Ch 4 Atomic Structure Electron Configurations Electron Configurations















- Slides: 35

Ch. 4 Atomic Structure Electron Configurations

Electron Configurations Ø the arrangement of electrons in an atom Ø each type of atom has a unique electron configuration Ø electrons tend to assume positions that create the lowest possible energy for atom Ø ground state electron configuration- lowest energy arrangement of electrons

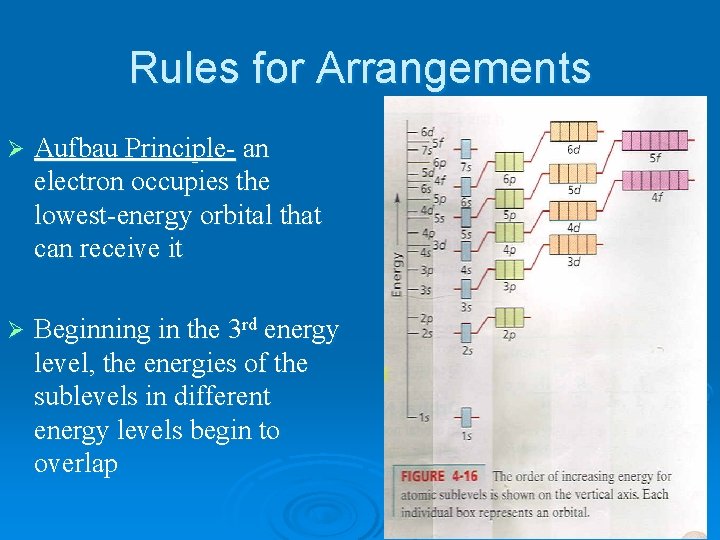
Rules for Arrangements Ø Aufbau Principle- an electron occupies the lowest-energy orbital that can receive it Ø Beginning in the 3 rd energy level, the energies of the sublevels in different energy levels begin to overlap

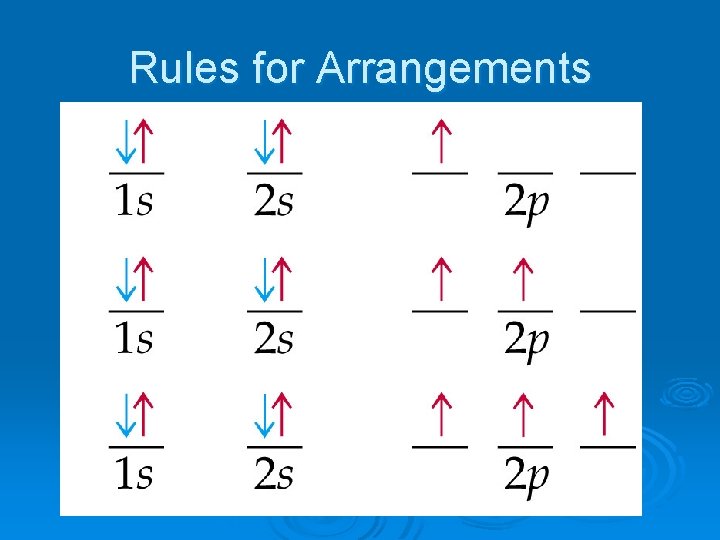
Rules for Arrangements Ø Pauli Exclusion Principle- no two electrons in the same atom can have the same set of 4 quantum numbers Ø Hund’s Rule- orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second Ø all unpaired electrons must have the same spin

Rules for Arrangements

Writing Configurations Ø Orbital Notation: l l l an orbital is written as a line each orbital has a name written below it electrons are drawn as arrows (up and down) Ø Electron Configuration Notation l number of electrons in sublevel is added as a superscript

