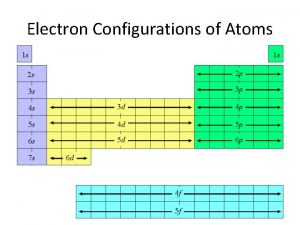
4 5 Electron Configurations of Ions To write













- Slides: 27

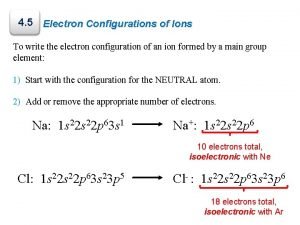
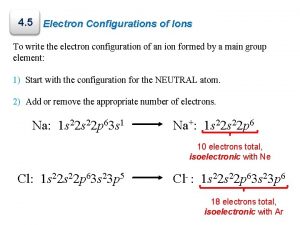
4. 5 Electron Configurations of Ions To write the electron configuration of an ion formed by a main group element: 1) Start with the configuration for the NEUTRAL atom. 2) Add or remove the appropriate number of electrons. Na: 1 s 22 p 63 s 1 Na+: 1 s 22 p 6 10 electrons total, isoelectronic with Ne Cl: 1 s 22 p 63 s 23 p 5 Cl : 1 s 22 p 63 s 23 p 6 − 18 electrons total, isoelectronic with Ar

Worked Example 4. 7 Write electron configurations for the following ions of main group elements: (a) N 3 -, (b) Ba 2+, and (c) Be 2+. Strategy First write electron configurations for the atoms. Then add electrons (for anions) and remove electrons (for cations) to account for the charge.

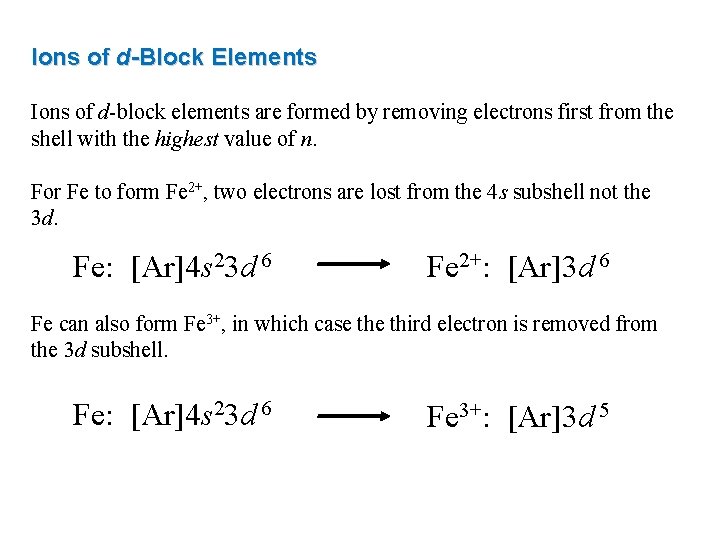
Ions of d-Block Elements Ions of d-block elements are formed by removing electrons first from the shell with the highest value of n. For Fe to form Fe 2+, two electrons are lost from the 4 s subshell not the 3 d. Fe: [Ar]4 s 23 d 6 Fe 2+: [Ar]3 d 6 Fe can also form Fe 3+, in which case third electron is removed from the 3 d subshell. Fe: [Ar]4 s 23 d 6 Fe 3+: [Ar]3 d 5

Worked Example 4. 8 Write electron configurations for the following ions of d-block elements: (a) Zn 2+, (b) Mn 2+, and (c) Cr 3+. Strategy First write electron configurations for the atoms. Then add electrons (for anions) and remove electrons (for cations) to account for the charge. The electrons removed from a d-block element must come first from the outermost s subshell, not the partially filled d subshell.

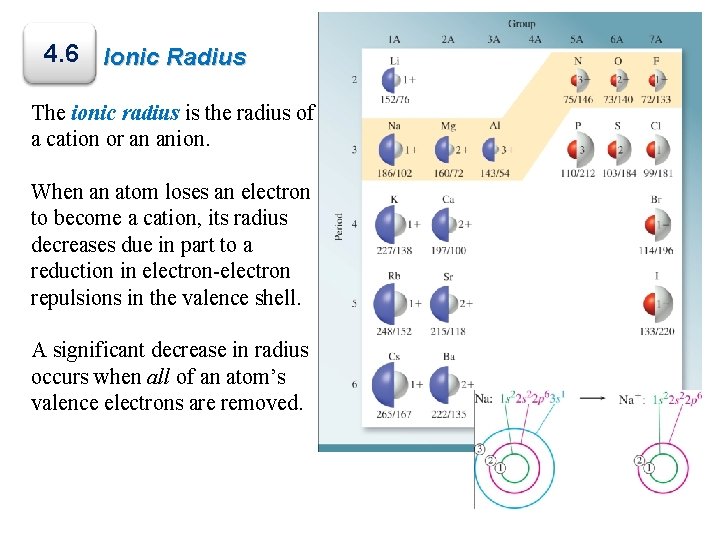
4. 6 Ionic Radius The ionic radius is the radius of a cation or an anion. When an atom loses an electron to become a cation, its radius decreases due in part to a reduction in electron-electron repulsions in the valence shell. A significant decrease in radius occurs when all of an atom’s valence electrons are removed.

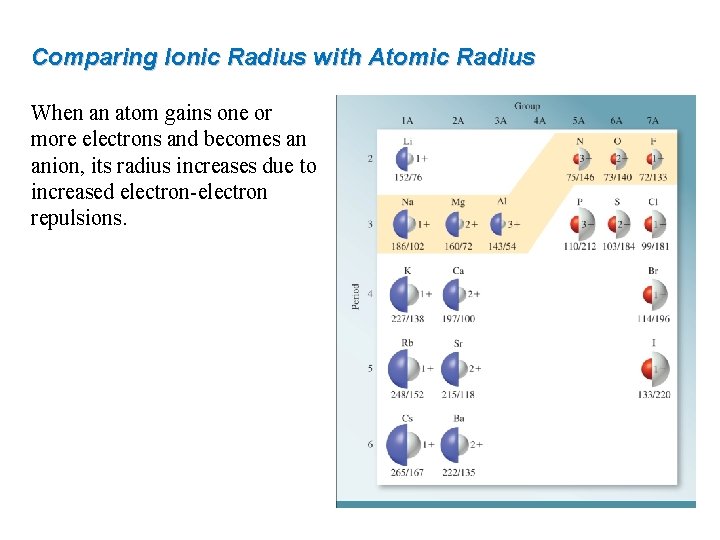
Comparing Ionic Radius with Atomic Radius When an atom gains one or more electrons and becomes an anion, its radius increases due to increased electron-electron repulsions.

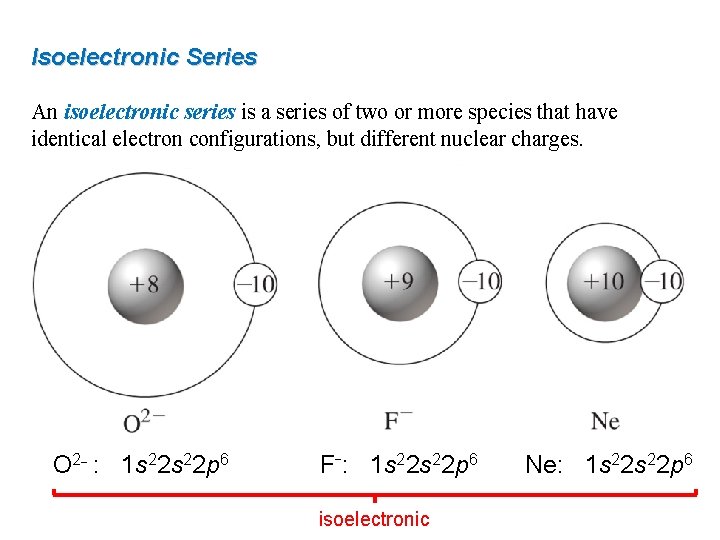
Isoelectronic Series An isoelectronic series is a series of two or more species that have identical electron configurations, but different nuclear charges. O 2− : 1 s 22 p 6 F−: 1 s 22 p 6 isoelectronic Ne: 1 s 22 p 6

