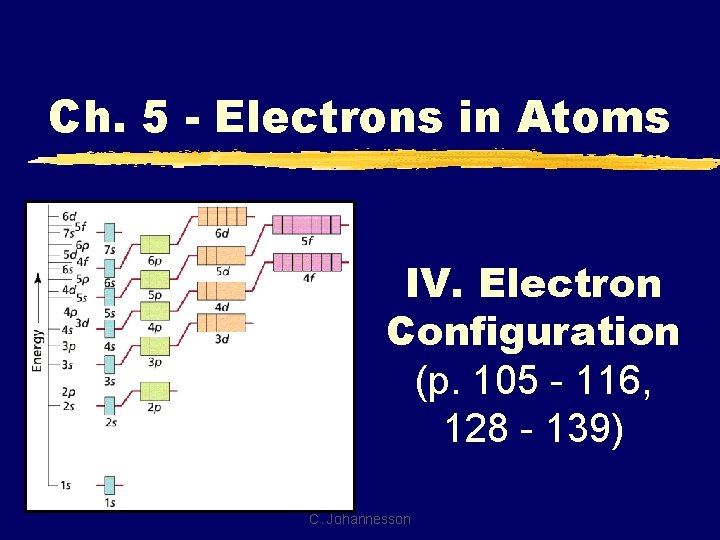
Ch 5 Electrons in Atoms IV Electron Configuration

![C. Periodic Patterns z. Example - Germanium [Ar] 2 4 s 10 3 d C. Periodic Patterns z. Example - Germanium [Ar] 2 4 s 10 3 d](https://slidetodoc.com/presentation_image/32d4053b013ef95674bcf4cf2ec3f795/image-10.jpg)
![D. Stability z. Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 D. Stability z. Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image/32d4053b013ef95674bcf4cf2ec3f795/image-12.jpg)
![D. Stability z. Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 D. Stability z. Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image/32d4053b013ef95674bcf4cf2ec3f795/image-13.jpg)

- Slides: 31

Ch. 5 - Electrons in Atoms IV. Electron Configuration (p. 105 - 116, 128 - 139) C. Johannesson

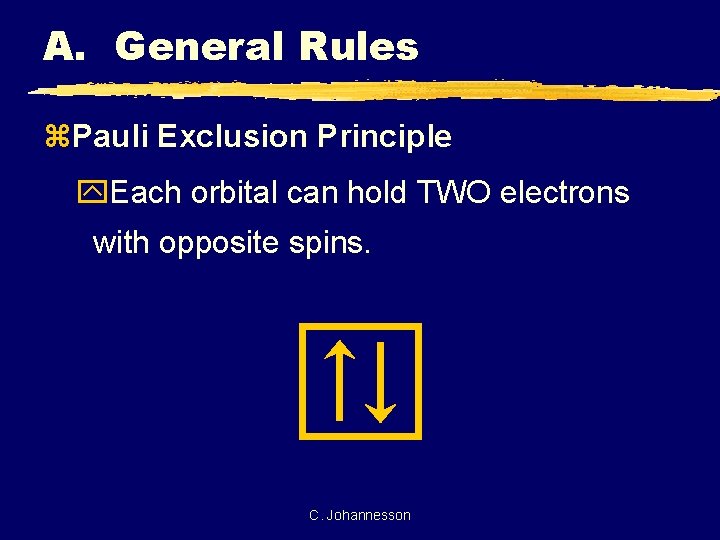
A. General Rules z. Pauli Exclusion Principle y. Each orbital can hold TWO electrons with opposite spins. C. Johannesson

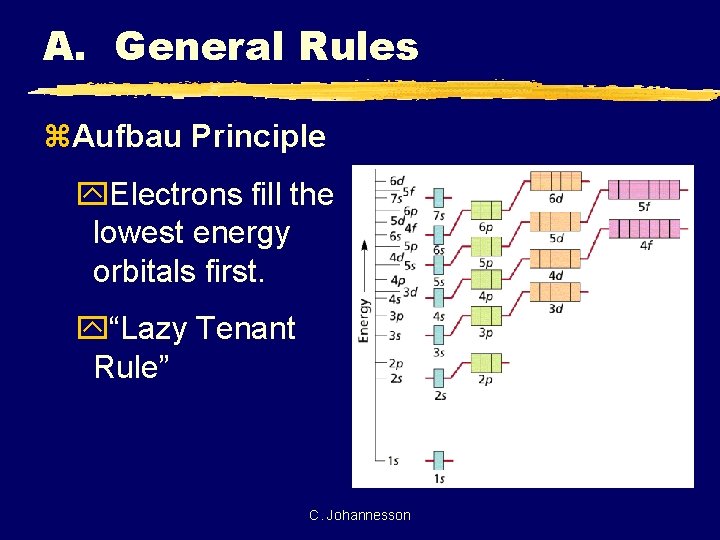
A. General Rules z. Aufbau Principle y. Electrons fill the lowest energy orbitals first. y“Lazy Tenant Rule” C. Johannesson