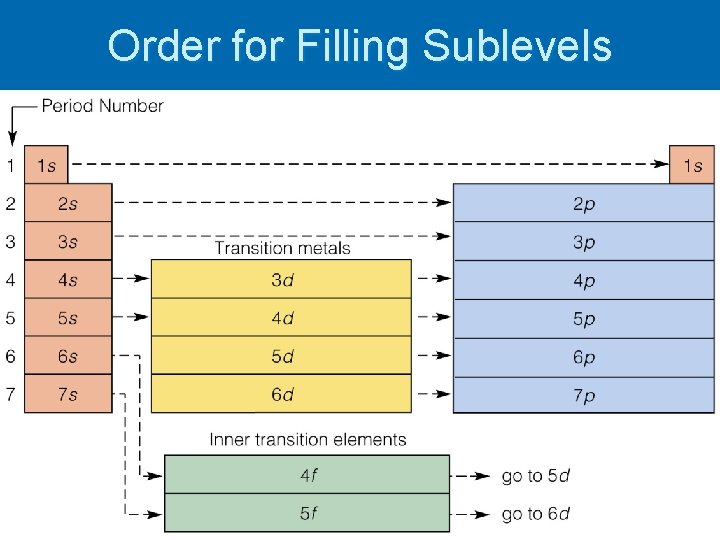
Order for Filling Sublevels

Writing Configurations Start by finding the number of electrons in the atom Ø Identify the sublevel that the last electron added is in by looking at the location in periodic table Ø Draw out lines for each orbital beginning with 1 s and ending with the sublevel identified Ø Add arrows individually to the orbitals until all electrons have been drawn Ø

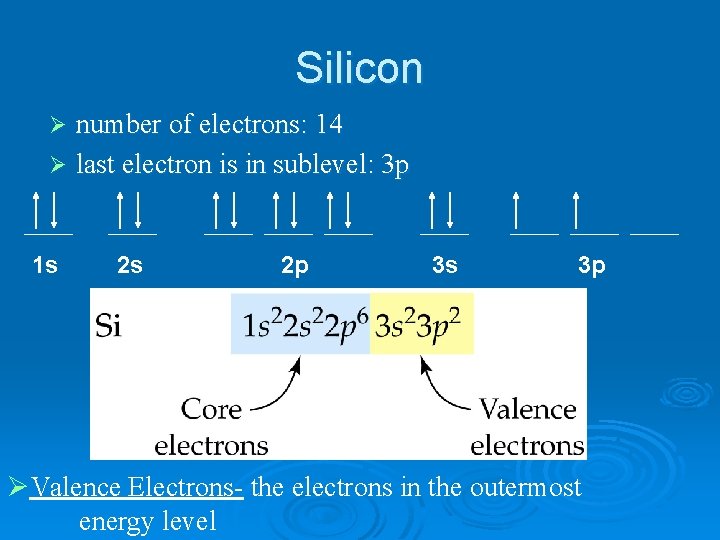
Silicon number of electrons: 14 Ø last electron is in sublevel: 3 p Ø 1 s 2 s 2 p 3 s 3 p ØValence Electrons- the electrons in the outermost energy level

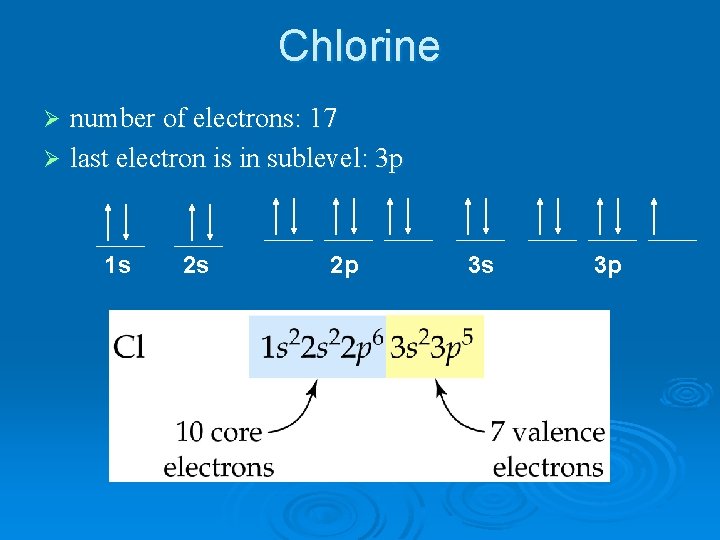
Chlorine number of electrons: 17 Ø last electron is in sublevel: 3 p Ø 1 s 2 s 2 p 3 s 3 p

