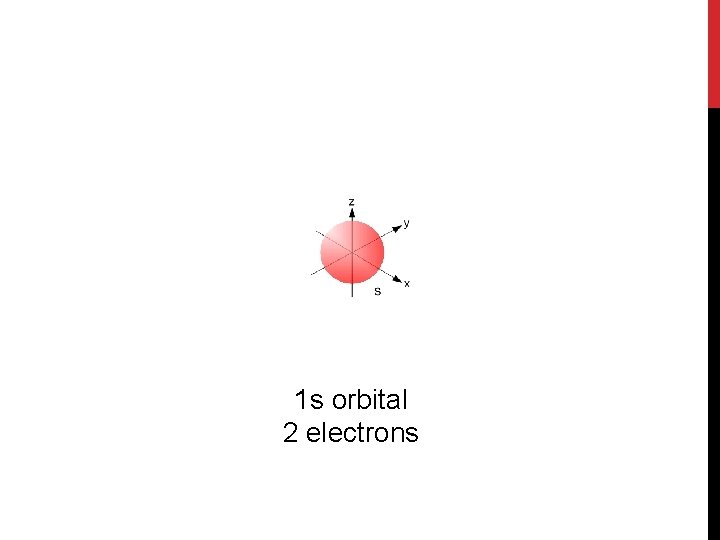
1 s orbital 2 electrons

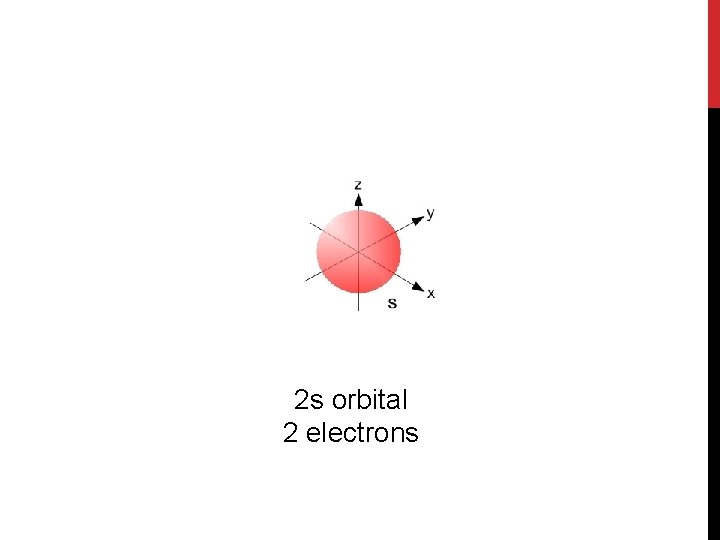
2 s orbital 2 electrons

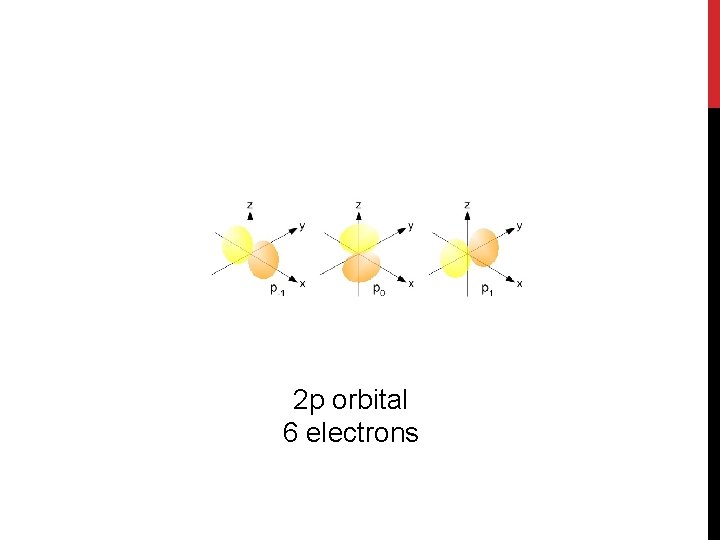
2 p orbital 6 electrons

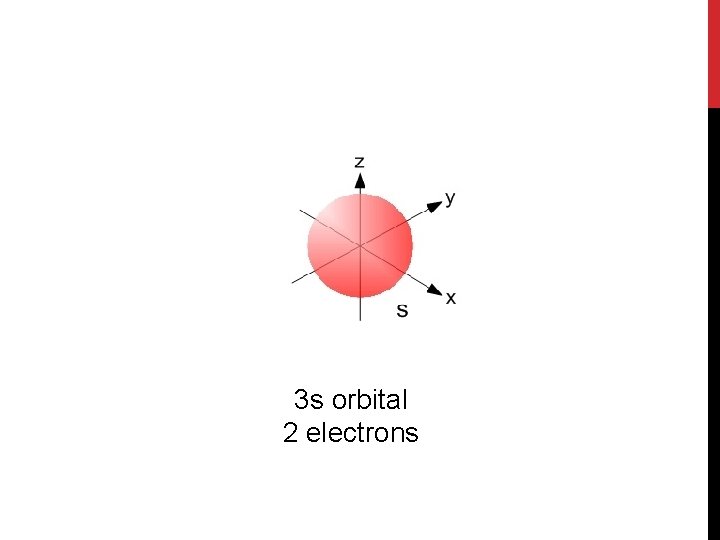
3 s orbital 2 electrons

3 p orbital 6 electrons

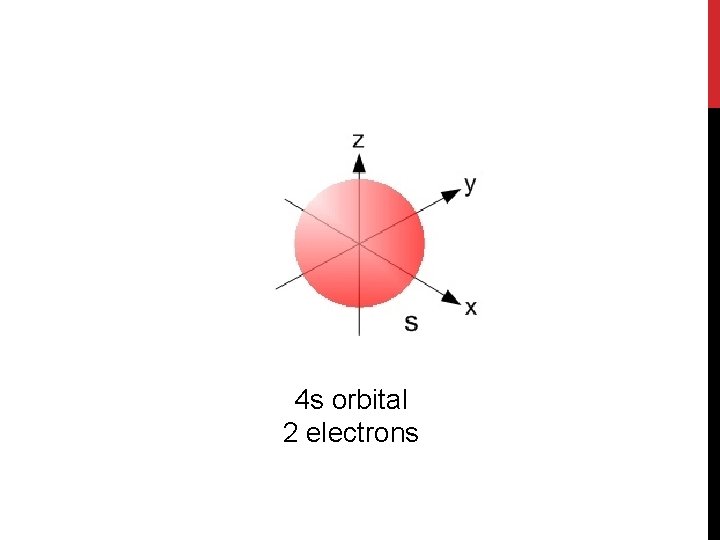
4 s orbital 2 electrons