A. General Rules z. Hund’s Rule y. Within a sublevel, place one e- per orbital before pairing them. y“Empty Bus Seat Rule” WRONG C. Johannesson RIGHT

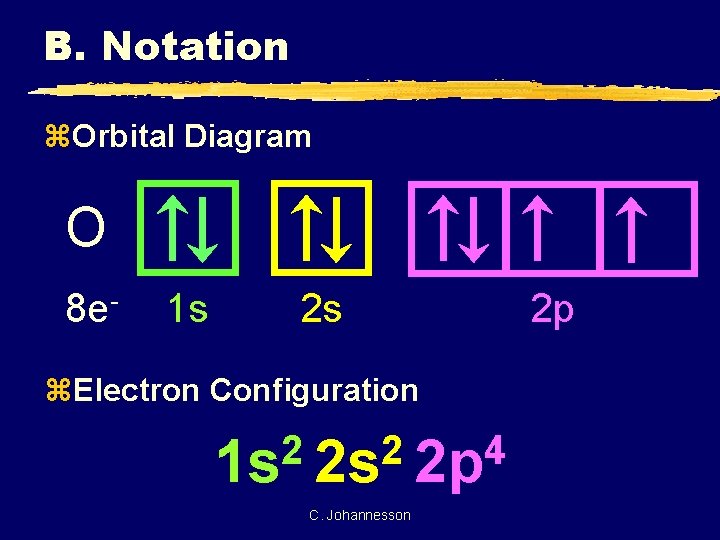
B. Notation z. Orbital Diagram O 8 e- 1 s 2 s z. Electron Configuration 2 2 4 1 s 2 s 2 p C. Johannesson 2 p

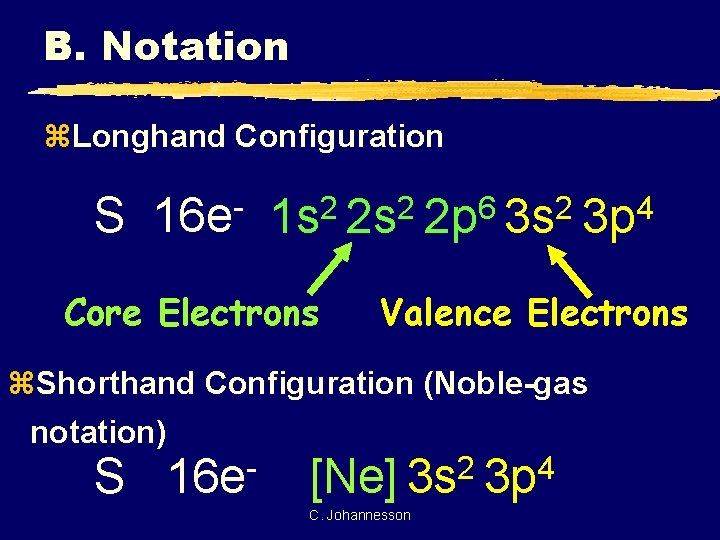
B. Notation z. Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons 4 3 p Valence Electrons z. Shorthand Configuration (Noble-gas notation) S 16 e 2 4 [Ne] 3 s 3 p C. Johannesson

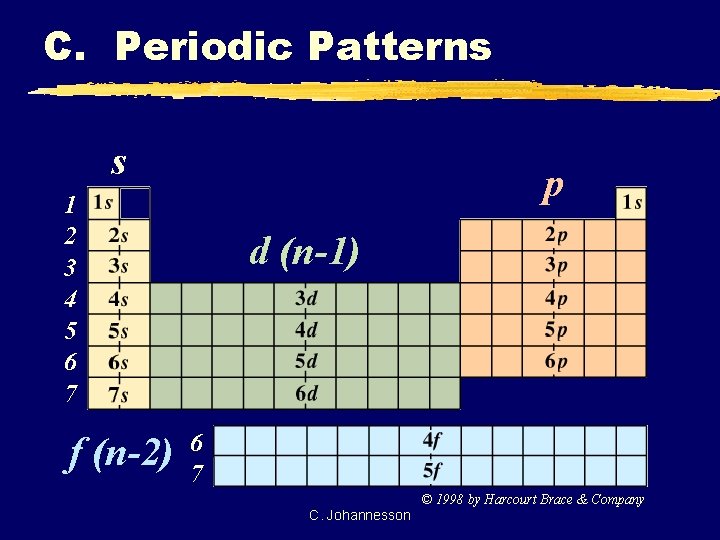
C. Periodic Patterns s p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company C. Johannesson