Worked Example 4. 9 Identify the isoelectronic series in the following group of species, and arrange them in order of increasing radius: K+, Ne, Ar, Kr, P 3 -, S 2 -, and Cl-. Strategy Isoelectronic series are species with identical electron configurations but different nuclear charges. Determine the number of electrons in each species. The radii of isoelectronic series members decreases with increasing nuclear charge. Text Practice: 4. 68 4. 72 4. 74 4. 78

Chemistry: Atoms First Second Edition Julia Burdge & Jason Overby Chapter 5 Ionic and Covalent Compounds M. Stacey Thomson Pasco-Hernando State College Copyright (c) The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

5. 1 Compounds A compound is a substance composed of two or more elements combined in a specific ratio and held together by chemical bonds. Familiar examples of compounds are water and salt (sodium chloride).

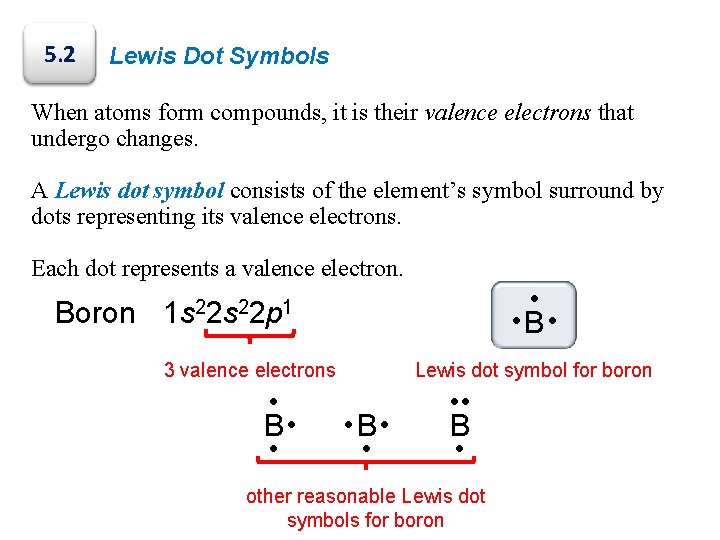
5. 2 Lewis Dot Symbols When atoms form compounds, it is their valence electrons that undergo changes. A Lewis dot symbol consists of the element’s symbol surround by dots representing its valence electrons. Each dot represents a valence electron. • • B • Boron 1 s 22 p 1 3 valence electrons • B • • Lewis dot symbol for boron • B • • • • B • other reasonable Lewis dot symbols for boron

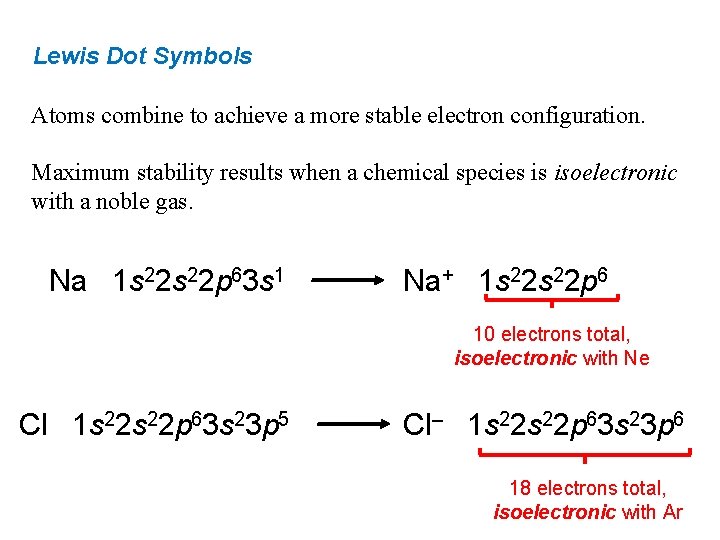
Lewis Dot Symbols Atoms combine to achieve a more stable electron configuration. Maximum stability results when a chemical species is isoelectronic with a noble gas. Na 1 s 22 p 63 s 1 Na+ 1 s 22 p 6 10 electrons total, isoelectronic with Ne Cl 1 s 22 p 63 s 23 p 5 Cl‒ 1 s 22 p 63 s 23 p 6 18 electrons total, isoelectronic with Ar

Lewis Dot Symbols Lewis dot symbols of the main group elements.

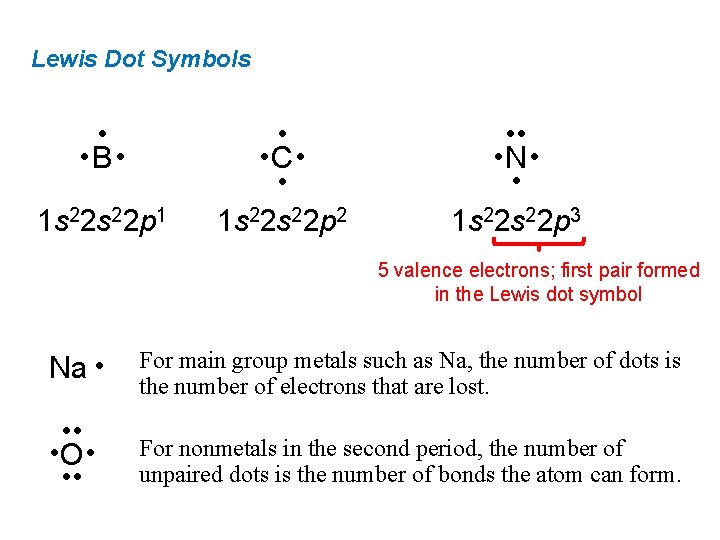
Lewis Dot Symbols • • B • 1 s 22 p 1 • • C • • 1 s 22 p 2 • • • N • • 1 s 22 p 3 5 valence electrons; first pair formed in the Lewis dot symbol Na • For main group metals such as Na, the number of dots is the number of electrons that are lost. • • • O • • • For nonmetals in the second period, the number of unpaired dots is the number of bonds the atom can form.

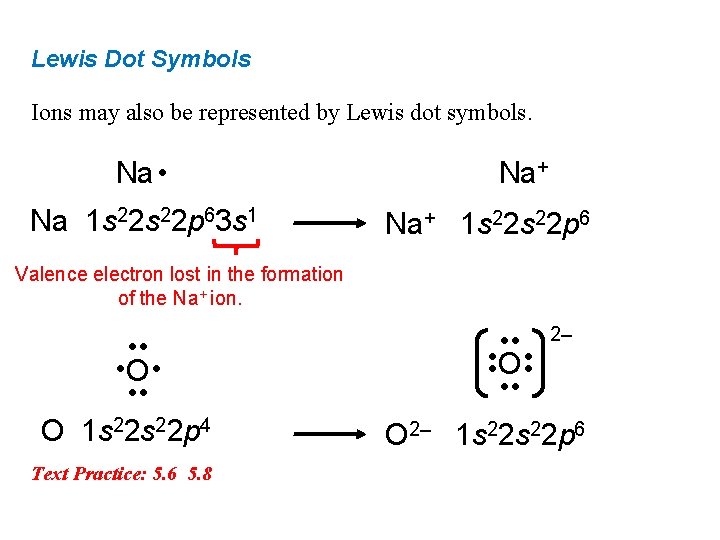
Lewis Dot Symbols Ions may also be represented by Lewis dot symbols. Na • Na 1 s 22 p 63 s 1 Na+ 1 s 22 p 6 Valence electron lost in the formation of the Na+ ion. • • • O • • • O 1 s 22 p 4 Text Practice: 5. 6 5. 8 • • O • • 2‒ O 2‒ 1 s 22 p 6

Activity: For each symbol, draw a Lewis symbol and predict whether it is the most commonly found form of the element in nature. H O 3 Ca 3+ Fe 3+ KAr

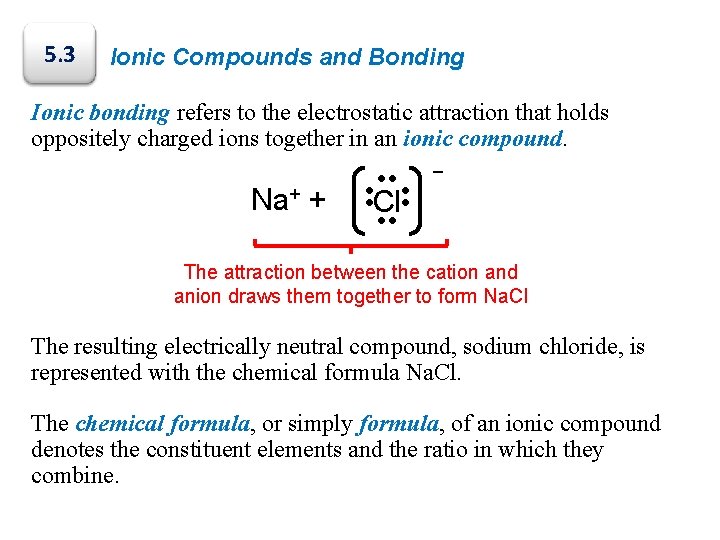
5. 3 Ionic Compounds and Bonding Ionic bonding refers to the electrostatic attraction that holds oppositely charged ions together in an ionic compound. Na+ + • • • • • Cl • • • − The attraction between the cation and anion draws them together to form Na. Cl The resulting electrically neutral compound, sodium chloride, is represented with the chemical formula Na. Cl. The chemical formula, or simply formula, of an ionic compound denotes the constituent elements and the ratio in which they combine.

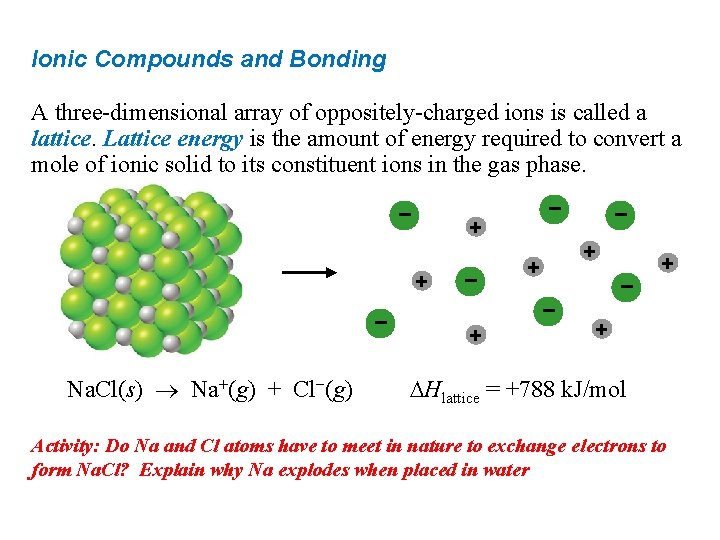
Ionic Compounds and Bonding A three-dimensional array of oppositely-charged ions is called a lattice. Lattice energy is the amount of energy required to convert a mole of ionic solid to its constituent ions in the gas phase. − + + − Na. Cl(s) Na+(g) + Cl−(g) − − + + Hlattice = +788 k. J/mol Activity: Do Na and Cl atoms have to meet in nature to exchange electrons to form Na. Cl? Explain why Na explodes when placed in water

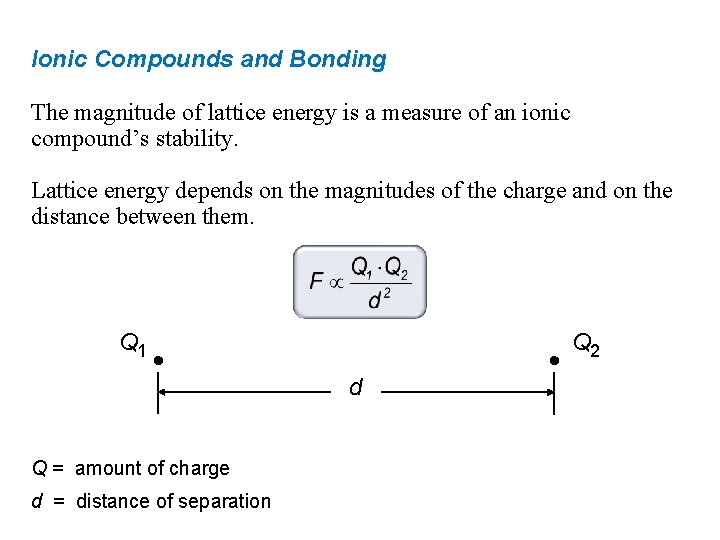
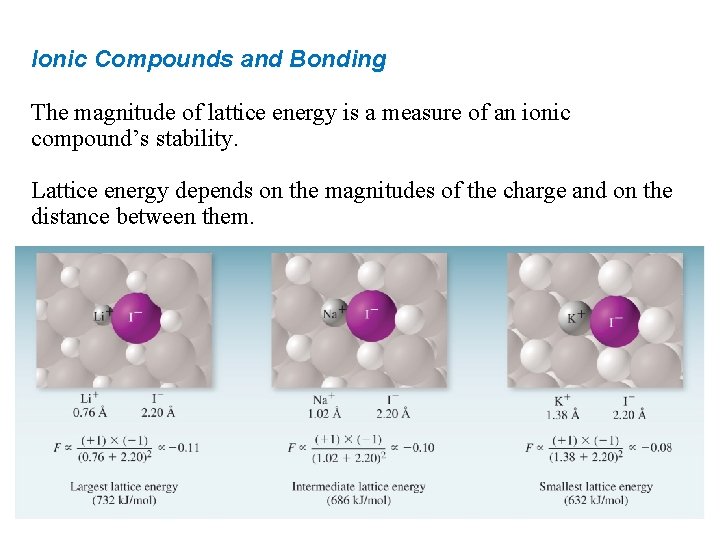
Ionic Compounds and Bonding The magnitude of lattice energy is a measure of an ionic compound’s stability. Lattice energy depends on the magnitudes of the charge and on the distance between them. Q 1 d Q = amount of charge d = distance of separation Q 2

Ionic Compounds and Bonding The magnitude of lattice energy is a measure of an ionic compound’s stability. Lattice energy depends on the magnitudes of the charge and on the distance between them.

Ionic Compounds and Bonding

Naming Ions and Ionic Compounds

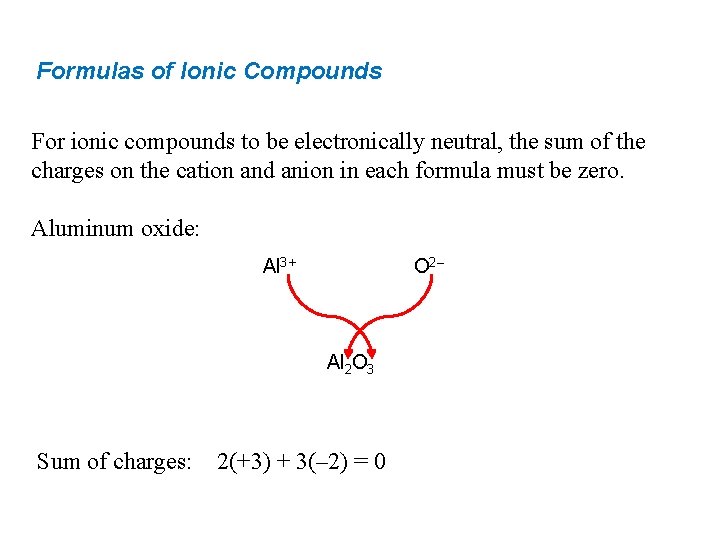
Formulas of Ionic Compounds For ionic compounds to be electronically neutral, the sum of the charges on the cation and anion in each formula must be zero. Aluminum oxide: Al 3+ O 2– Al 2 O 3 Sum of charges: 2(+3) + 3(– 2) = 0

Worked Example 5. 4 Deduce the formulas of the following ionic compounds: (a) mercury(II) chloride, (b) lead(II) bromide, and (c) potassium nitride. Strategy Identify the ions in each compound, and determine their ratios of combination using the charges on the cation and anion in each. Text Practice: 5. 12 5. 24

Study Guide for Sections 4. 5 -4. 6, 5. 1 -5. 4 DAY 10, Terms to know: Sections 4. 5 -4. 6, 5. 1 -5. 4: ionic radii, isoelectronic, compound, Lewis dot symbol, ionic bonding, ionic compound, chemical formula, lattice energy DAY 10, Specific outcomes and skills that may be tested on exam 2: Sections 4. 5 -4. 6, 5. 1 -5. 4 • Be able to give a complete or abbreviated electron configuration for an ion in either its ground state or a possible excited state • Be able to give a complete or abbreviated orbital diagram for an ion either its ground state or a possible excited state • Be able to rank the radii of isoelectronic particles and explain your answer • Be able to rank relative radii, electron affinity, and ionization energy ionic radii for a series of particles including atoms and ions, and explain WHY they are ranked based on attractions and repulsions within the atom • Be able to give the Lewis dot symbol for any main group element or ion • Be able to predict the chemical formula for an ionic compound resulting from any two given ions

Extra Practice Problems for Sections 4. 5 -4. 6, 5. 1 -5. 4 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 4. 69 4. 71 4. 73 4. 75 4. 77 4. 79 4. 81 4. 83 4. 85 4. 89 4. 91 4. 93 4. 99 4. 111 4. 121 5. 7 5. 9 5. 23 (just formulas) 5. 123

Prep for Day 11 Must Watch videos: http: //www. youtube. com/watch? v=PKA 4 CZwb. ZWU (Tyler De. Witt: ionic vs. molecular) https: //www. youtube. com/watch? v=QXT 4 OVM 4 v. XI (Crash Course Chemistry: bonds) http: //www. youtube. com/watch? v=Kj 3 o 0 Xvh. Vq. Q (electronegativity) https: //www. youtube. com/watch? v=a 8 LF 7 JEb 0 IA (Lewis structures, crash course chemistry) https: //www. youtube. com/watch? v=1 Zlnzy. Hahvo (Lewis structures) Other helpful videos: http: //www. learnerstv. com/video/Free-video-Lecture-29328 -Chemistry. htm (covalent versus ionic) http: //ocw. mit. edu/courses/chemistry/5 -111 -principles-of-chemical-science-fall-2008/video-lectures/ (MIT lecture 10: covalent bonds) http: //www. youtube. com/watch? v=b 2 p-Bt. At 1 T 8 (Lewis Structures) http: //echem 1 a. cchem. berkeley. edu/modules/module-4/ (UC-Berkeley lesson 10) https: //www. youtube. com/watch? v=6 Gj. YGdk 32 U&list=PLq. OZ 6 FD_RQ 7 k. Tj. N 4 O 2 MNzf 5 Yfei. Ix 7 SGI (UC-Irvine start at 15 min mark) http: //ocw. mit. edu/courses/chemistry/5 -111 -principles-of-chemical-science-fall-2008/video-lectures/ (MIT lecture 11) Read sections 5. 5, 6. 1, 6. 2 (pages 193 -195), 6. 3
 Electron configuration of ions
Electron configuration of ions Ccechs
Ccechs Orbital diagram for ca
Orbital diagram for ca Stable electron configurations are likely to contain
Stable electron configurations are likely to contain 1s 22 s22 p63 s2
1s 22 s22 p63 s2 Stable electron configurations
Stable electron configurations Calcium ion formula
Calcium ion formula Positive ions and negative ions table
Positive ions and negative ions table Electron configuration of transition metals
Electron configuration of transition metals Ceedar innovation configurations
Ceedar innovation configurations Electrons configurations
Electrons configurations Flexible and rigid pavement
Flexible and rigid pavement S electrons
S electrons Icq transistor
Icq transistor Uses of bjt transistor
Uses of bjt transistor Electrons configurations
Electrons configurations Electron configuration rules
Electron configuration rules When adding a subscript to a polyatomic ion you should
When adding a subscript to a polyatomic ion you should Which neutral atom is isoelectronic with o+
Which neutral atom is isoelectronic with o+ How to read electron configuration
How to read electron configuration Teknik mapping pada memori
Teknik mapping pada memori Heavy ions
Heavy ions What are ions
What are ions Ions
Ions Sulfate anion test
Sulfate anion test Polyatomic ions
Polyatomic ions Atoms molecules and ions
Atoms molecules and ions As the distance between ions in an ionic bond is shortened
As the distance between ions in an ionic bond is shortened