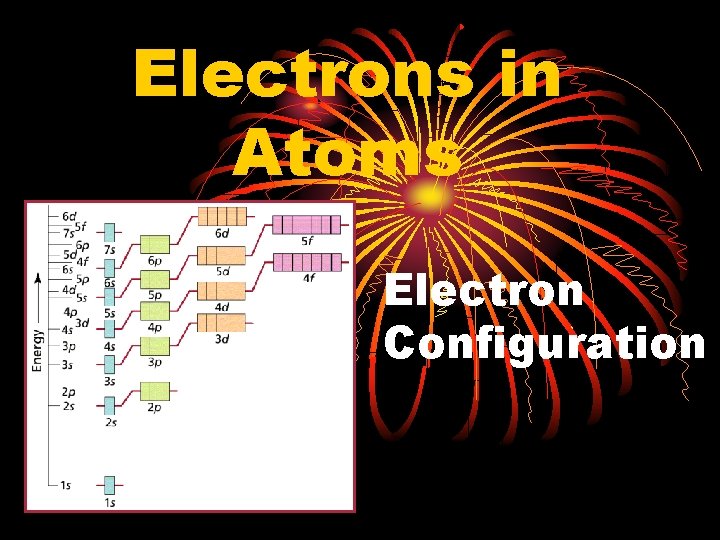
Electrons in Atoms Electron Configuration General Rules Pauli


![Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d 2 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d 2](https://slidetodoc.com/presentation_image_h2/18d4f3be03dfd31b6d55216f20ee2c8d/image-11.jpg)

![Stability • Electron Configuration Exceptions • Copper EXPECT: [Ar] 4 s 2 3 d Stability • Electron Configuration Exceptions • Copper EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/18d4f3be03dfd31b6d55216f20ee2c8d/image-15.jpg)
![Stability • Electron Configuration Exceptions • Chromium EXPECT: ACTUALLY: [Ar] 4 s 2 3 Stability • Electron Configuration Exceptions • Chromium EXPECT: ACTUALLY: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image_h2/18d4f3be03dfd31b6d55216f20ee2c8d/image-16.jpg)

- Slides: 18

Electrons in Atoms Electron Configuration

General Rules • Pauli Exclusion Principle • Each orbital can hold TWO electrons with opposite spins.

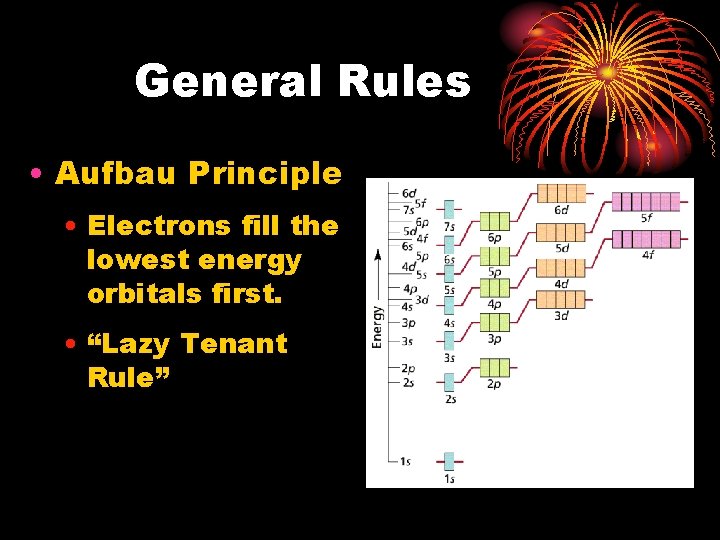
General Rules • Aufbau Principle • Electrons fill the lowest energy orbitals first. • “Lazy Tenant Rule”

General Rules • Hund’s Rule • Within a sublevel, place one eper orbital before pairing them. • “Empty Bus Seat Rule” WRONG RIGHT

