Electron Configurations a Review and More Electron Configurations
































- Slides: 57

Electron Configurations – a Review and More…

Electron Configurations e- configuration notation: • Reminder – this notation uses # of e- in a sublevel as a superscript over the sublevel (block) designation • Write complete e- configuration notation for element 16

Electron Configurations e- configuration notation (noble gas shortcut): • Reminder – this version uses a noble gas (group 18) “core” instead of beginning at 1 s • Write noble gas shortcut for elements 12, 21, & 35.

Electron Configurations e- dot notation: • simplest notation, only shows valence e- (outer shell e- that may be lost, gained, or shared when chemical compounds are formed - they are from s & p blocks) • Draw dot diagrams for elements 1 -10

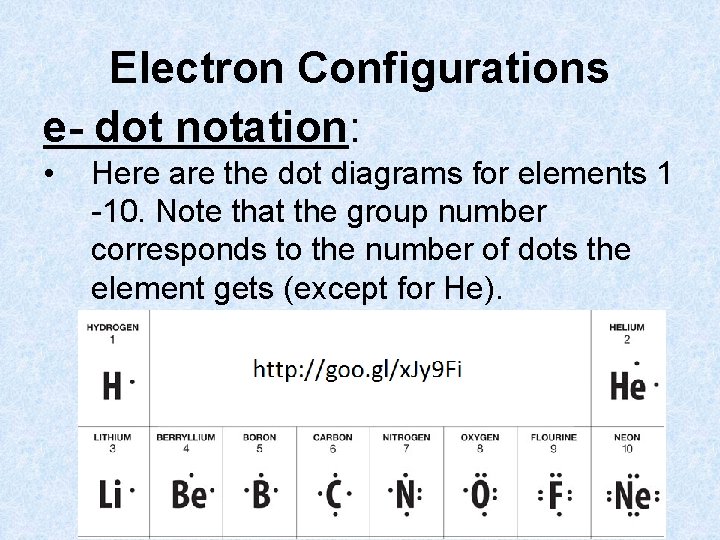
Electron Configurations e- dot notation: • Here are the dot diagrams for elements 1 -10. Note that the group number corresponds to the number of dots the element gets (except for He).

Ion Formation…

Ion Formation… Octet rule: • atoms are most stable when they have a filled outer shell of valence e- (usually 8 e-) • noble gases have this configuration without any help – other atoms lose, gain, or share e- to fill their outer shell

Ion Formation… Ions: • Atoms that have either gained or lost e-. –Gain of e gives a negative ion called an anion. –Loss of e- gives a positive ion called a cation.

Ion Formation… Ion examples: • The magnesium ion is Mg 2+. + How many p and e does it have?

Ion Formation… Ion examples: • The oxide ion is O 2 -. How + many p and e does it have?

Ion Formation… Ion examples: • An ion has 7 p+ and 10 e-. What ion is it? Give your answer as a dot diagram.

Ion Formation… Ion examples: • An ion has 4 p+ and 2 e-. What ion is it? Give your answer as a dot diagram.

Bonding

Chemical Bonds • link between atoms due to mutual attraction of nuclei for e -

Chemical Bonds Why bond? ? ? • Bonding can result in lower potential energy (this is usually associated with a release of energy) • Lower energy gives greater stability (greatest stability @ completed energy level)

Chemical Bonds are classified by how the valence e- are distributed around nuclei of combined atoms

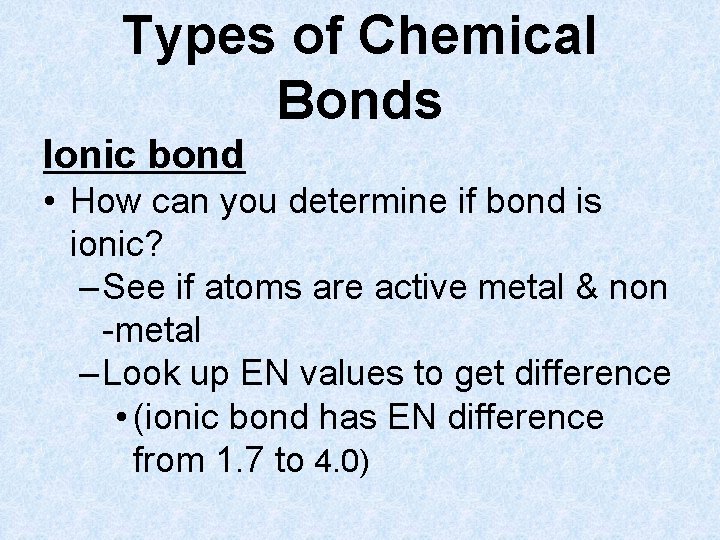
Types of Chemical Bonds Ionic bond – results from electrostatic attraction between positive and negative ions (usually done when metal bonds w/ nonmetal)