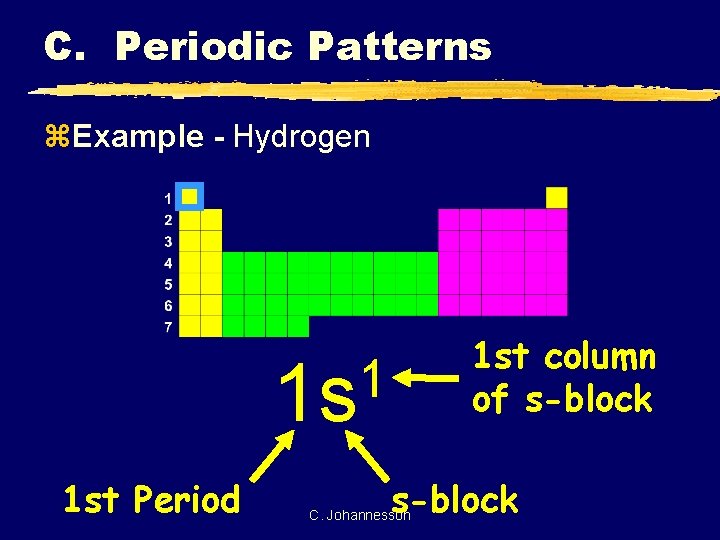
C. Periodic Patterns z. Example - Hydrogen 1 st column of s-block 1 1 s 1 st Period s-block C. Johannesson

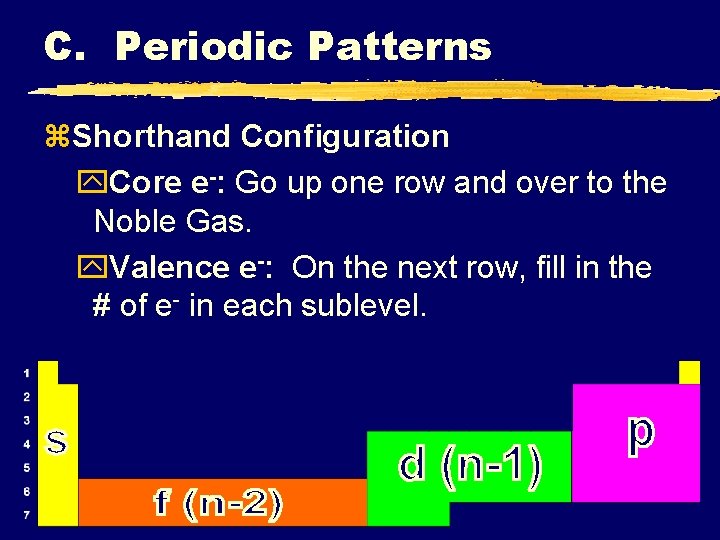
C. Periodic Patterns z. Shorthand Configuration y. Core e-: Go up one row and over to the Noble Gas. y. Valence e-: On the next row, fill in the # of e- in each sublevel. C. Johannesson
![C Periodic Patterns z Example Germanium Ar 2 4 s 10 3 d C. Periodic Patterns z. Example - Germanium [Ar] 2 4 s 10 3 d](https://slidetodoc.com/presentation_image/32d4053b013ef95674bcf4cf2ec3f795/image-10.jpg)
C. Periodic Patterns z. Example - Germanium [Ar] 2 4 s 10 3 d C. Johannesson 2 4 p