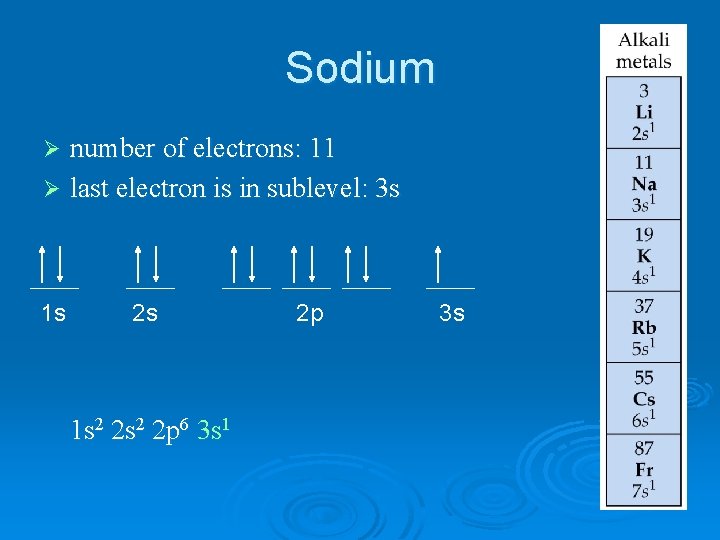
Sodium number of electrons: 11 Ø last electron is in sublevel: 3 s Ø 1 s 2 s 1 s 2 2 p 6 3 s 1 2 p 3 s

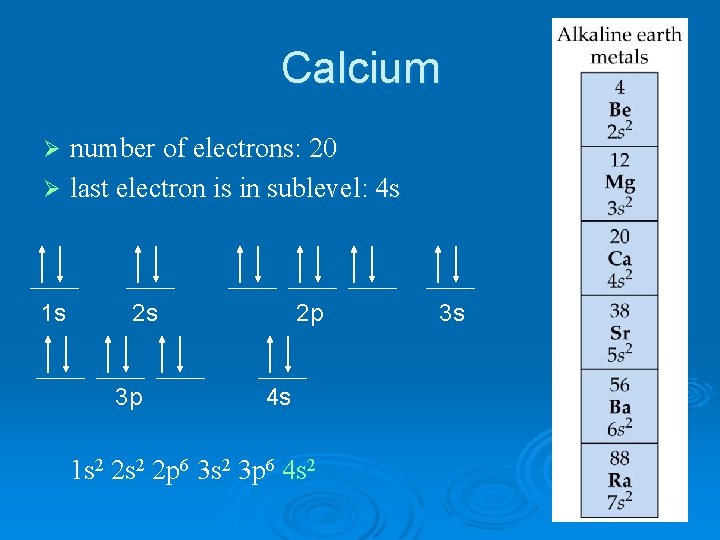
Calcium number of electrons: 20 Ø last electron is in sublevel: 4 s Ø 1 s 2 s 3 p 2 p 4 s 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 s