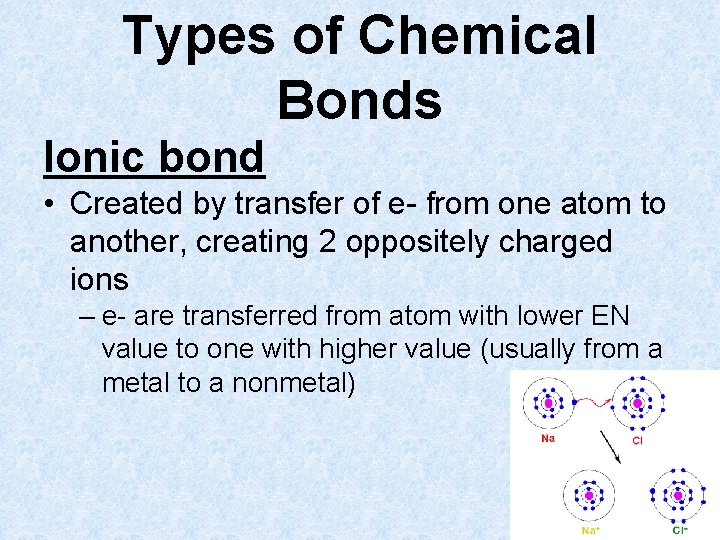
Types of Chemical Bonds Ionic bond • Created by transfer of e- from one atom to another, creating 2 oppositely charged ions – e- are transferred from atom with lower EN value to one with higher value (usually from a metal to a nonmetal)

Types of Chemical Bonds Ionic bond • How can you determine if bond is ionic? – See if atoms are active metal & non -metal – Look up EN values to get difference • (ionic bond has EN difference from 1. 7 to 4. 0)

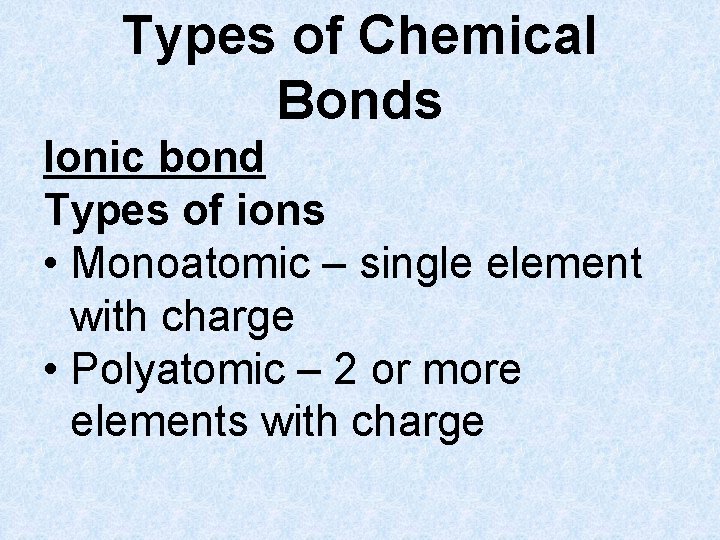
Types of Chemical Bonds Ionic bond Types of ions • Monoatomic – single element with charge • Polyatomic – 2 or more elements with charge

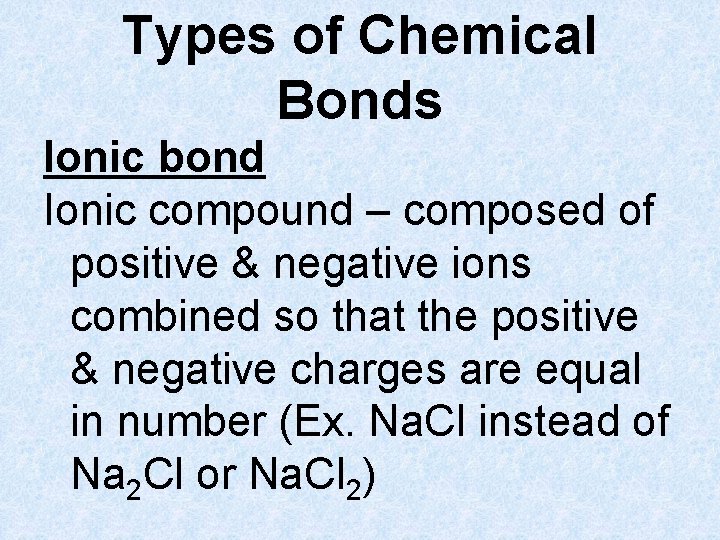
Types of Chemical Bonds Ionic bond Ionic compound – composed of positive & negative ions combined so that the positive & negative charges are equal in number (Ex. Na. Cl instead of Na 2 Cl or Na. Cl 2)

