Electrons Configuration in Atoms Where are the electrons








- Slides: 22

Electrons Configuration in Atoms Where are the electrons located?

Essential Question o. Where are the electrons located in an atom?

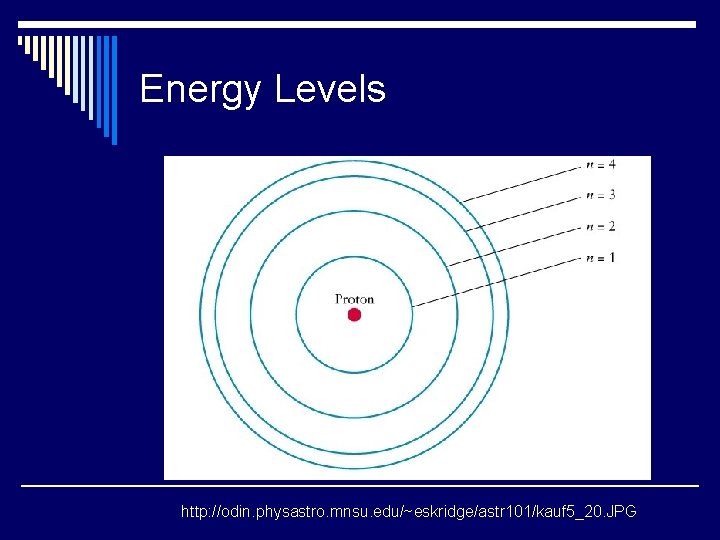
Energy Levels http: //odin. physastro. mnsu. edu/~eskridge/astr 101/kauf 5_20. JPG

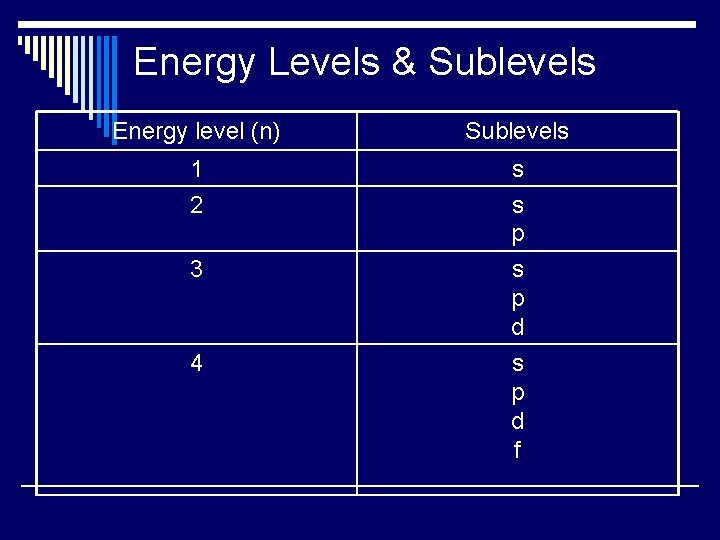
Energy Levels & Sublevels Energy level (n) Sublevels 1 2 s s p 3 s p d 4 s p d f