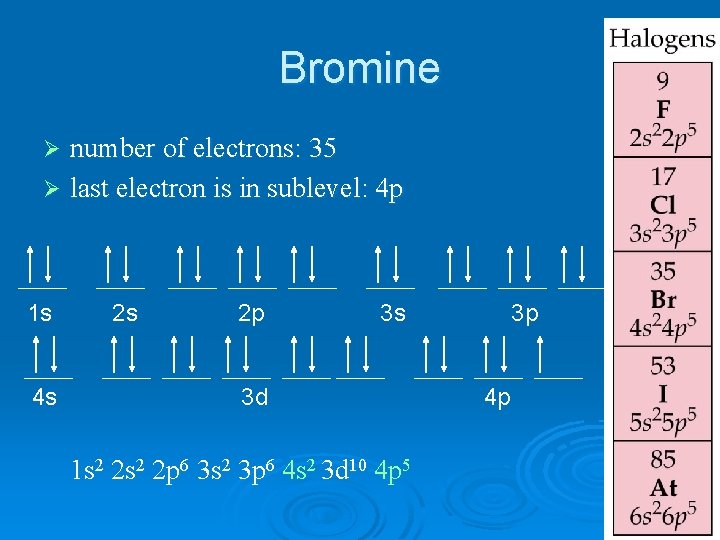
Bromine number of electrons: 35 Ø last electron is in sublevel: 4 p Ø 1 s 4 s 2 s 2 p 3 s 3 d 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 3 p 4 p

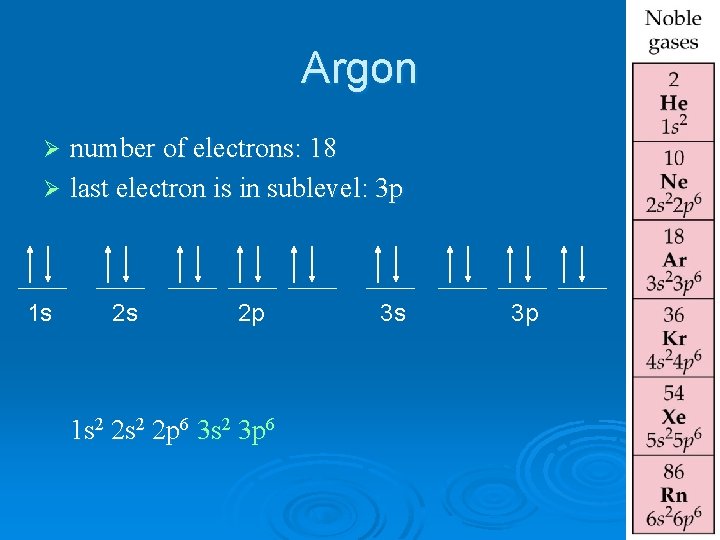
Argon number of electrons: 18 Ø last electron is in sublevel: 3 p Ø 1 s 2 s 2 p 1 s 2 2 p 6 3 s 2 3 p 6 3 s 3 p

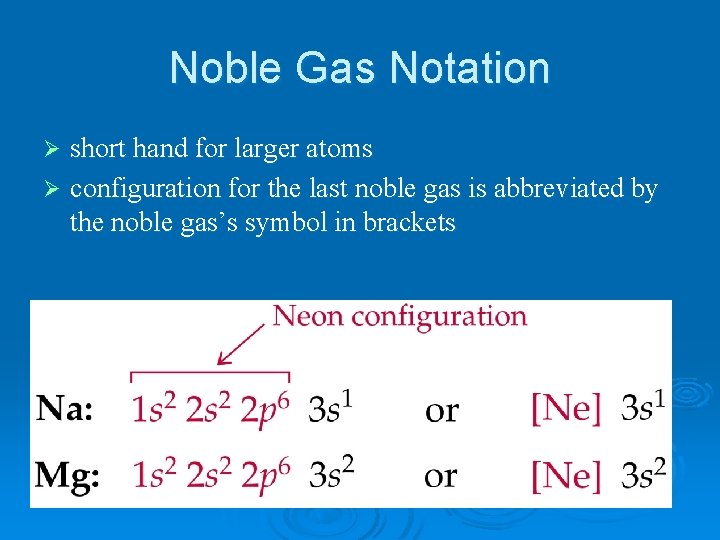
Noble Gas Notation short hand for larger atoms Ø configuration for the last noble gas is abbreviated by the noble gas’s symbol in brackets Ø

Ch. 5: Atomic Structure Periodic Table and Periodic Trends

Periodic Table Ø Russian, Dmitri Mendeleev Ø when he arranged them by atomic mass, he found similar properties at certain intervals Ø published the first periodic table in 1869 Ø left empty spaces where he predicted undiscovered elements should be Ø confirmed his predictions and persuaded other chemists