Types of Chemical Bonds Covalent (molecular) bond – results from the sharing of ebetween two atoms (usually done w/ nonmetal atoms) • The e- are not always equally shared (like tug of war)

Covalent Bonds • Bonds between 2 unlike atoms are never completely covalent • Non-polar covalent – e- are shared equally ( which only happens between two identical atoms) • Polar covalent – e- are not equally shared (due to differences in electronegativity)

Covalent Bonds • May share 1 or more pairs of e • Single bond – single pair of shared e- between two atoms, longest/weakest covalent bond • Double bond – two pairs of shared e- between two atoms • Triple bond – three pairs of shared e- between two atoms, shortest/strongest covalent bond

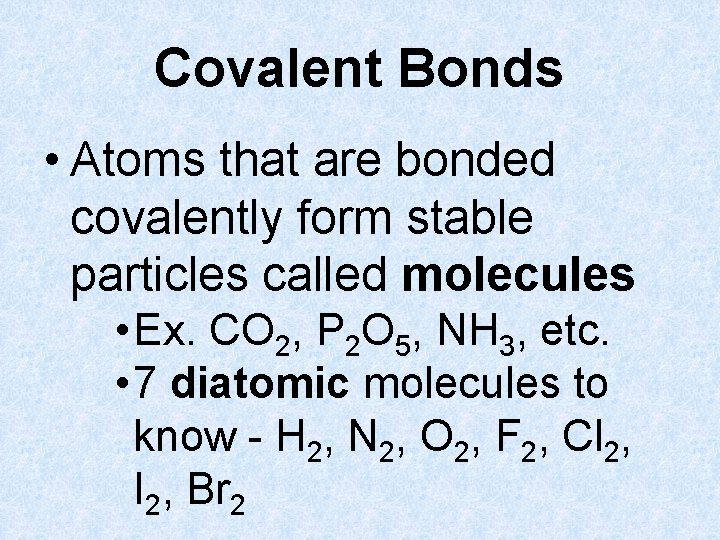
Covalent Bonds • Atoms that are bonded covalently form stable particles called molecules • Ex. CO 2, P 2 O 5, NH 3, etc. • 7 diatomic molecules to know - H 2, N 2, O 2, F 2, Cl 2, I 2, Br 2

Covalent Bonds • Molecular compound – chemical compound whose simplest formulas are molecules

Comparing Properties… Ionic Compounds • Held together tightly (due to attraction of charges) –High melting point –High boiling point –Hard & brittle crystalline solids –Dissolve in water –Carry a current (very well) in water

Comparing Properties… Molecular Compounds • Most are not tightly held –Most have low melting point (due to weak attractions between molecules) –Most have low boiling point –Usually soft, amorphous solids –Some dissolve in water –Do not carry current well in water

Determining Bond Type… Using Periodic Table • Metal Element (left of staircase) + Nonmetal Element (right of staircase) – Ionic Bond • Two Nonmetal Elements (right of staircase) – Covalent Bond

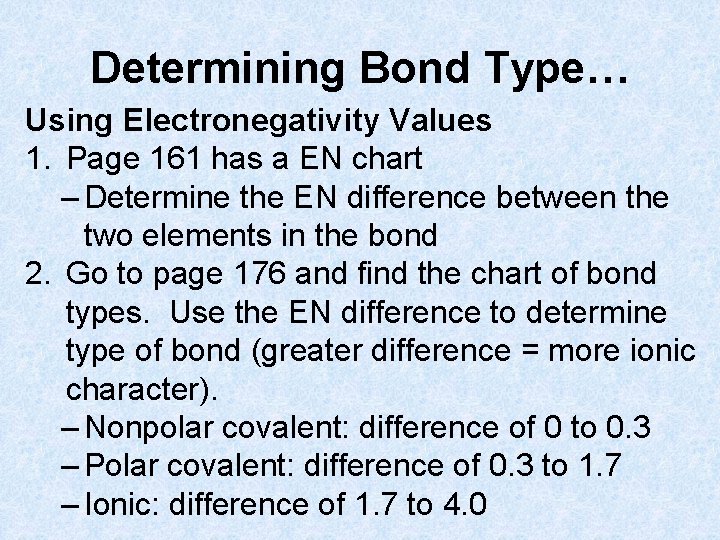
Determining Bond Type… Using Electronegativity Values 1. Page 161 has a EN chart – Determine the EN difference between the two elements in the bond 2. Go to page 176 and find the chart of bond types. Use the EN difference to determine type of bond (greater difference = more ionic character). – Nonpolar covalent: difference of 0 to 0. 3 – Polar covalent: difference of 0. 3 to 1. 7 – Ionic: difference of 1. 7 to 4. 0