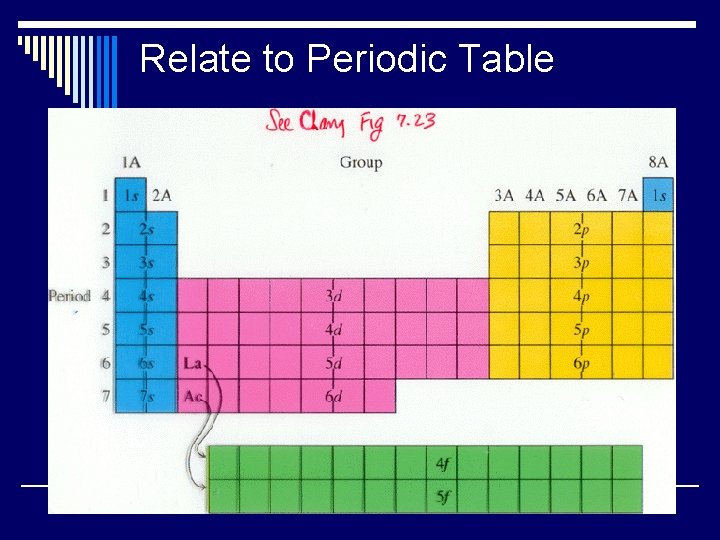
Relate to Periodic Table

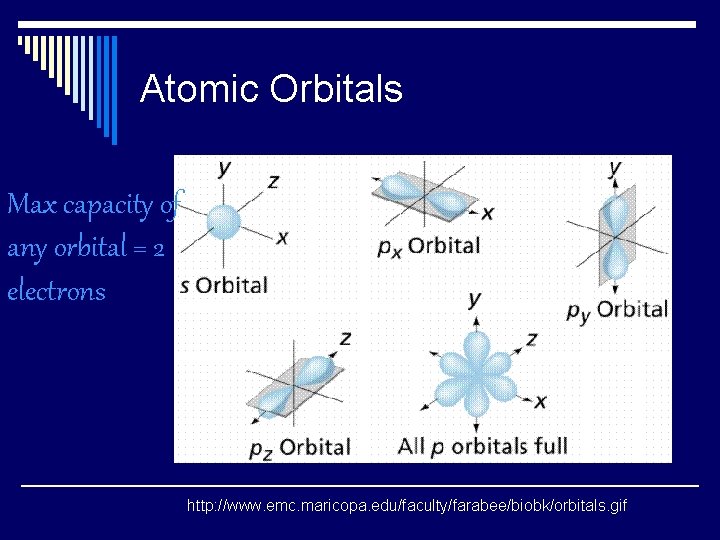
Atomic Orbitals Max capacity of any orbital = 2 electrons http: //www. emc. maricopa. edu/faculty/farabee/biobk/orbitals. gif

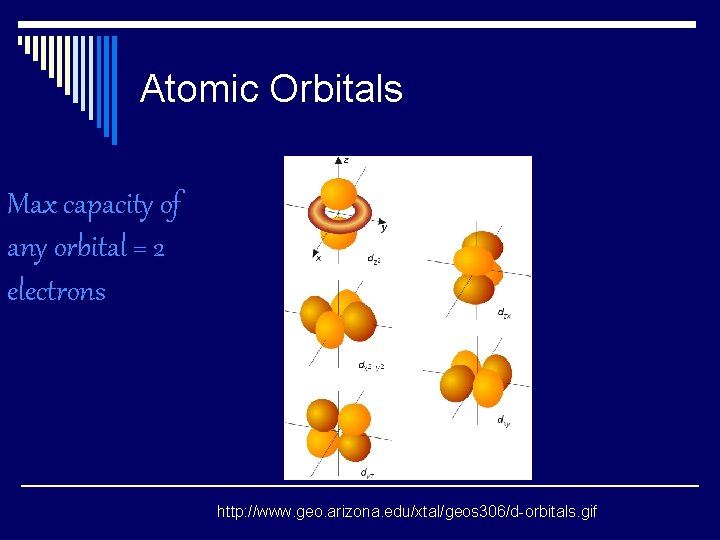
Atomic Orbitals Max capacity of any orbital = 2 electrons http: //www. geo. arizona. edu/xtal/geos 306/d-orbitals. gif