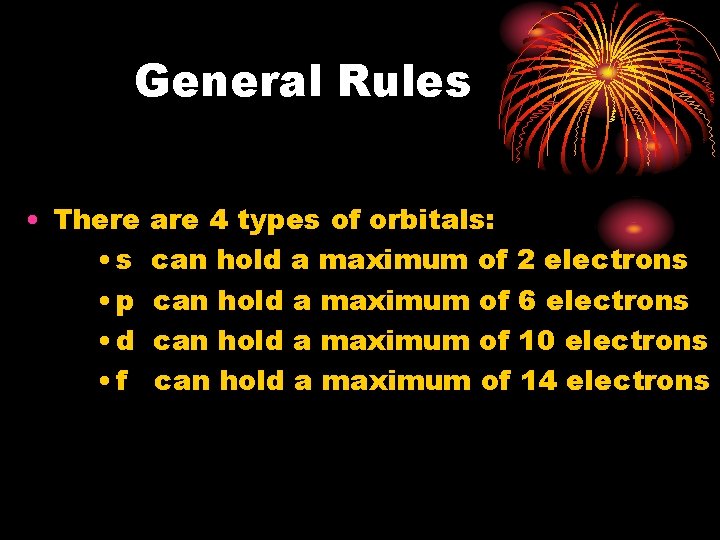
General Rules • There • s • p • d • f are 4 types of orbitals: can hold a maximum of 2 electrons can hold a maximum of 6 electrons can hold a maximum of 10 electrons can hold a maximum of 14 electrons

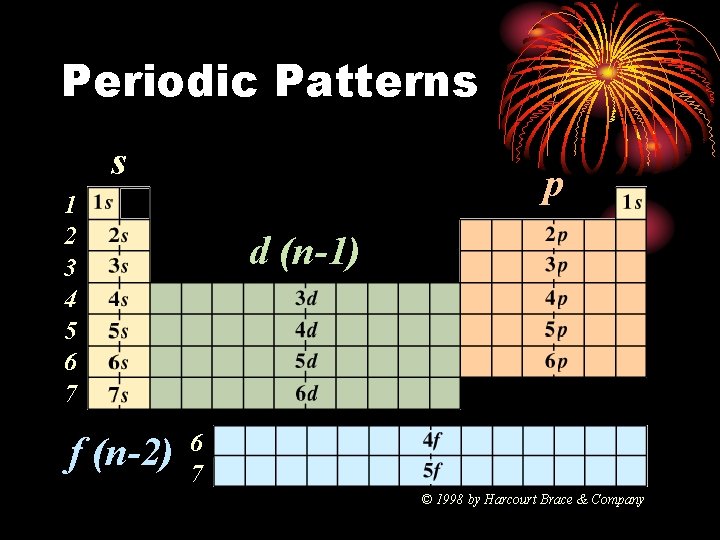
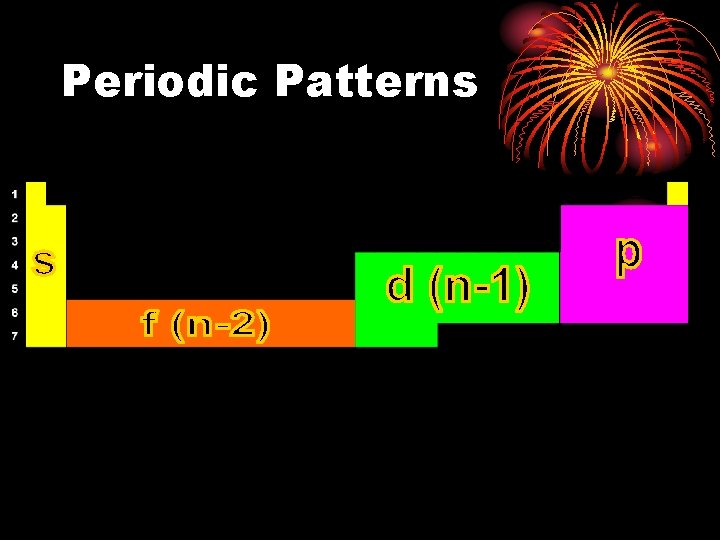
Periodic Patterns s p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company

Periodic Patterns

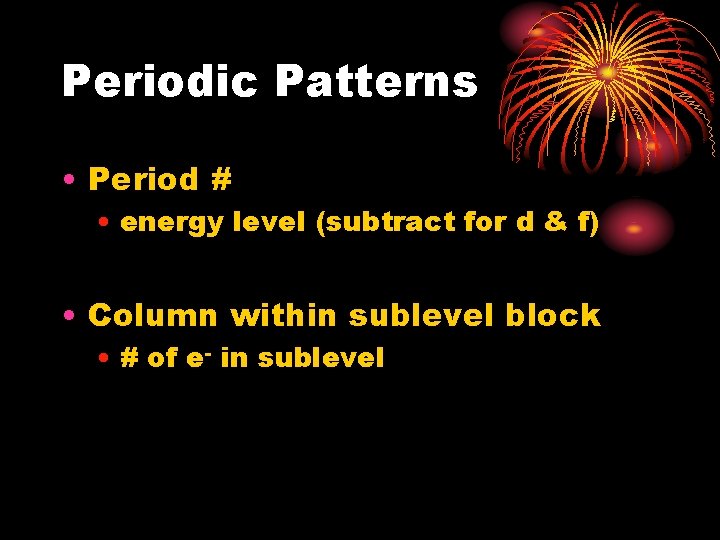
Periodic Patterns • Period # • energy level (subtract for d & f) • Column within sublevel block • # of e- in sublevel

Periodic Patterns • Example - Hydrogen 1 1 s 1 st Period 1 st column of s-block

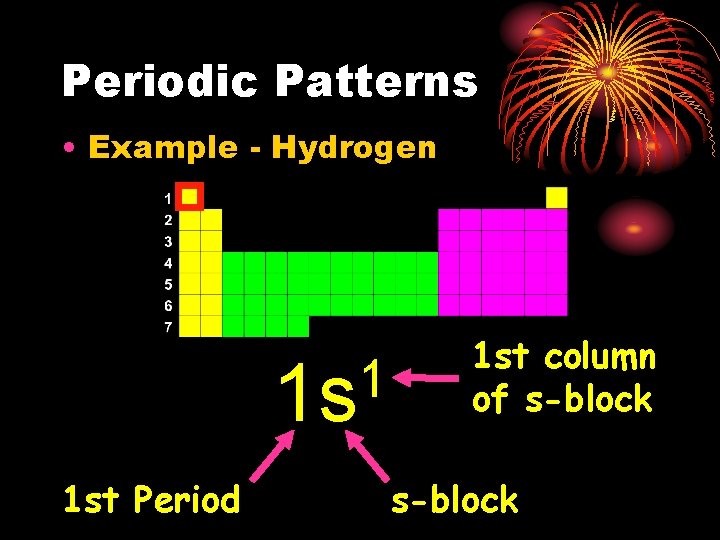
Notation • Orbital Diagram O 8 e- 1 s 2 s • Electron Configuration 2 2 4 1 s 2 s 2 p 2 p
![Periodic Patterns Example Germanium Ar 2 4 s 10 3 d 2 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d 2](https://slidetodoc.com/presentation_image_h2/18d4f3be03dfd31b6d55216f20ee2c8d/image-11.jpg)
Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d 2 4 p

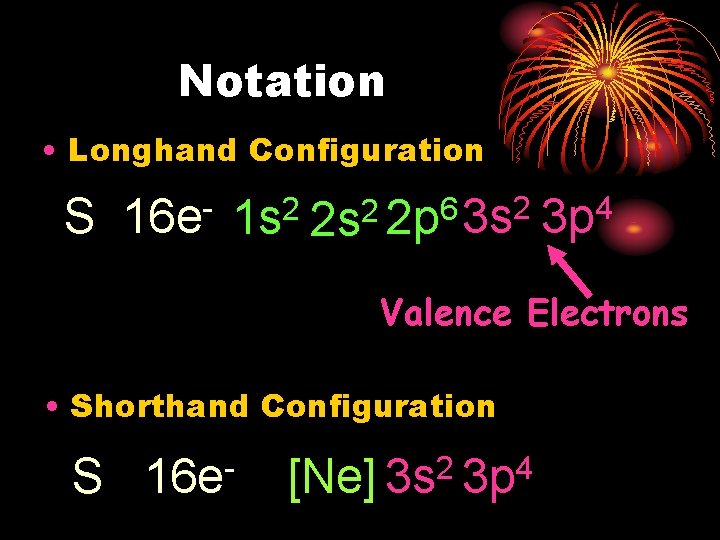
Notation • Longhand Configuration S 16 e 2 1 s 2 6 2 2 s 2 p 3 s 4 3 p Valence Electrons • Shorthand Configuration S 16 e 2 4 [Ne] 3 s 3 p