Determining Bond Type… Using Observed Properties from Lab Activity 1. Conducts electricity while dry – Metallic bonding (valence electrons are free to move from one atom to another) 2. Dissolves in water – Rule for dissolving is “like dissolves like” • Compounds with either polar covalent bonds or ionic bonds (which are very polar bonds) may dissolve because water has polar covalent bonds

Determining Bond Type… Using Observed Properties from Lab Activity 3. Conducts electricity while dissolved in H 2 O – Ionic bonds • Dissolving ionic compounds allows ions to separate and this lets electric current flow from one ion to the next

Representing Compounds Ionic Empirical formula or Formula unit – indicates lowest whole number ratio of cations to anions in any sample of an ionic compound (ex. Na. F = 1 Na+ ion + 1 F- ion)

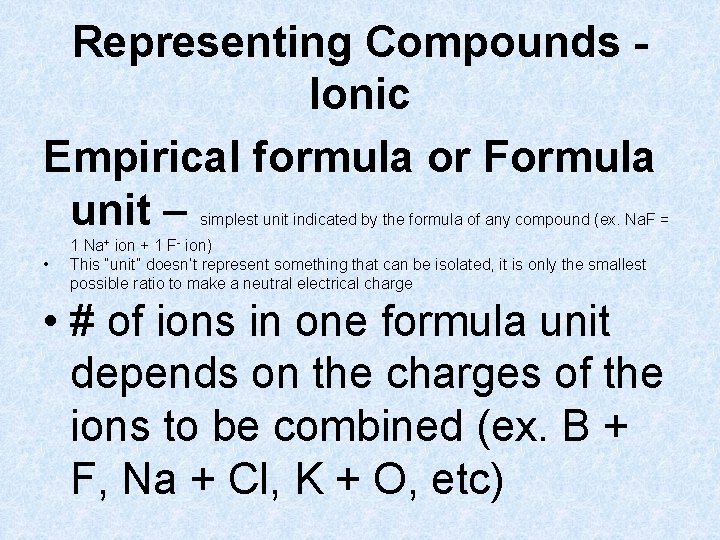
Representing Compounds Ionic Empirical formula or Formula unit – simplest unit indicated by the formula of any compound (ex. Na. F = 1 Na+ ion + 1 F- ion) • This “unit” doesn’t represent something that can be isolated, it is only the smallest possible ratio to make a neutral electrical charge

Representing Compounds Ionic Empirical formula or Formula unit – simplest unit indicated by the formula of any compound (ex. Na. F = • 1 Na+ ion + 1 F- ion) This “unit” doesn’t represent something that can be isolated, it is only the smallest possible ratio to make a neutral electrical charge • # of ions in one formula unit depends on the charges of the ions to be combined (ex. B + F, Na + Cl, K + O, etc)

Representing Compounds Ionic Just a thought… Can you use the periodic table to determine the charge of an ion?

Representing Compounds Ionic Determining formula units by the crisscross method • Ca + Br becomes

Representing Compounds Ionic Determining formula units by the crisscross method • K + P becomes

Representing Compounds Ionic Determining formula units by the crisscross method • Al + O becomes

Representing Compounds Ionic Determining formula units by the crisscross method • Ca + O becomes

Representing Compounds Ionic Determining formula units by the crisscross method 3+ • Al + 1 OH becomes

Representing Compounds Ionic Determining formula units by the crisscross method 2+ • Mg + PO 4 becomes 3 -