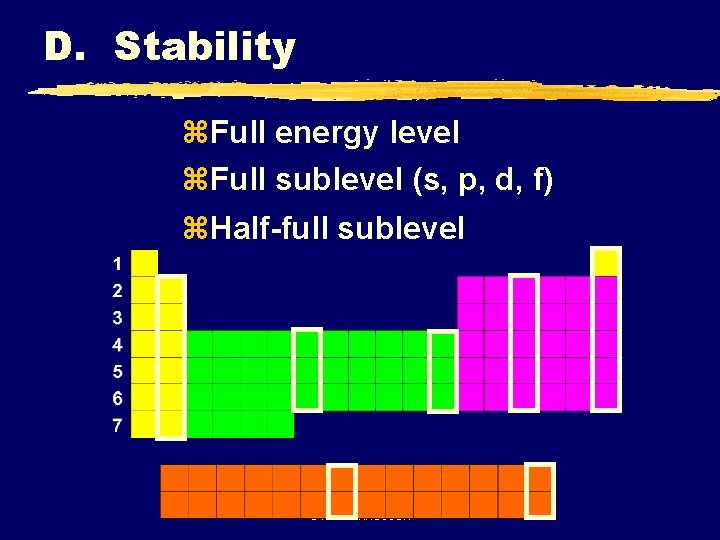
D. Stability z. Full energy level z. Full sublevel (s, p, d, f) z. Half-full sublevel C. Johannesson
![D Stability z Electron Configuration Exceptions y Copper EXPECT Ar 4 s 2 3 D. Stability z. Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image/32d4053b013ef95674bcf4cf2ec3f795/image-12.jpg)
D. Stability z. Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 y. Copper gains stability with a full d-sublevel. C. Johannesson
![D Stability z Electron Configuration Exceptions y Chromium EXPECT Ar 4 s 2 3 D. Stability z. Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image/32d4053b013ef95674bcf4cf2ec3f795/image-13.jpg)
D. Stability z. Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d 4 ACTUALLY: [Ar] 4 s 1 3 d 5 y. Chromium gains stability with a half-full d-sublevel. C. Johannesson

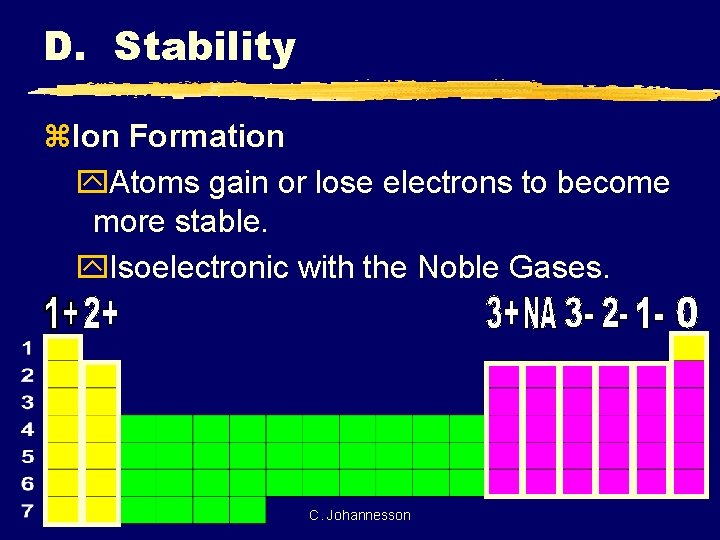
D. Stability z. Ion Formation y. Atoms gain or lose electrons to become more stable. y. Isoelectronic with the Noble Gases. C. Johannesson

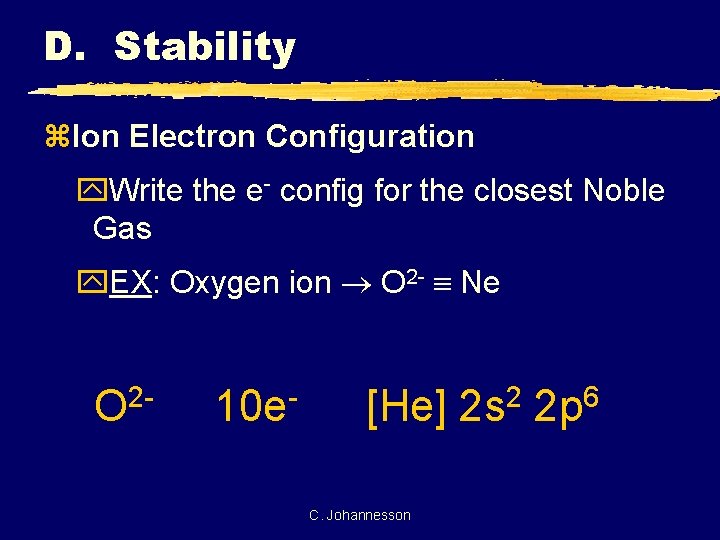
D. Stability z. Ion Electron Configuration y. Write the e- config for the closest Noble Gas y. EX: Oxygen ion O 2 - Ne 2 O 10 e [He] C. Johannesson 2 2 s 6 2 p