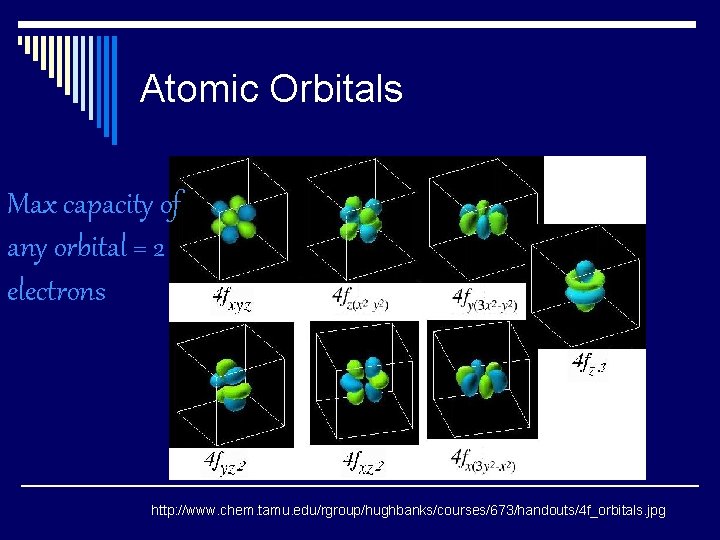
Atomic Orbitals Max capacity of any orbital = 2 electrons http: //www. chem. tamu. edu/rgroup/hughbanks/courses/673/handouts/4 f_orbitals. jpg

Energy Levels, Sublevels, Atomic Orbitals Energy level Sublevels Number of Atomic Orbitals Total Number of orbitals 1 1 2 s p 1 3 4 3 s p d 1 3 5 9 4 s p d f 1 3 5 7 16

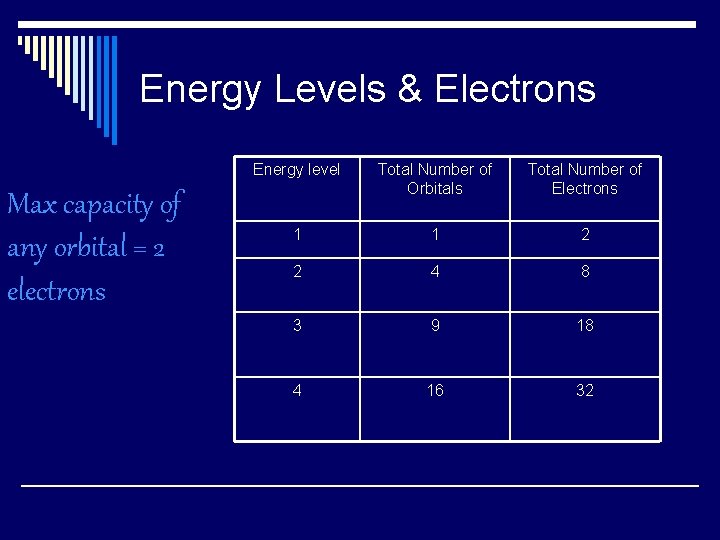
Energy Levels & Electrons Max capacity of any orbital = 2 electrons Energy level Total Number of Orbitals Total Number of Electrons 1 1 2 2 4 8 3 9 18 4 16 32

AUFBAU principle o Electrons are LAZY o Stated scientifically: n www. smugmug. com Electrons will occupy the lowest energy level available