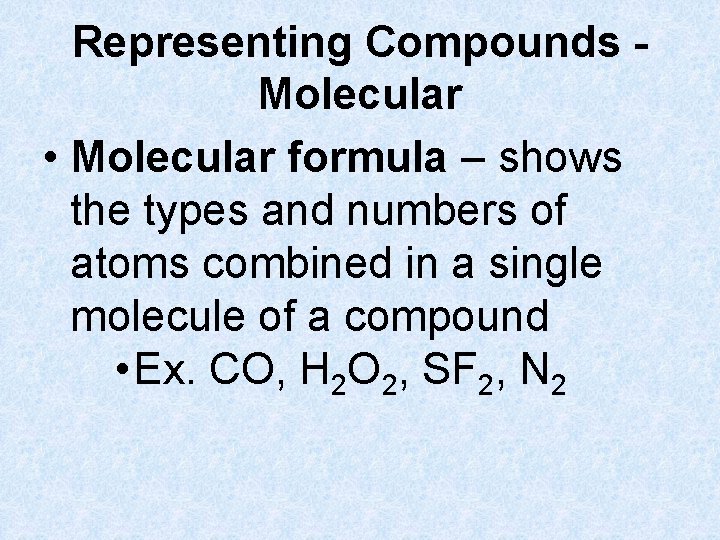
Representing Compounds Molecular • Molecular formula – shows the types and numbers of atoms combined in a single molecule of a compound • Ex. CO, H 2 O 2, SF 2, N 2

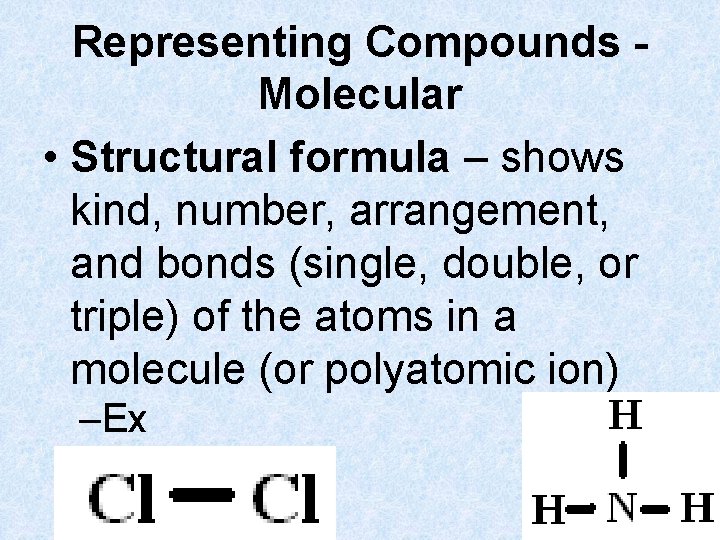
Representing Compounds Molecular • Structural formula – shows kind, number, arrangement, and bonds (single, double, or triple) of the atoms in a molecule (or polyatomic ion) –Ex

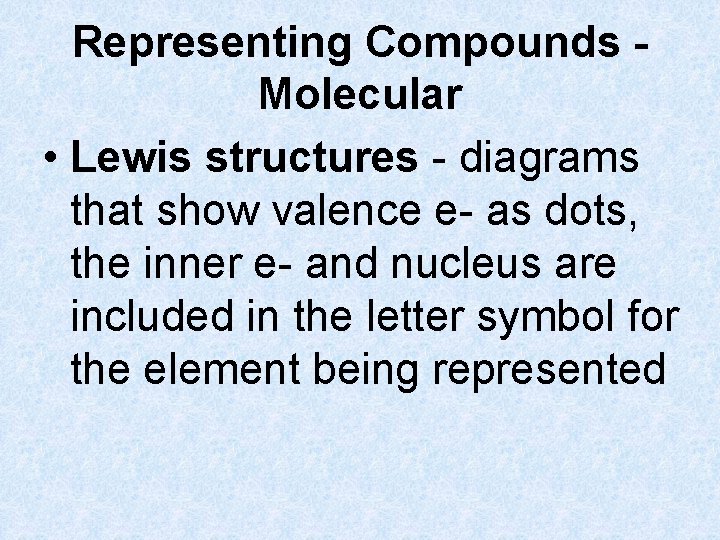
Representing Compounds Molecular • Lewis structures - diagrams that show valence e- as dots, the inner e- and nucleus are included in the letter symbol for the element being represented

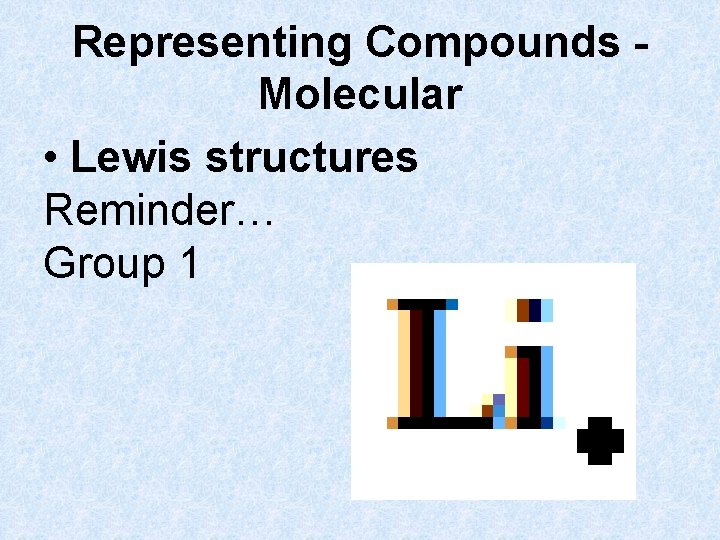
Representing Compounds Molecular • Lewis structures Reminder… Group 1