Periodic Table Ø In 1911, Henry Moseley (English) found that the pattern worked best if arranged by number of protons Ø Our current periodic tables use this method or arrangement

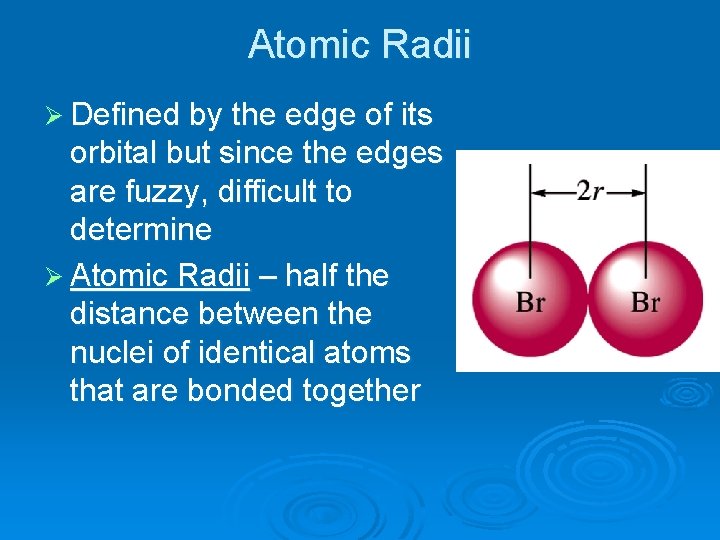
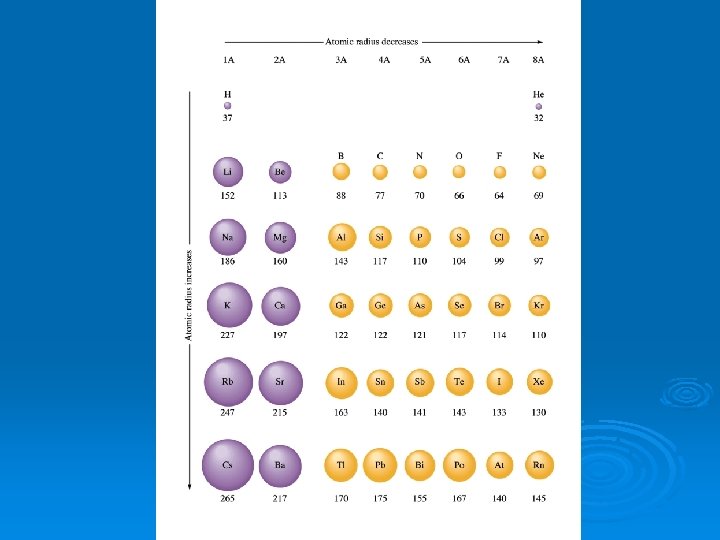
Atomic Radii Ø Defined by the edge of its orbital but since the edges are fuzzy, difficult to determine Ø Atomic Radii – half the distance between the nuclei of identical atoms that are bonded together


Atomic/Ionic Radii

Which is bigger? Ø Na or Rb? Ø Na or S? Ø S or Te?

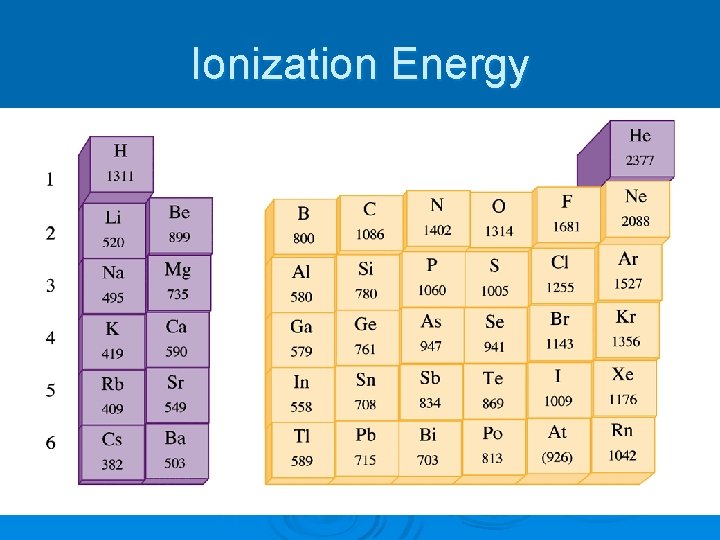
Ionization Energy Ø An electron can be removed from an atom if enough energy is used Ø Ionization energy – the energy required to remove one electron from a gaseous neutral atom A + energy A+ + e-

Ionization Energy

Ionization Energy

Which has higher IE? Ø Li or F? Ø Ca or P? Ø Ba or Li?

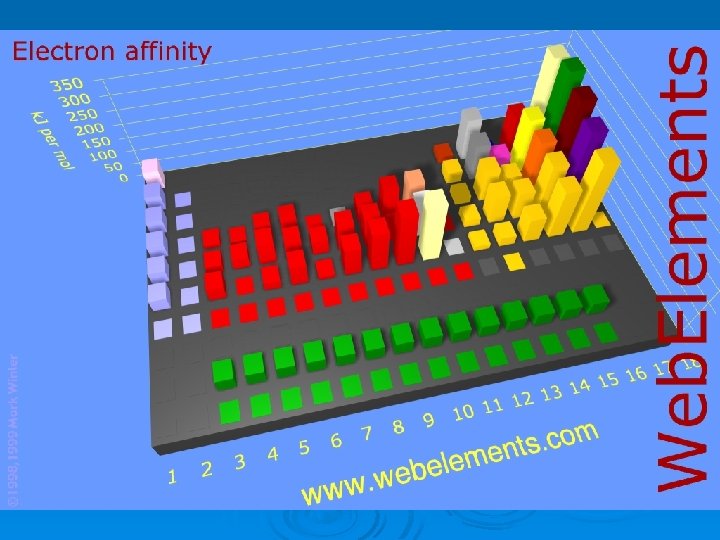
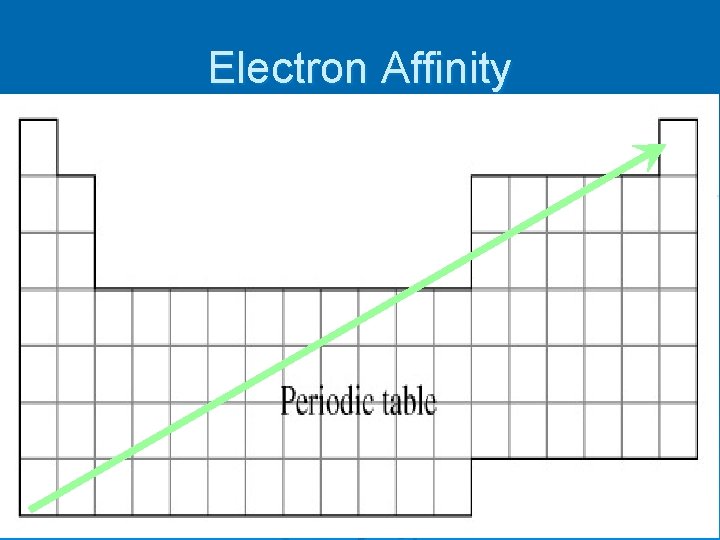
Electron Affinity – the energy change when an electron is added to a gaseous neutral atom l exothermic (-) A + e- A- + energy


Electron Affinity

Which has higher EA? Ø Ge or C? Ø In or I? Ø Mg or F?