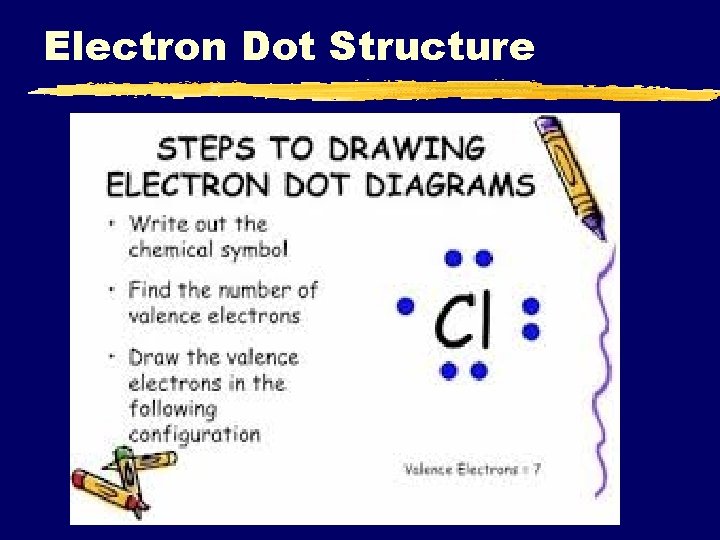
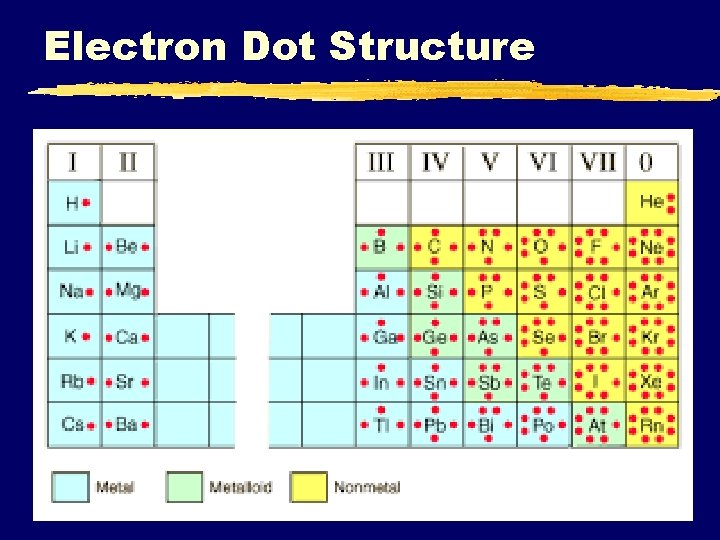
Electron Dot Structure C. Johannesson

Electron Dot Structure C. Johannesson

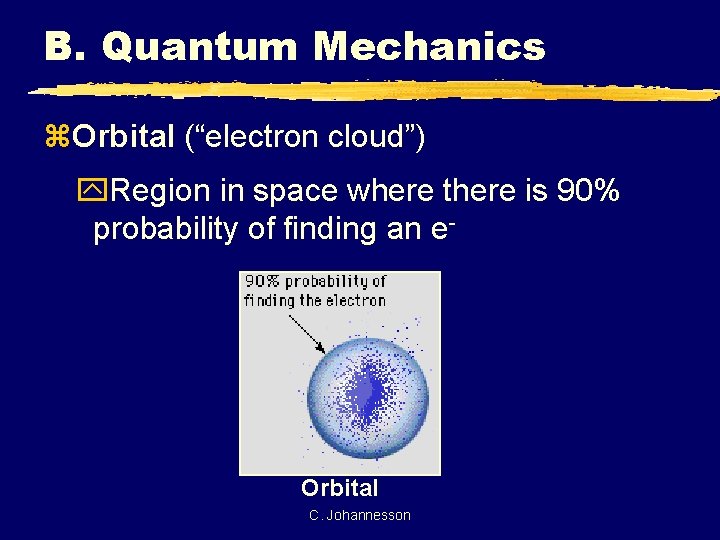
B. Quantum Mechanics z. Orbital (“electron cloud”) y. Region in space where there is 90% probability of finding an e- Orbital C. Johannesson

C. Quantum Numbers z. Four Quantum Numbers: y. Specify the “address” of each electron in an atom UPPER LEVEL C. Johannesson

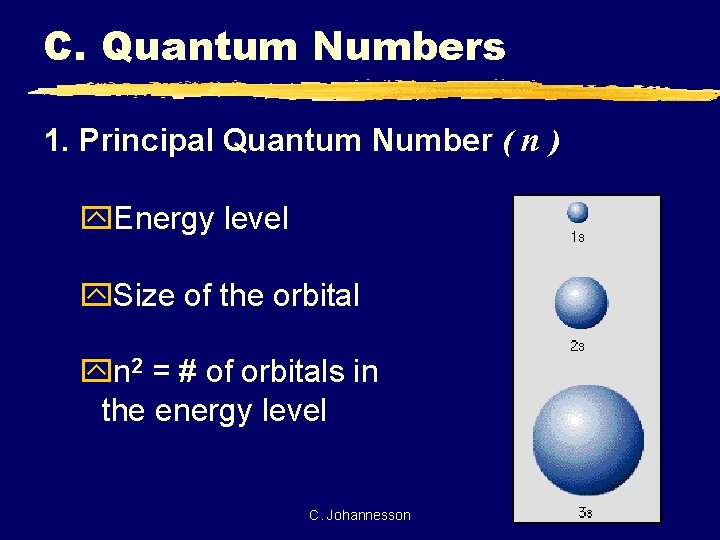
C. Quantum Numbers 1. Principal Quantum Number ( n ) y. Energy level y. Size of the orbital yn 2 = # of orbitals in the energy level C. Johannesson

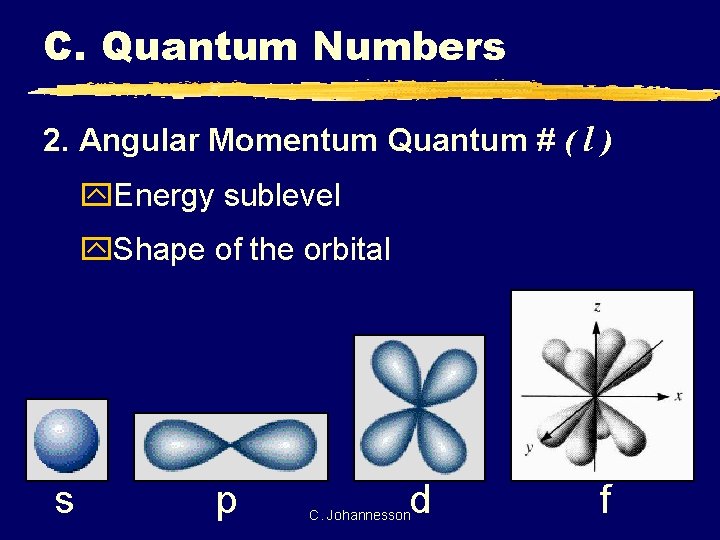
C. Quantum Numbers 2. Angular Momentum Quantum # ( l ) y. Energy sublevel y. Shape of the orbital s p d C. Johannesson f

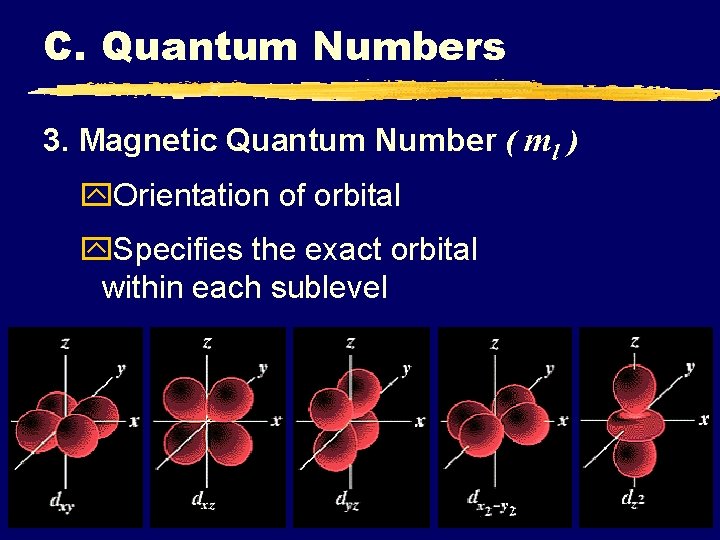
C. Quantum Numbers 3. Magnetic Quantum Number ( ml ) y. Orientation of orbital y. Specifies the exact orbital within each sublevel C. Johannesson

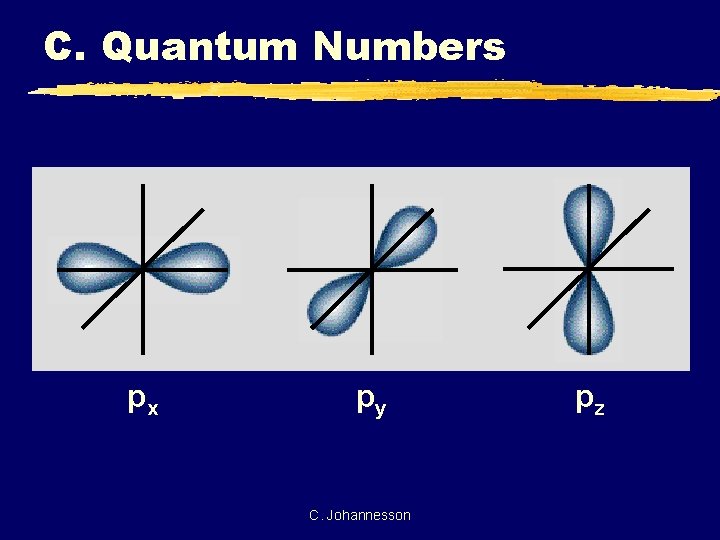
C. Quantum Numbers px py C. Johannesson pz

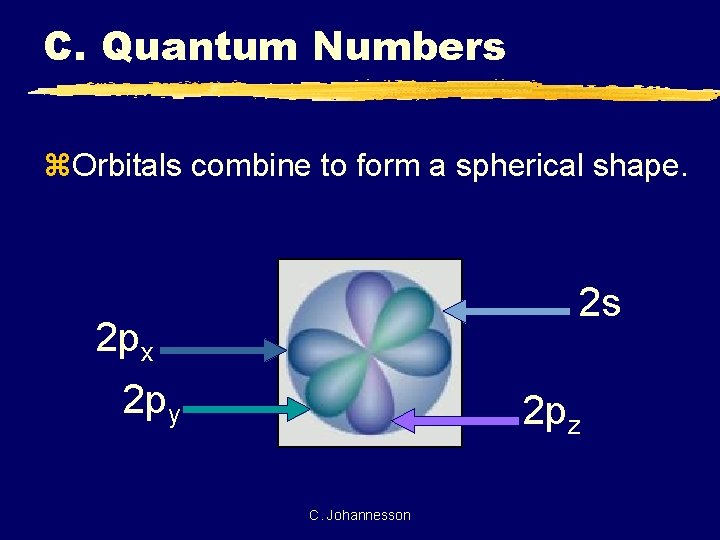
C. Quantum Numbers z. Orbitals combine to form a spherical shape. 2 s 2 px 2 py 2 pz C. Johannesson

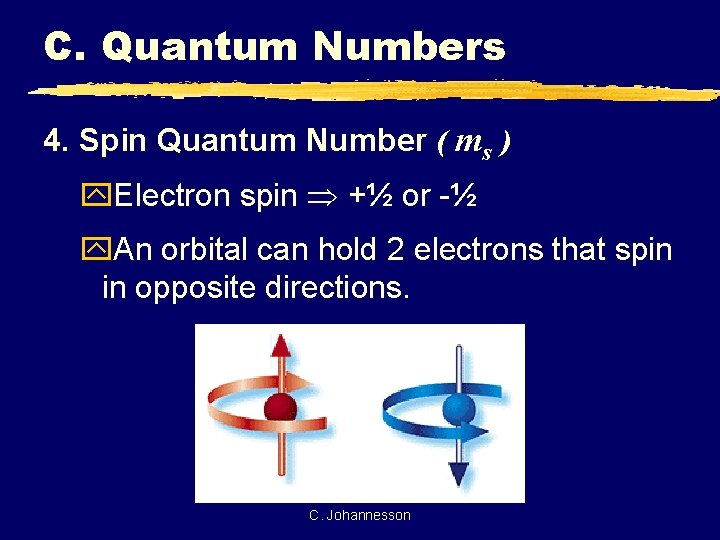
C. Quantum Numbers 4. Spin Quantum Number ( ms ) y. Electron spin +½ or -½ y. An orbital can hold 2 electrons that spin in opposite directions. C. Johannesson