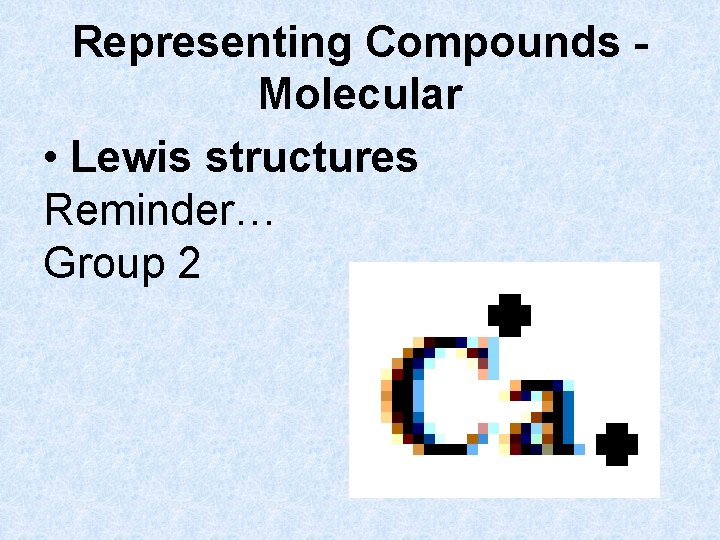
Representing Compounds Molecular • Lewis structures Reminder… Group 2

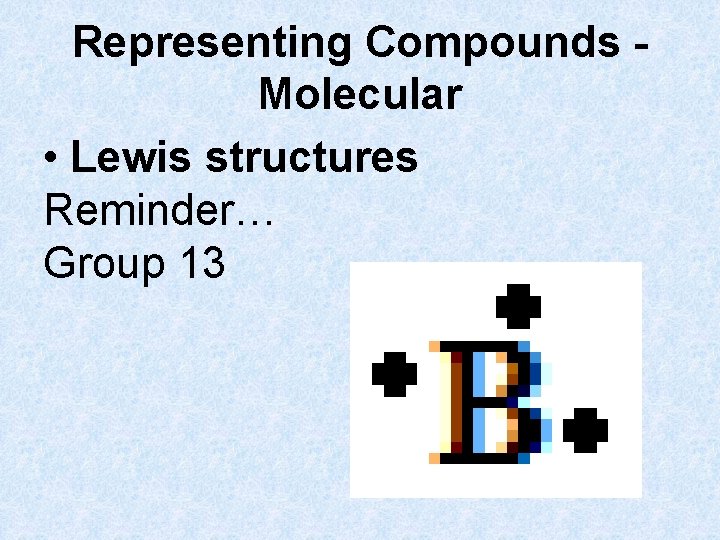
Representing Compounds Molecular • Lewis structures Reminder… Group 13

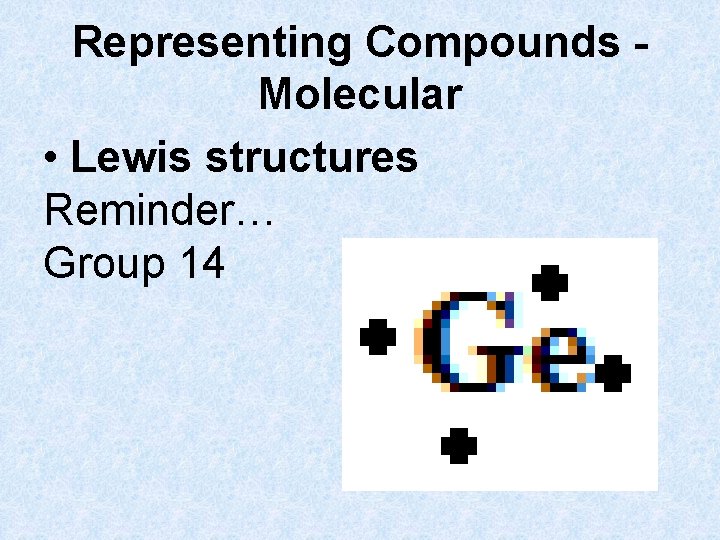
Representing Compounds Molecular • Lewis structures Reminder… Group 14