Practice Time • Give the longhand shorthand configurations for the following: • Al • Co • As • Sr • Sn • Pb 1 s 22 p 63 s 23 p 1 or [Ne] 3 s 23 p 1 1 s 22 p 63 s 23 p 64 s 23 d 7 or [Ar] 4 s 23 d 7 1 s 22 p 63 s 23 p 64 s 23 d 104 p 3 or [Ar] 4 s 23 d 104 p 3 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 2 or [Kr] 5 s 2 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 2 or [Kr] 5 s 24 d 105 p 2 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 145 d 106 p 2 or [Xe] 6 s 24 f 145 d 106 p 2

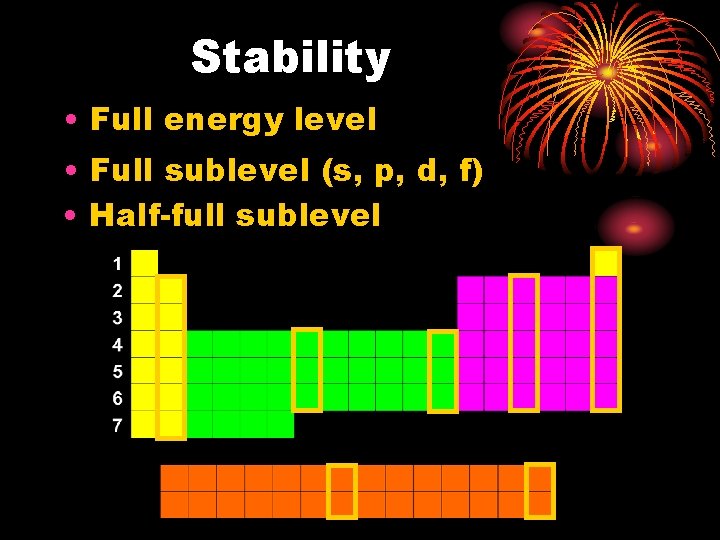
Stability • Full energy level • Full sublevel (s, p, d, f) • Half-full sublevel
![Stability Electron Configuration Exceptions Copper EXPECT Ar 4 s 2 3 d Stability • Electron Configuration Exceptions • Copper EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/18d4f3be03dfd31b6d55216f20ee2c8d/image-15.jpg)
Stability • Electron Configuration Exceptions • Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 • Copper gains stability with a full d-sublevel.
![Stability Electron Configuration Exceptions Chromium EXPECT ACTUALLY Ar 4 s 2 3 Stability • Electron Configuration Exceptions • Chromium EXPECT: ACTUALLY: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image_h2/18d4f3be03dfd31b6d55216f20ee2c8d/image-16.jpg)
Stability • Electron Configuration Exceptions • Chromium EXPECT: ACTUALLY: [Ar] 4 s 2 3 d 4 [Ar] 4 s 1 3 d 5 • Chromium gains stability with a halffull d-sublevel.

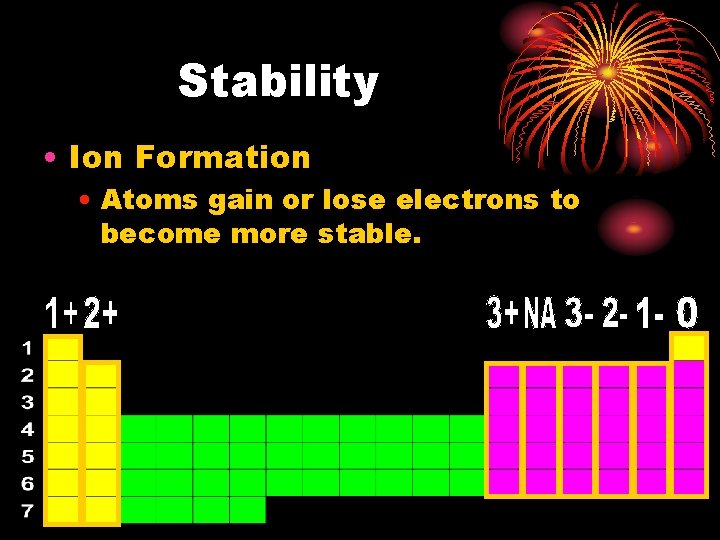
Stability • Ion Formation • Atoms gain or lose electrons to become more stable.

Stability • Ion Electron Configuration • Write the e- config for the closest Noble Gas • EX: Oxygen ion O 2 - Ne O 2 - 10 e- [He] 2 s 2 2 p 6