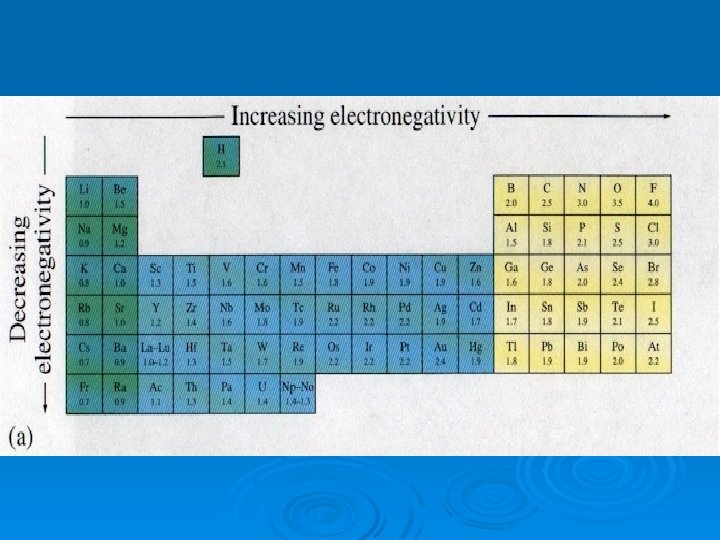
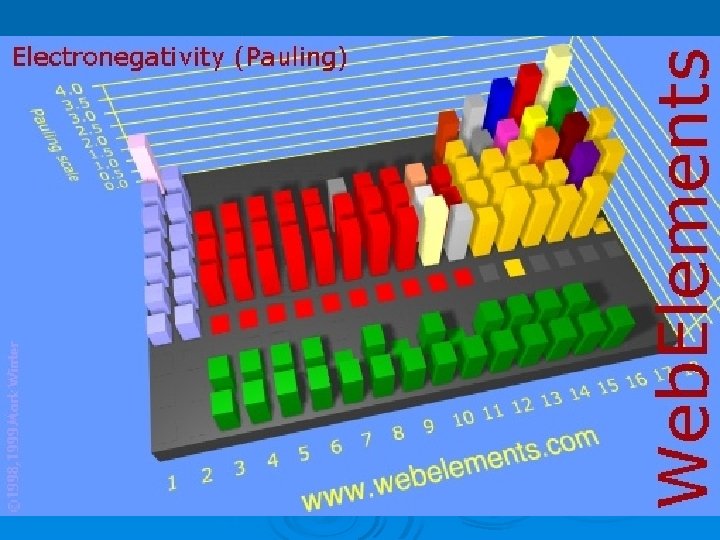
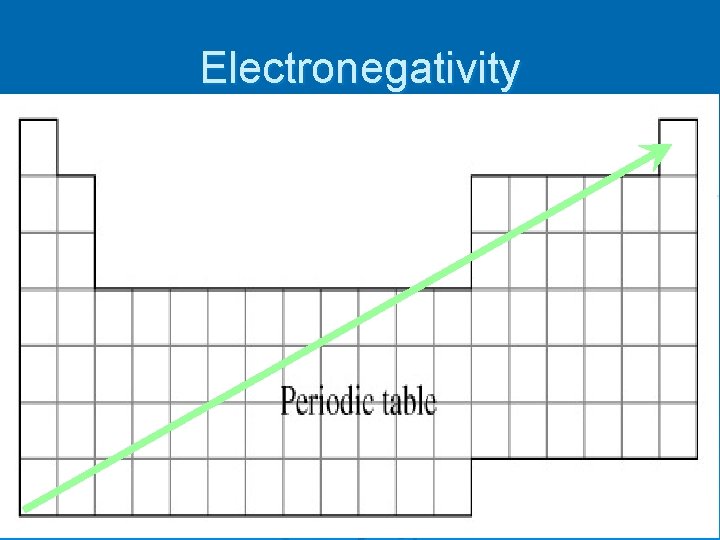
Electronegativity Ø applies when an atom is in a compound NOT alone Ø Electronegativity – measure of how strongly an atom attracts electrons when it is in a compound Ø Fluorine (the most electronegative element) is assigned a 4. 0 and then all the others were determined by comparison



Electronegativity

Which has higher electronegativity? Ø Sr or Be? Ø P or O? Ø Si or Cl?