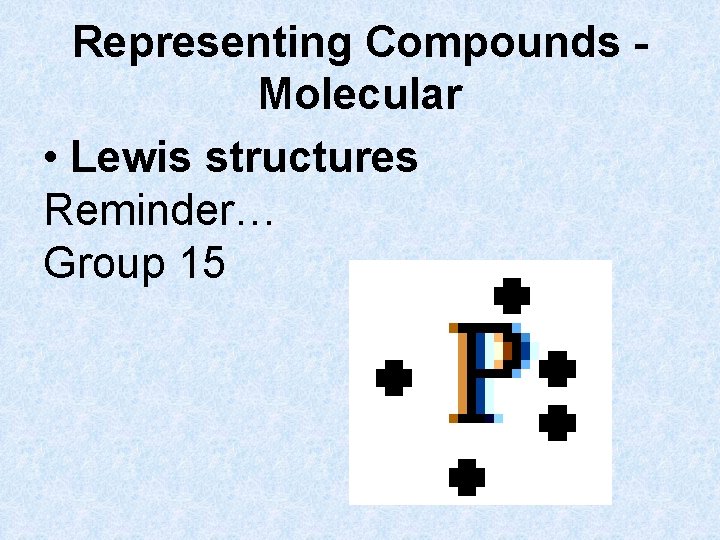
Representing Compounds Molecular • Lewis structures Reminder… Group 15

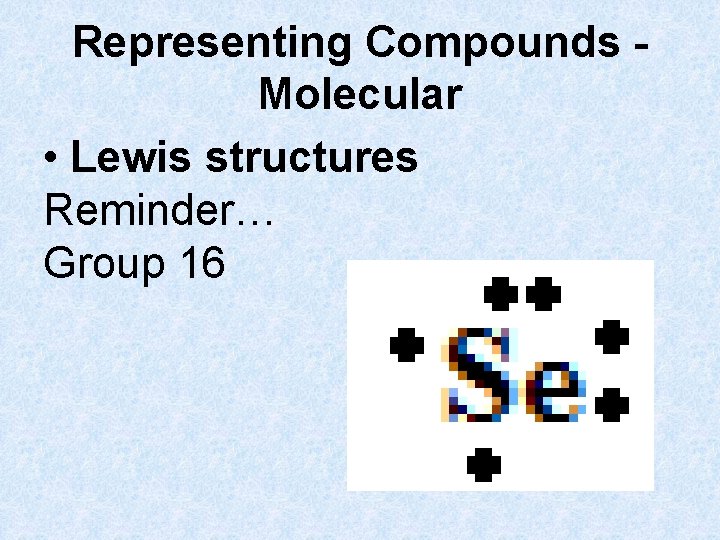
Representing Compounds Molecular • Lewis structures Reminder… Group 16

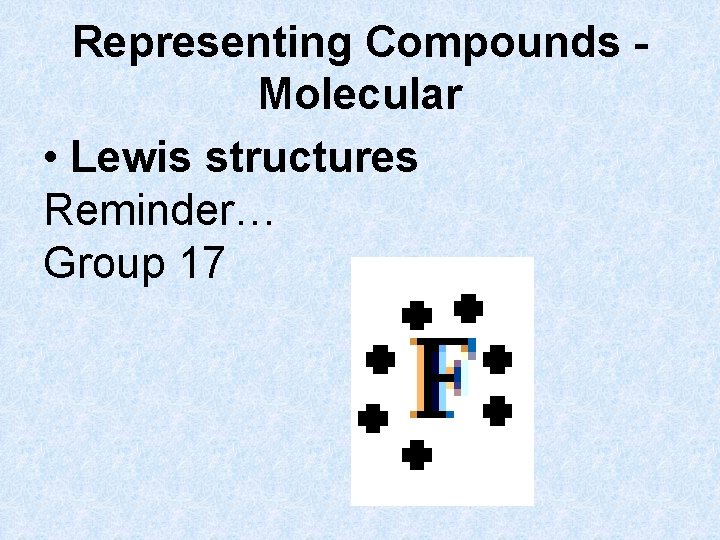
Representing Compounds Molecular • Lewis structures Reminder… Group 17