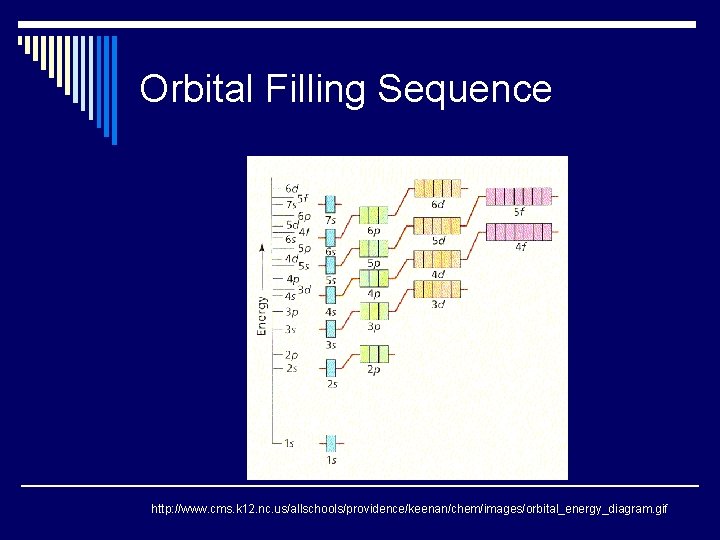
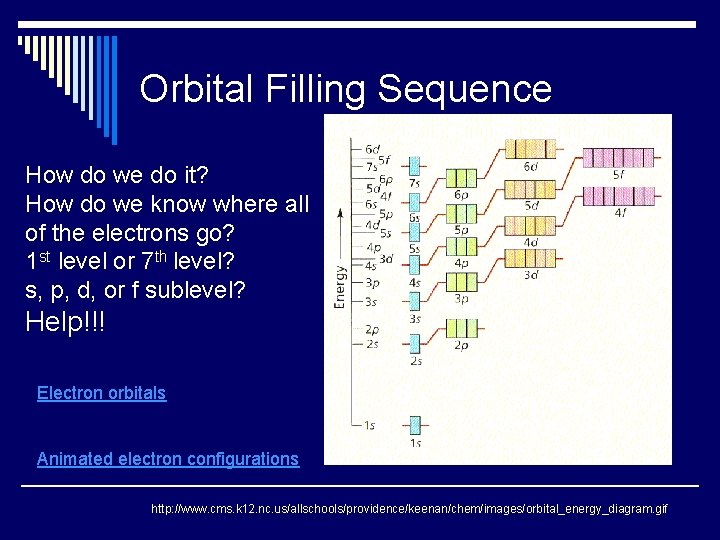
Orbital Filling Sequence http: //www. cms. k 12. nc. us/allschools/providence/keenan/chem/images/orbital_energy_diagram. gif

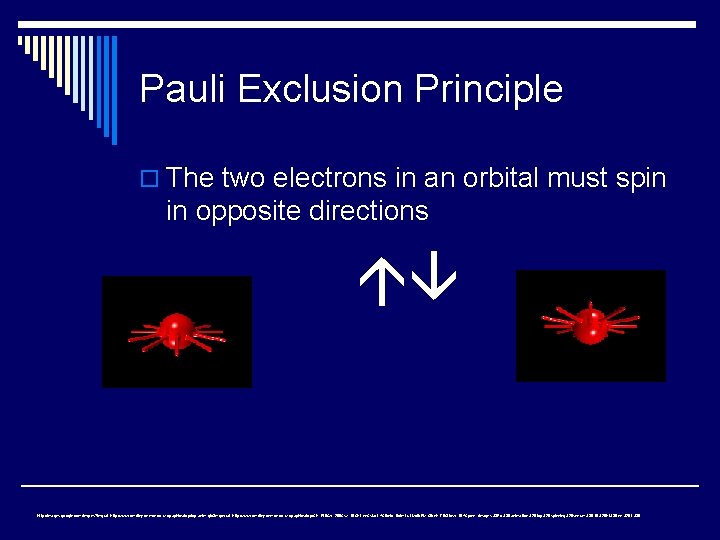
Pauli Exclusion Principle o The two electrons in an orbital must spin in opposite directions http: //images. google. com/imgres? imgurl=http: //www. dmriley. demon. co. uk/graphics/top-anim. gif&imgrefurl=http: //www. dmriley. demon. co. uk/graphics/top/&h=150&w=200&sz=18&hl=en&start=4&tbnid=9 xlnk. Lu. LMx. Ib. EM: &tbnh=78&tbnw=104&prev=/images%3 Fq%3 Danimation%2 Btop%2 Bspinning%26 svnum%3 D 10%26 hl%3 Den%26 lr%3 D

Weird! http: //www. 2 atoms. com/images/weird/illusions/055 b. gif

Hund’s Rule o Electrons won’t pair up unless they have to o Once there is one electron in every orbital…the pairing will begin! http: //www. tangischools. org/schools/hhs/Hammond%20 High%202001 -2002/Superbowl_files/Kids%20 in%20 bus. JPG

Putting It All Together o The … n n n aufbau principle, Pauli exclusion principle, And Hund’s rule o Can be used to describe the structure of the electron cloud and the possible locations of the electrons in the cloud!