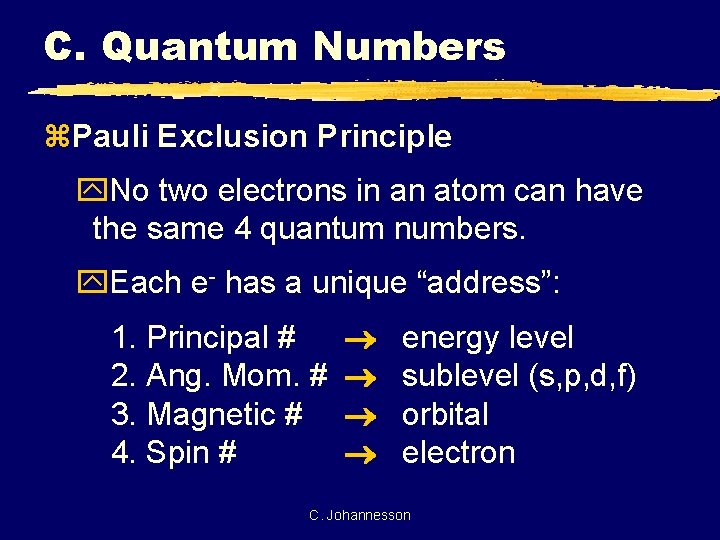
C. Quantum Numbers z. Pauli Exclusion Principle y. No two electrons in an atom can have the same 4 quantum numbers. y. Each e- has a unique “address”: 1. Principal # 2. Ang. Mom. # 3. Magnetic # 4. Spin # energy level sublevel (s, p, d, f) orbital electron C. Johannesson

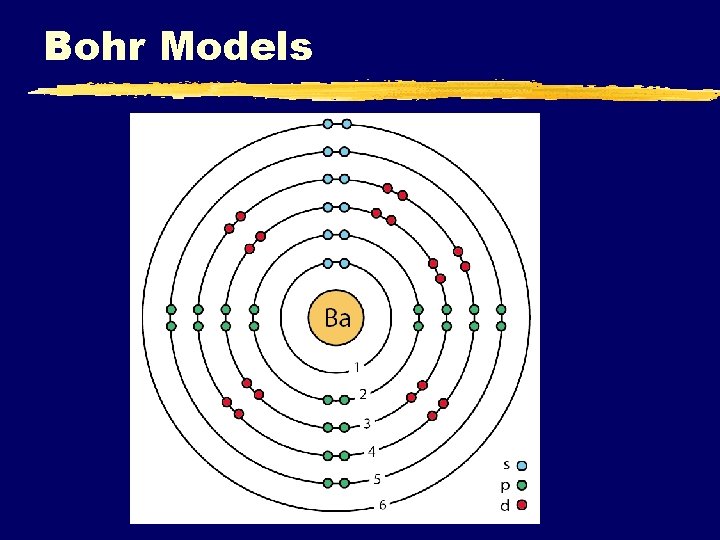
Bohr Models C. Johannesson

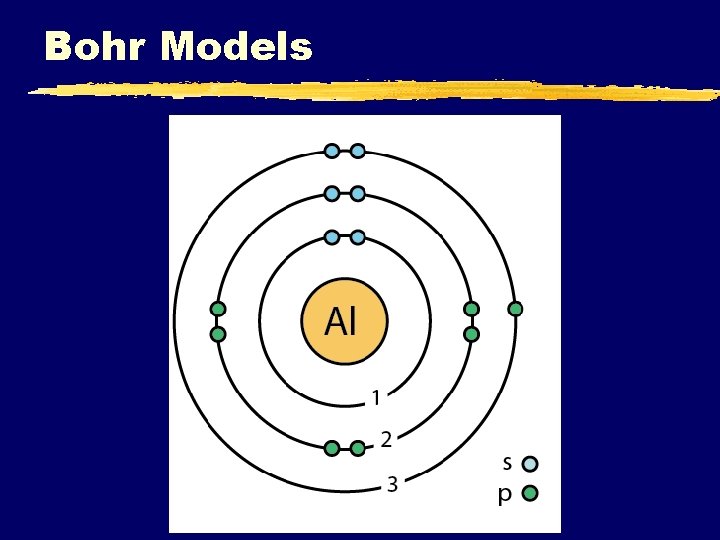
Bohr Models C. Johannesson

Bohr Models C. Johannesson

Classwork z#24 on page 160 z#29 on page 162 (don’t draw the orbital diagrams) z#85 on 167 z#87 on page 168 C. Johannesson

Classwork z. ELECTRON DOT CONFIGURATION z. Page 162 do #33 z. Page 168 do #90, 92, and 93 z. Page 979 do #5, 6, 7, 8, 9 (b, c, d) for Chapter 5 C. Johannesson