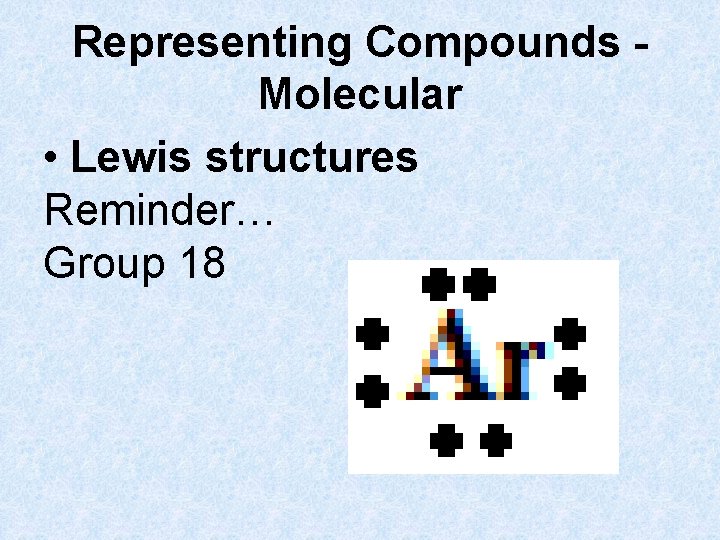
Representing Compounds Molecular • Lewis structures Reminder… Group 18

Representing Compounds Molecular • Lewis structures – Structures of individual elements may be joined to form compounds – a dash (sometimes a pair of dots instead) between symbols represent bonds (or electron pairs), dots adjacent only to one symbol are unshared e- (also called lone pairs)

Representing Compounds Molecular • Lewis structures Ex. F 2, NH 3, H 2 O, CH 4, O 2, CO 2, N 2, CH 2 O, C 2 H 2, PI 3 (must draw structures)

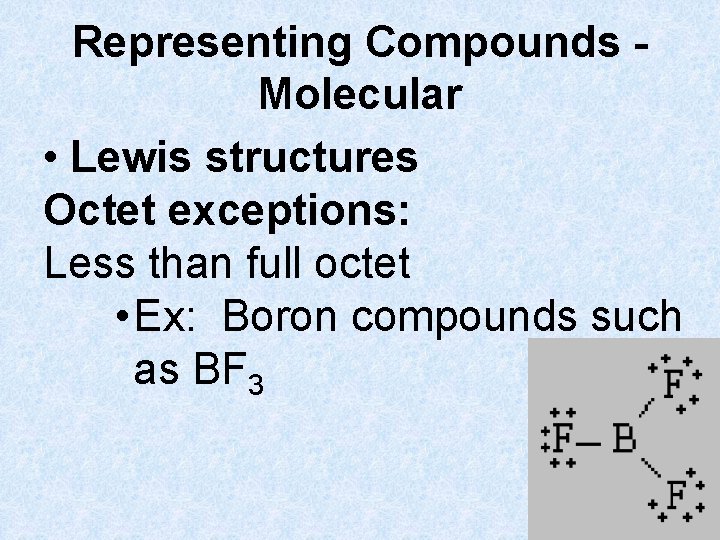
Representing Compounds Molecular • Lewis structures Octet exceptions: Less than full octet • Ex: Boron compounds such as BF 3

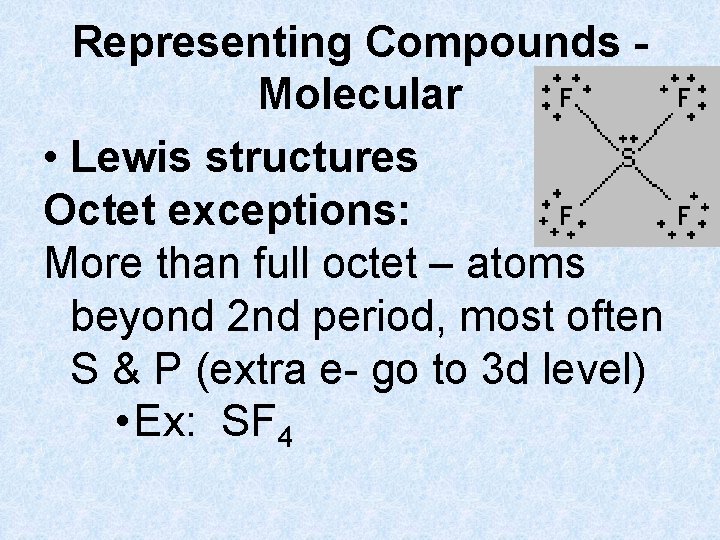
Representing Compounds Molecular • Lewis structures Octet exceptions: More than full octet – atoms beyond 2 nd period, most often S & P (extra e- go to 3 d level) • Ex: SF 4