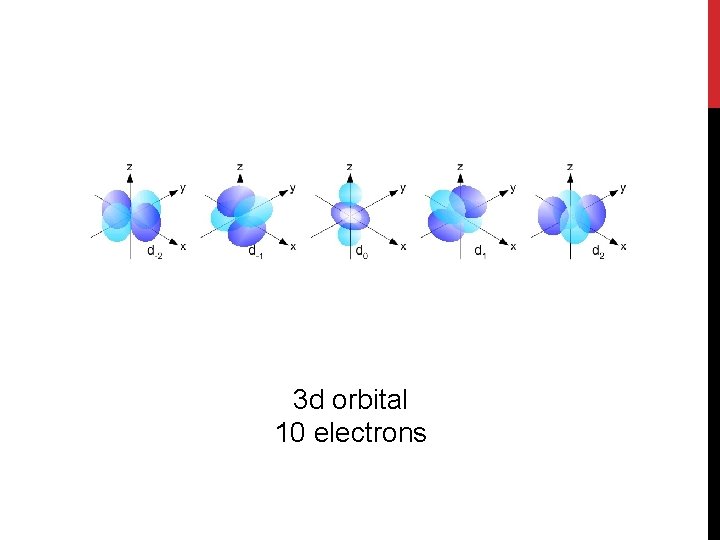
3 d orbital 10 electrons

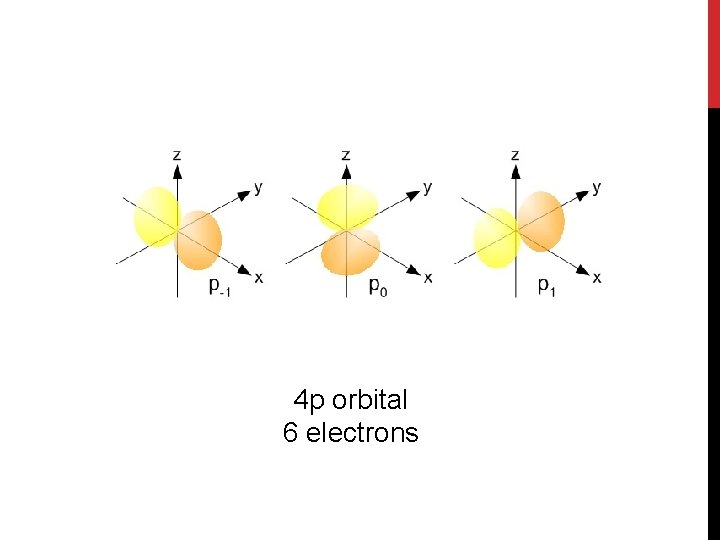
4 p orbital 6 electrons

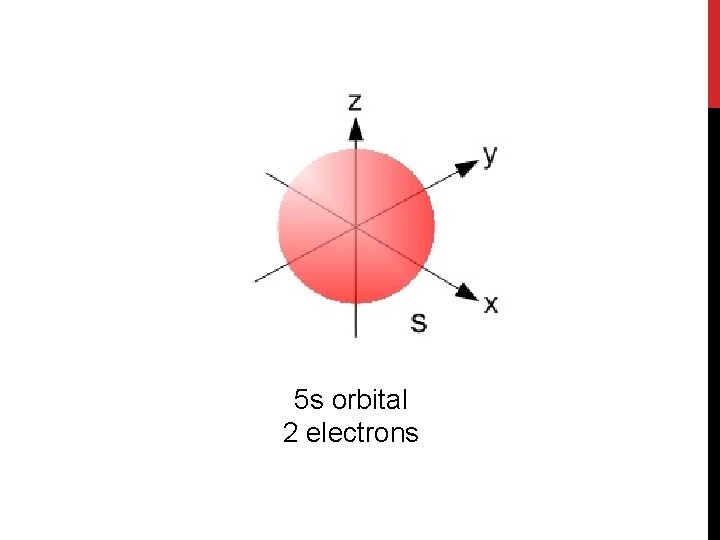
5 s orbital 2 electrons

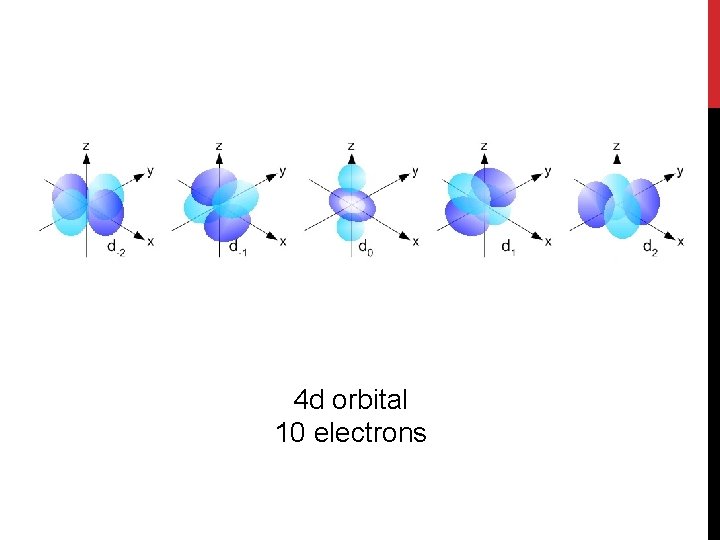
4 d orbital 10 electrons

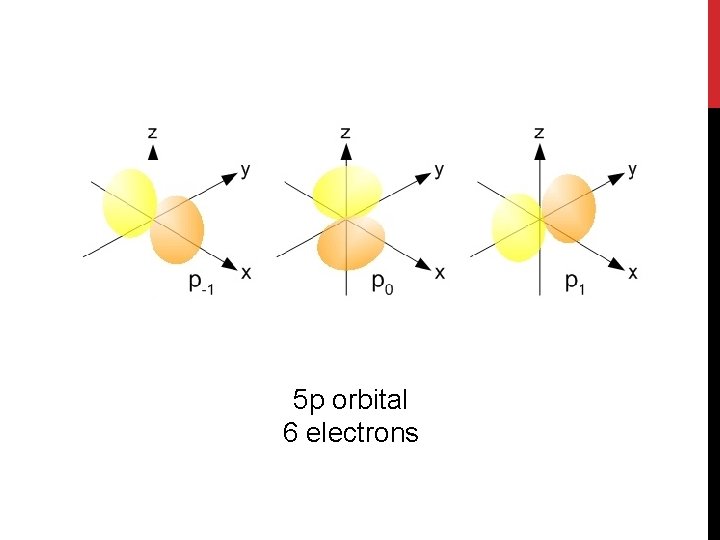
5 p orbital 6 electrons

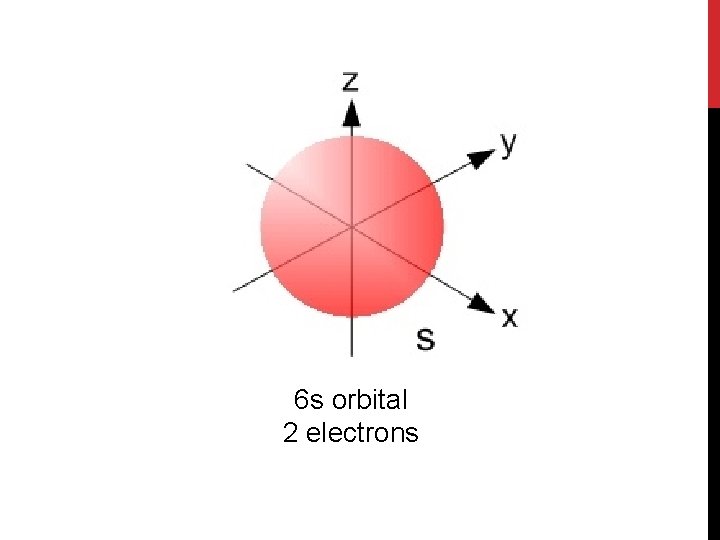
6 s orbital 2 electrons

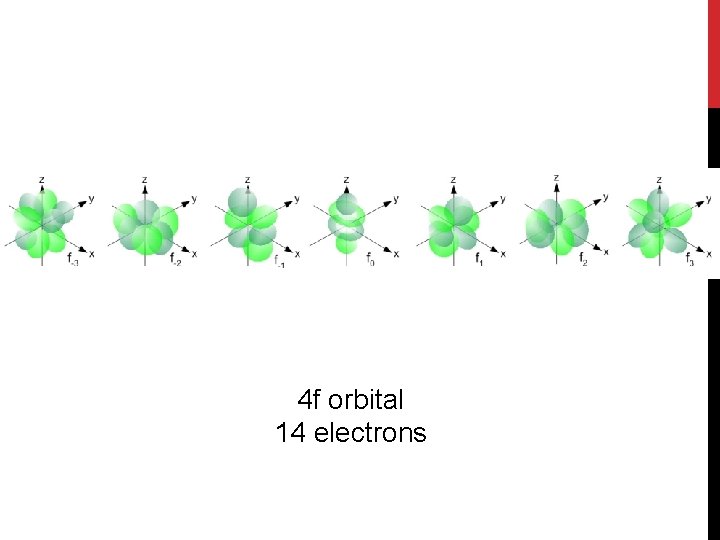
4 f orbital 14 electrons

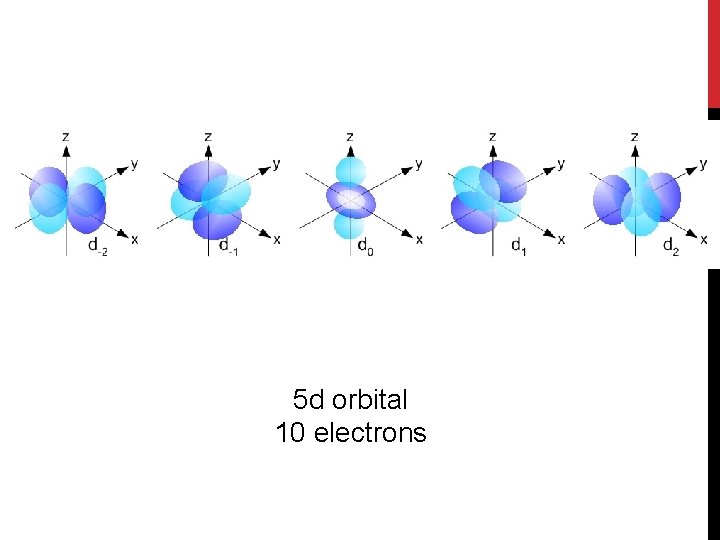
5 d orbital 10 electrons

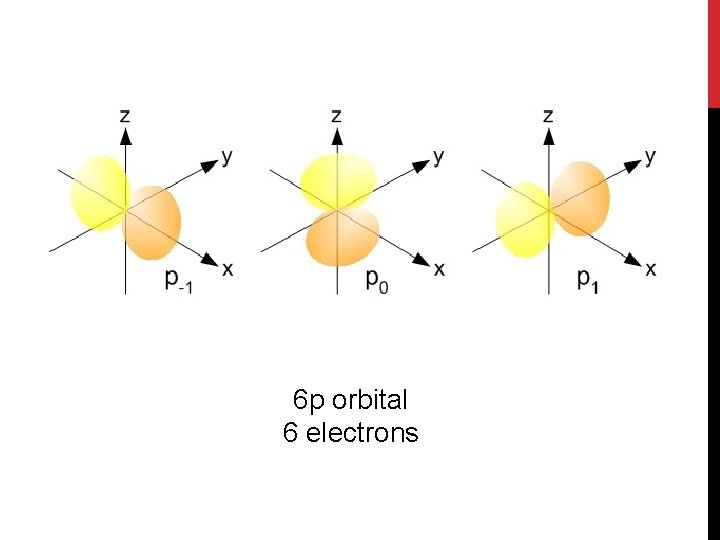
6 p orbital 6 electrons

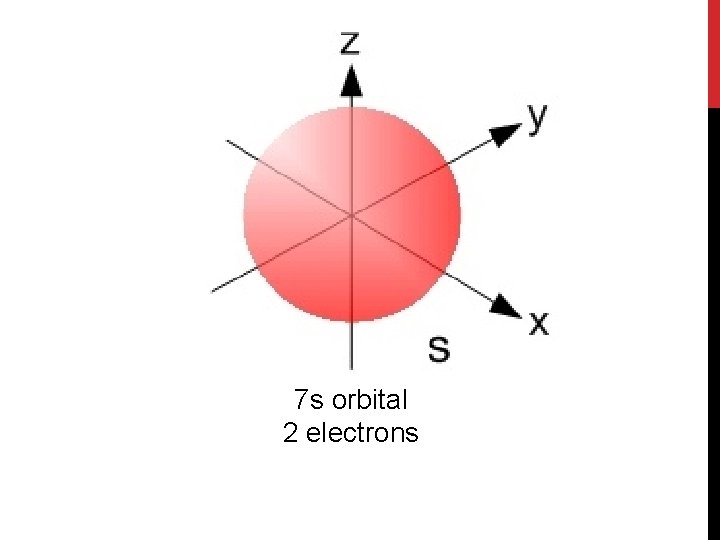
7 s orbital 2 electrons

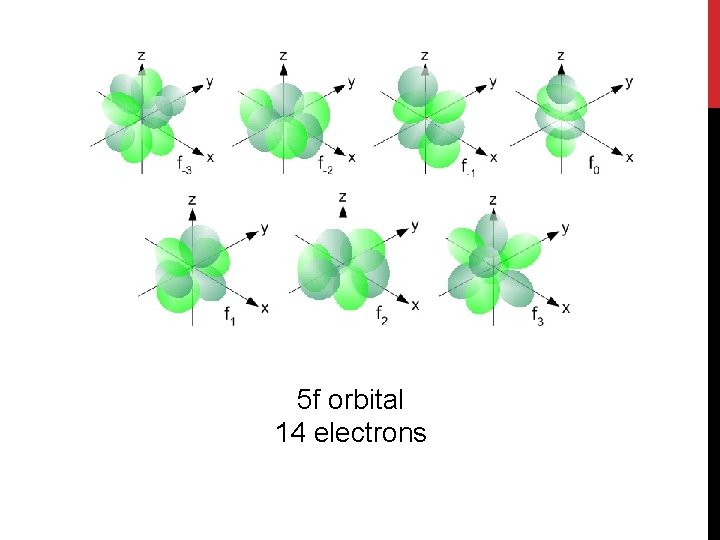
5 f orbital 14 electrons

6 d orbital 10 electrons

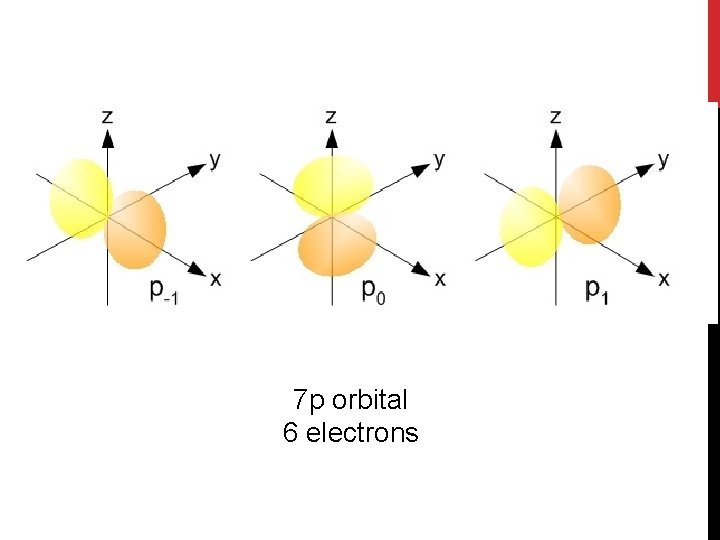
7 p orbital 6 electrons



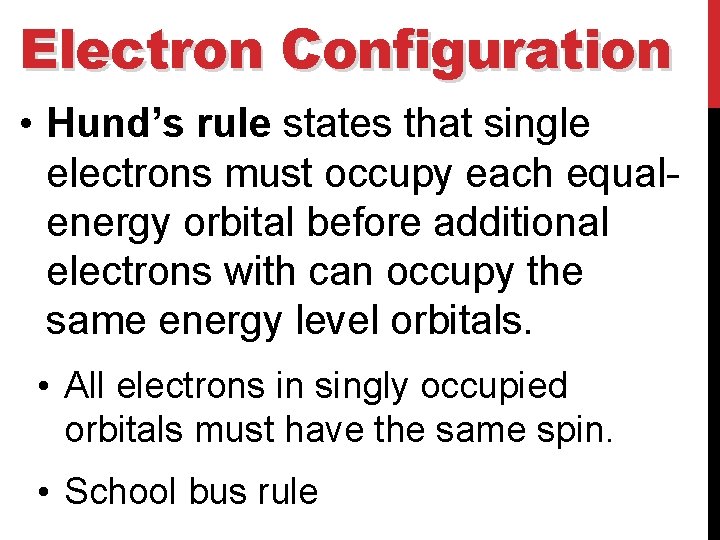
Electron Configuration • Hund’s rule states that single electrons must occupy each equalenergy orbital before additional electrons with can occupy the same energy level orbitals. • All electrons in singly occupied orbitals must have the same spin. • School bus rule


Electron Configuration • The Pauli exclusion principle states that a maximum of two electrons can occupy a single orbital, but only if the electrons have opposite spins. • We symbolize this as two arrows pointing in opposite directions.



Orbital Notation Examples • Write the orbital notation electron configuration for the following atoms or ions. State how many unpaired electrons are in each. a. N b. Cl

Orbital Notation Examples c. Al d. V

Orbital Notation Examples e. Li+ f. 2 O

Electron Configuration • Electron Configurations Using the Periodic Table: • Read the periodic table from left to right to determine the electron configuration.



Electron Configuration • Write the ground state electron configuration for the following: a. C b. Fe

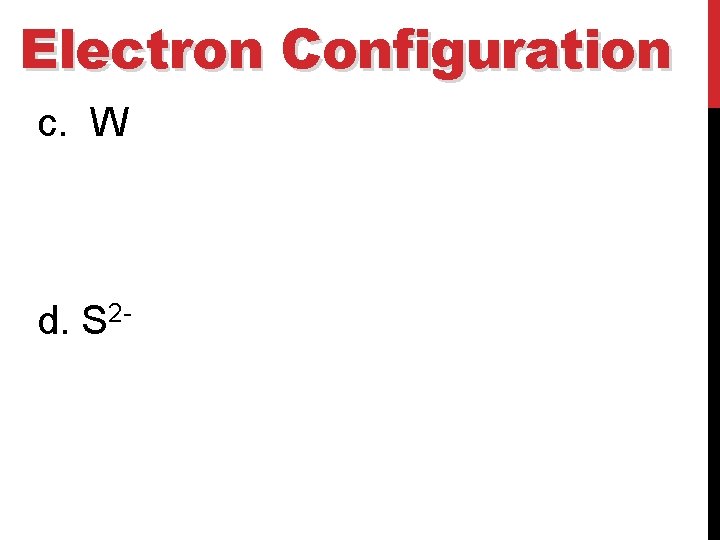
Electron Configuration c. W d. S 2 -


Electron Configuration • Noble gas notation uses noble gas symbols in brackets to shorten inner electron configurations of other elements. • The noble gas must have a lower atomic number than the atom or ion that the electron configuration is being written for.

Noble Gas Notation a. W b. Eu

Noble Gas Notation c. As d. O



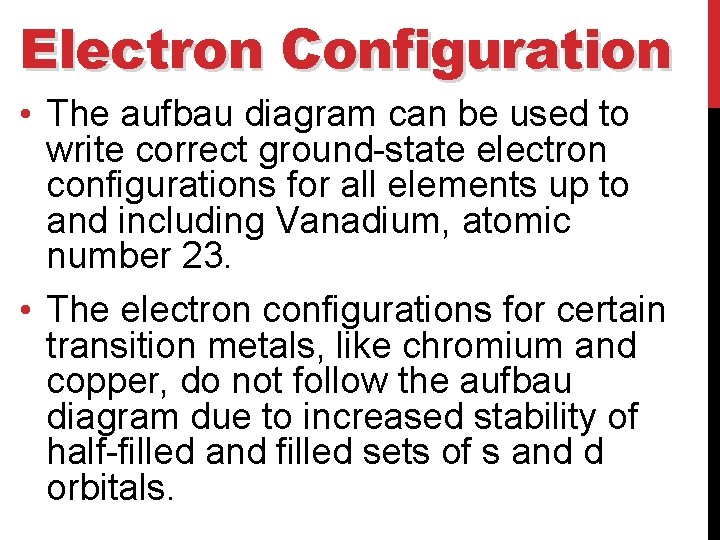
Electron Configuration • The aufbau diagram can be used to write correct ground-state electron configurations for all elements up to and including Vanadium, atomic number 23. • The electron configurations for certain transition metals, like chromium and copper, do not follow the aufbau diagram due to increased stability of half-filled and filled sets of s and d orbitals.
![Electron Configuration Chromium 1 5 Ar4 s 3 d Copper Ar4 s Electron Configuration • Chromium: 1 5 [Ar]4 s 3 d • Copper: [Ar]4 s](https://slidetodoc.com/presentation_image_h/91e9df93309af9dc382e8b12019a6b98/image-45.jpg)
Electron Configuration • Chromium: 1 5 [Ar]4 s 3 d • Copper: [Ar]4 s 13 d 10 • These exceptions occur because a half-filled d subshell and a completely filled d subshell are particularly stable

Electron Configuration • The number of outer-shell electrons in a transition series does not change as you move across a period. • The transition series represents the filling of core orbitals and the number of outershell electrons is mostly constant—either 2 or 1.

Electron Configuration (2 e–) for 4 s 23 dx (1 e–) for 1 5 4 s 3 d or 1 10 4 s 3 d