Orbital Filling Sequence How do we do it? How do we know where all of the electrons go? 1 st level or 7 th level? s, p, d, or f sublevel? Help!!! Electron orbitals Animated electron configurations http: //www. cms. k 12. nc. us/allschools/providence/keenan/chem/images/orbital_energy_diagram. gif

Orbital Diagrams 1 s 4 s 5 s 6 s 2 s 2 p 3 s 3 p 3 d 4 p 4 d 5 p 4 f 5 d 6 p

What’s the Point? o Orbital diagrams are just an easy way to figure out where the electrons are positioned in an atom o Helps us understand the structure of atoms o Which will help us understand the properties of atoms

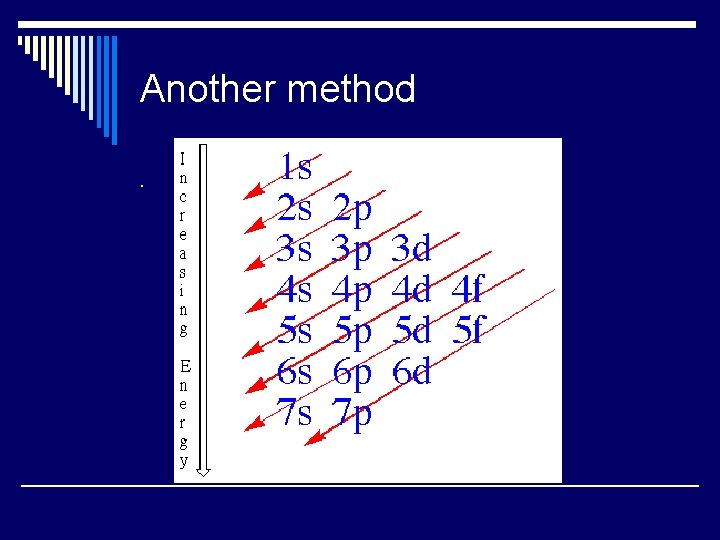
Another method.

Electron Configurations o Do the same thing as orbital diagrams, except without all the little boxes! 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 14 5 d 10 6 p 6 This is the max configuration we’ll learn…so when you get this…you’ve got it!

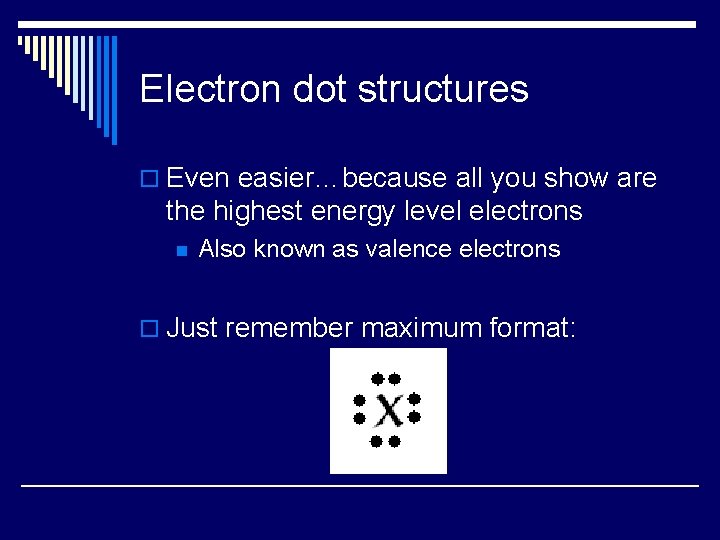
Electron dot structures o Even easier…because all you show are the highest energy level electrons n Also known as valence electrons o Just remember maximum format: