Chapter 5 ARRANGEMENT OF ELECTRONS IN ATOMS THE







- Slides: 24

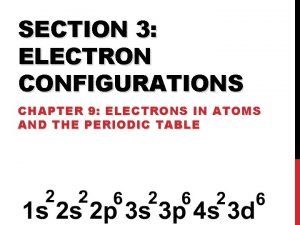
Chapter 5 ARRANGEMENT OF ELECTRONS IN ATOMS

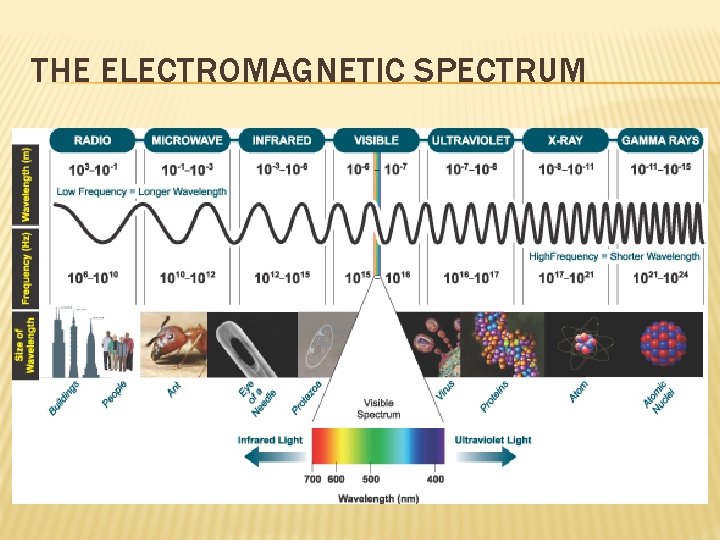
THE ELECTROMAGNETIC SPECTRUM � Light is electromagnetic radiation (energy that exhibits wavelike behavior). � ER moves at a constant speed (c) of 3. 0 x 108 m/s (through a vacuum).

THE ELECTROMAGNETIC SPECTRUM

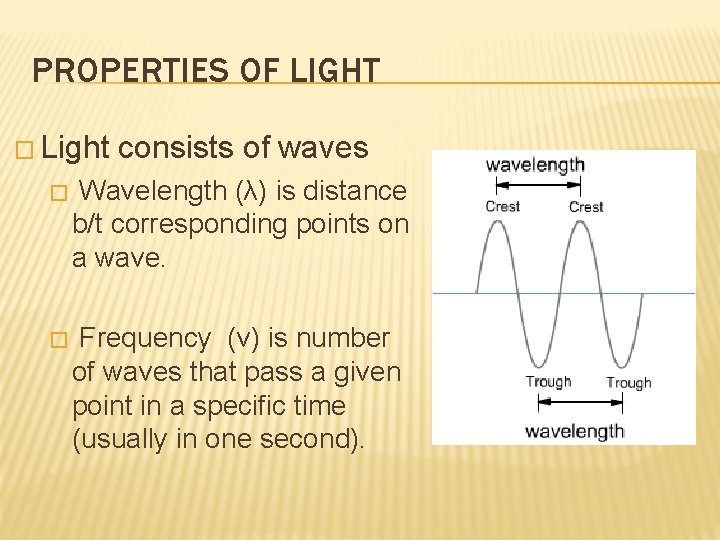
PROPERTIES OF LIGHT � Light consists of waves � Wavelength (λ) is distance b/t corresponding points on a wave. � Frequency (ν) is number of waves that pass a given point in a specific time (usually in one second).

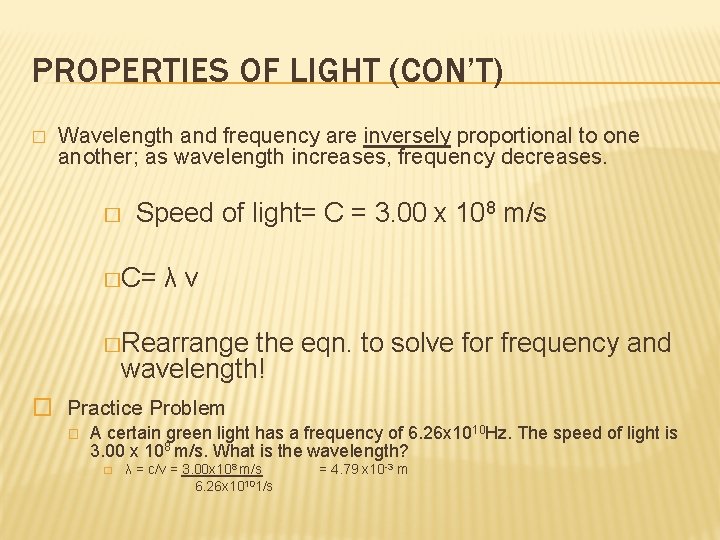
PROPERTIES OF LIGHT (CON’T) � Wavelength and frequency are inversely proportional to one another; as wavelength increases, frequency decreases. � Speed of light= C = 3. 00 x 108 m/s �C= λν �Rearrange the eqn. to solve for frequency and wavelength! � Practice Problem � A certain green light has a frequency of 6. 26 x 1010 Hz. The speed of light is 3. 00 x 108 m/s. What is the wavelength? � λ = c/v = 3. 00 x 108 m/s 6. 26 x 10101/s = 4. 79 x 10 -3 m

THE PHOTOELECTRIC EFFECT � Light has a dual wave-particle nature; it does exhibit wave like properties but it can be thought of as a stream of particles with each photon carrying a quantum of energy. �E = hv �h = Plank’s constant (6. 626 x 10 -34 J/Hz) �E = energy of a quantum �v = frequency in hertz

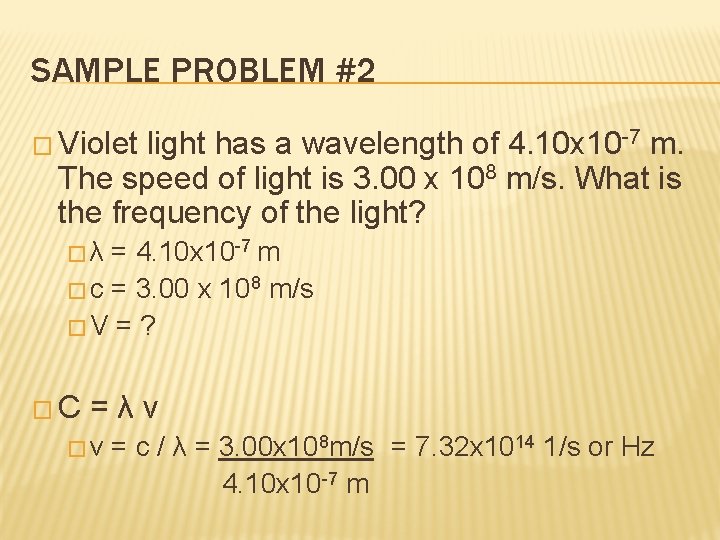
SAMPLE PROBLEM #2 � Violet light has a wavelength of 4. 10 x 10 -7 m. The speed of light is 3. 00 x 108 m/s. What is the frequency of the light? �λ = 4. 10 x 10 -7 m � c = 3. 00 x 108 m/s �V = ? �C =λν �v = c / λ = 3. 00 x 108 m/s = 7. 32 x 1014 1/s or Hz 4. 10 x 10 -7 m

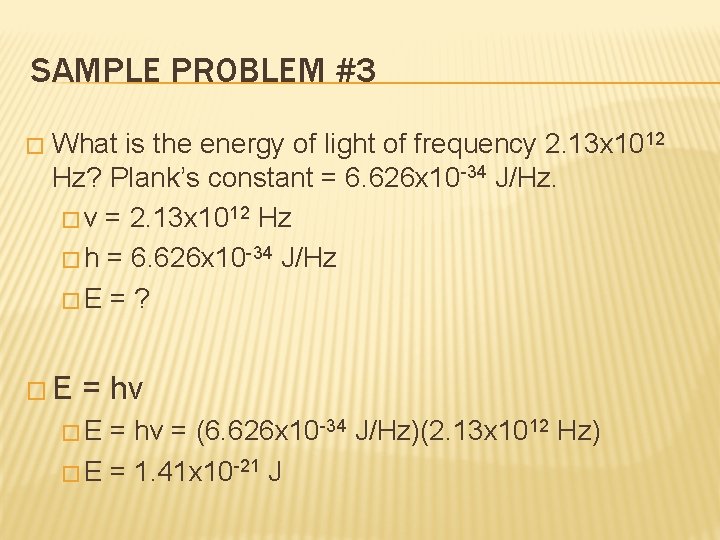
SAMPLE PROBLEM #3 � What is the energy of light of frequency 2. 13 x 1012 Hz? Plank’s constant = 6. 626 x 10 -34 J/Hz. � v = 2. 13 x 1012 Hz � h = 6. 626 x 10 -34 J/Hz �E = ? �E = hv = (6. 626 x 10 -34 J/Hz)(2. 13 x 1012 Hz) � E = 1. 41 x 10 -21 J

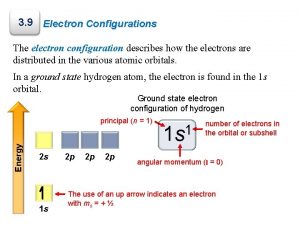
ATOMIC SPECTRA � � If a current is passed through a gas at low pressure, the electrons of the atoms become energized. � Ground state – lowest energy state of an atom � Excited state – condition in which the atom has more energy than its ground state. When the atom returns to ground state, it gives off electromagnetic radiation (light). � Example: Neon Signs

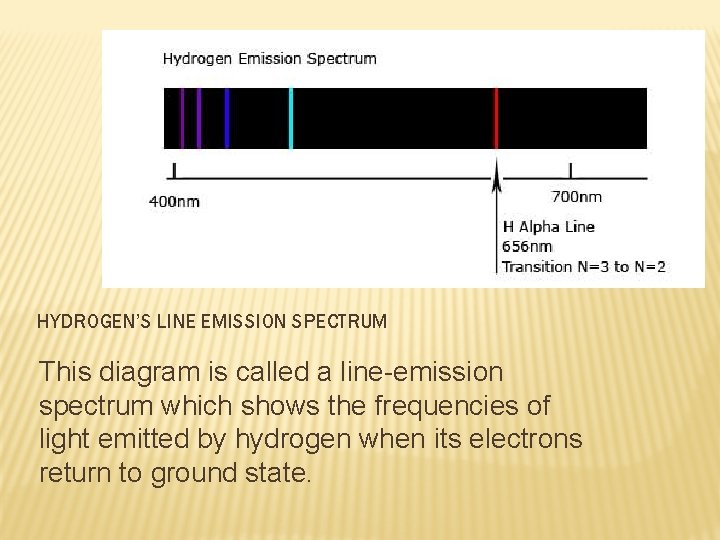
HYDROGEN’S LINE EMISSION SPECTRUM This diagram is called a line-emission spectrum which shows the frequencies of light emitted by hydrogen when its electrons return to ground state.

BOHR’S MODEL � Linked the atom’s electron with photon emission. � Electrons are allowed to circle the nucleus only in fixed paths (orbits). � Atom is in the lowest energy level when the electron is closest to the nucleus. � An electron can move to a higher orbital by gaining energy. � When an electron falls from an excited state, a photon is emitted � Bohr’s model mathematically explained the lineemission spectrum of hydrogen.

QUANTUM THEORY

QUANTUM THEORY � Electrons, like light, are found to have a dual wave-particle nature. But where are they? � Heisenberg uncertainty principle states that it is impossible to know the velocity and position of a particle at the same time. � This and Schrödinger’s wave equation lead to modern quantum mechanics, which describes the motions of subatomic particles as waves.

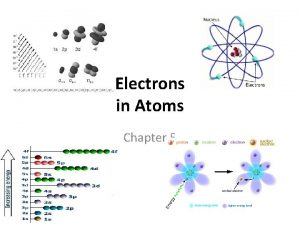
ORBITALS � Electrons are not in neat fixed orbits like Bohr proposed but in orbitals. � 3 -D region around the nucleus that indicates the probable location (90%) of an electron. � Each electron has an “address” consisting of four Quantum numbers.

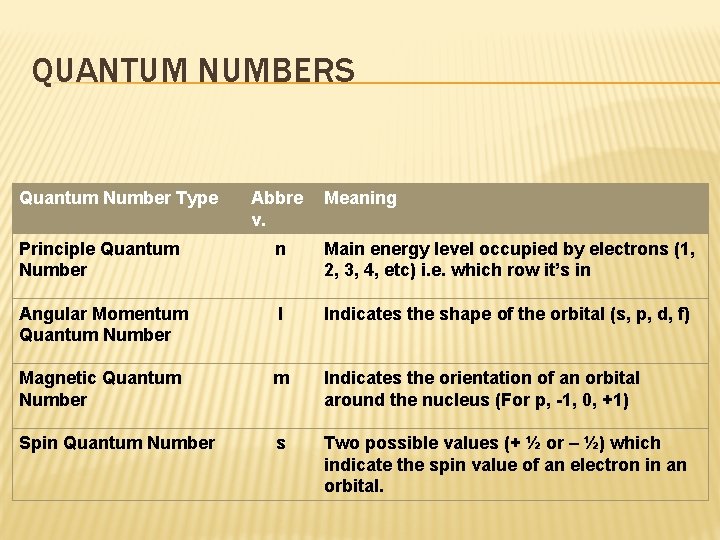
QUANTUM NUMBERS Quantum Number Type Abbre v. Meaning Principle Quantum Number n Main energy level occupied by electrons (1, 2, 3, 4, etc) i. e. which row it’s in Angular Momentum Quantum Number l Indicates the shape of the orbital (s, p, d, f) Magnetic Quantum Number m Indicates the orientation of an orbital around the nucleus (For p, -1, 0, +1) Spin Quantum Number s Two possible values (+ ½ or – ½) which indicate the spin value of an electron in an orbital.

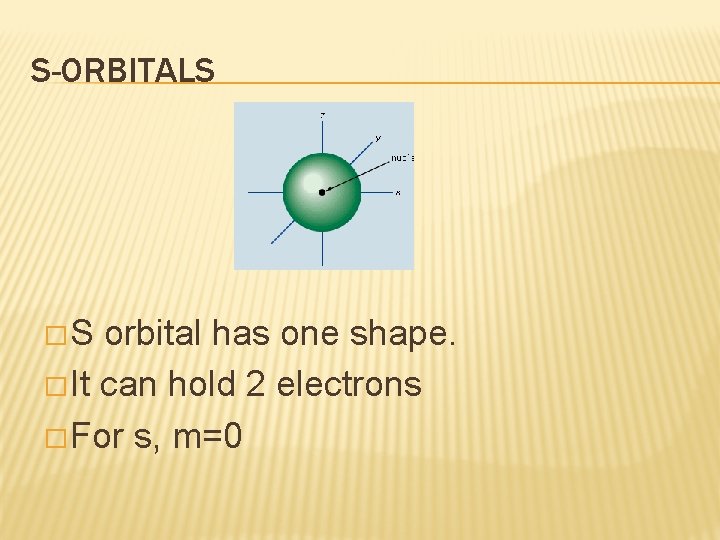
S-ORBITALS �S orbital has one shape. � It can hold 2 electrons � For s, m=0

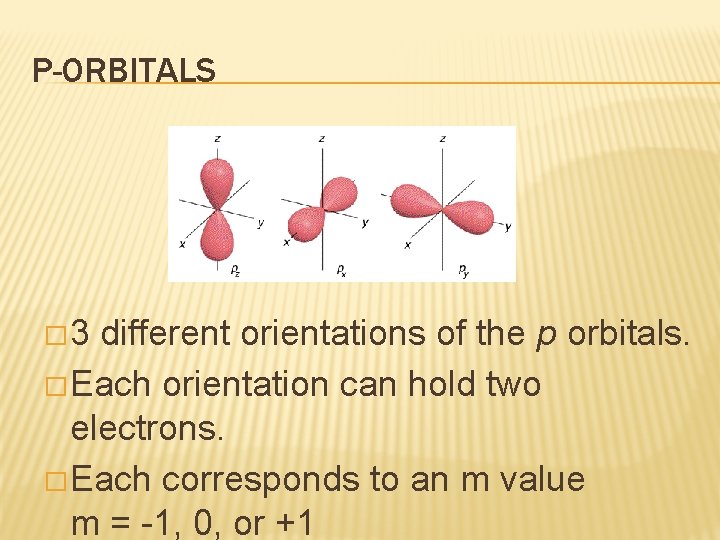
P-ORBITALS � 3 different orientations of the p orbitals. � Each orientation can hold two electrons. � Each corresponds to an m value m = -1, 0, or +1

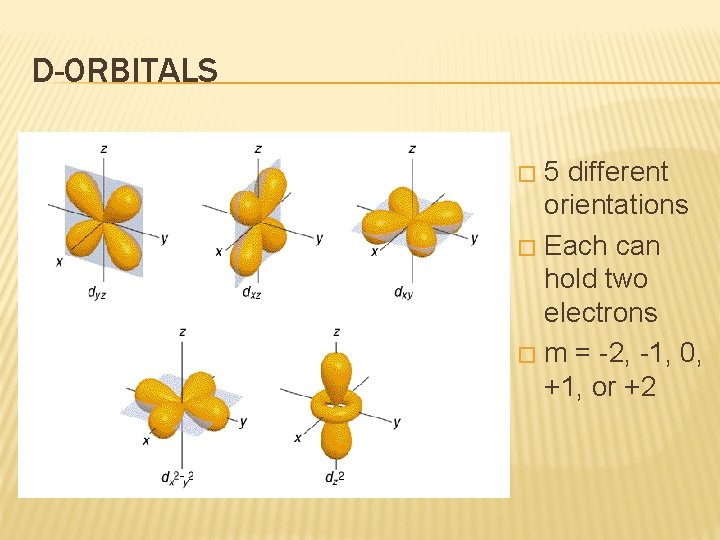
D-ORBITALS 5 different orientations � Each can hold two electrons � m = -2, -1, 0, +1, or +2 �

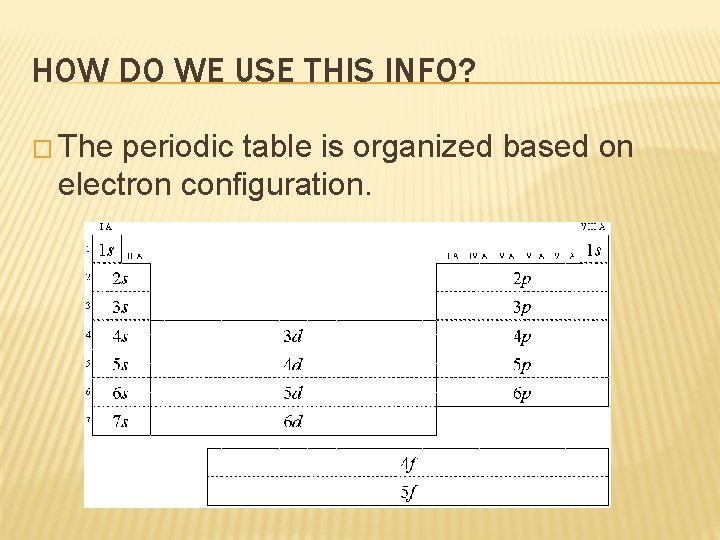
HOW DO WE USE THIS INFO? � The periodic table is organized based on electron configuration.

WRITING ELECTRON CONFIGURATIONS

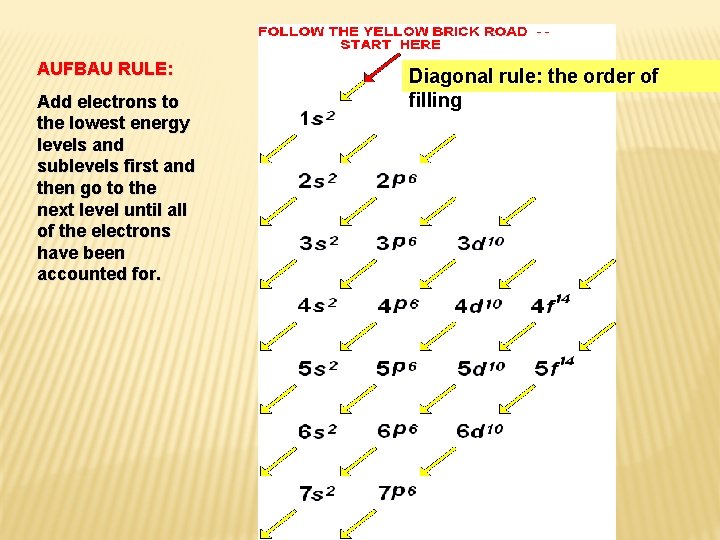
AUFBAU RULE: Add electrons to the lowest energy levels and sublevels first and then go to the next level until all of the electrons have been accounted for. Diagonal rule: the order of filling

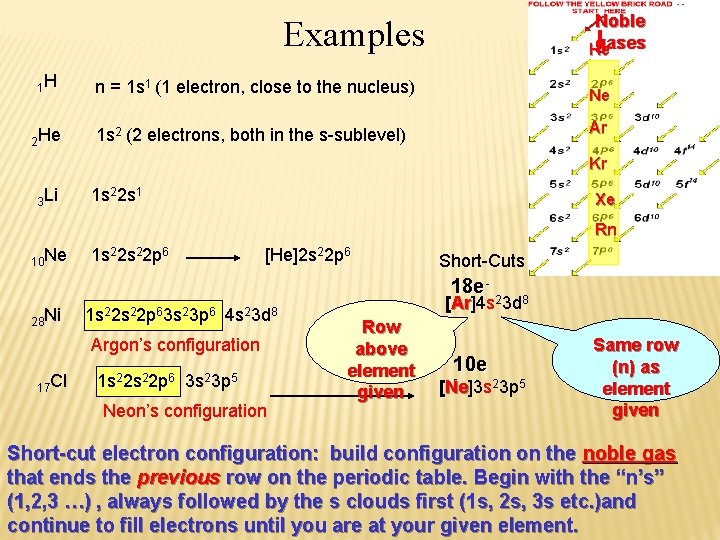
Noble gases He Examples 1 H 2 He n = 1 s 1 (1 electron, close to the nucleus) Ne 1 s 2 (2 electrons, both in the s-sublevel) Ar Kr 3 Li 1 s 22 s 1 Xe Rn 10 Ne 28 Ni 1 s 22 p 6 [He]2 s 22 p 6 1 s 22 p 63 s 23 p 6 4 s 23 d 8 Argon’s configuration 17 Cl 1 s 22 p 6 3 s 23 p 5 Neon’s configuration Short-Cuts 18 e 2 [Ar]4 s 3 d 8 Ar Row above element given 10 e - [Ne]3 s Ne 23 p 5 Same row (n) as element given Short-cut electron configuration: build configuration on the noble gas that ends the previous row on the periodic table. Begin with the “n’s” (1, 2, 3 …) , always followed by the s clouds first (1 s, 2 s, 3 s etc. )and continue to fill electrons until you are at your given element.

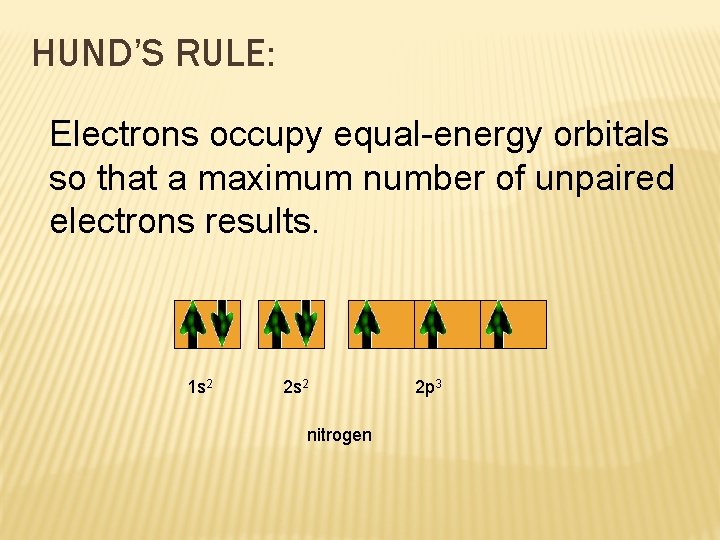
HUND’S RULE: Electrons occupy equal-energy orbitals so that a maximum number of unpaired electrons results. 1 s 2 2 s 2 nitrogen 2 p 3

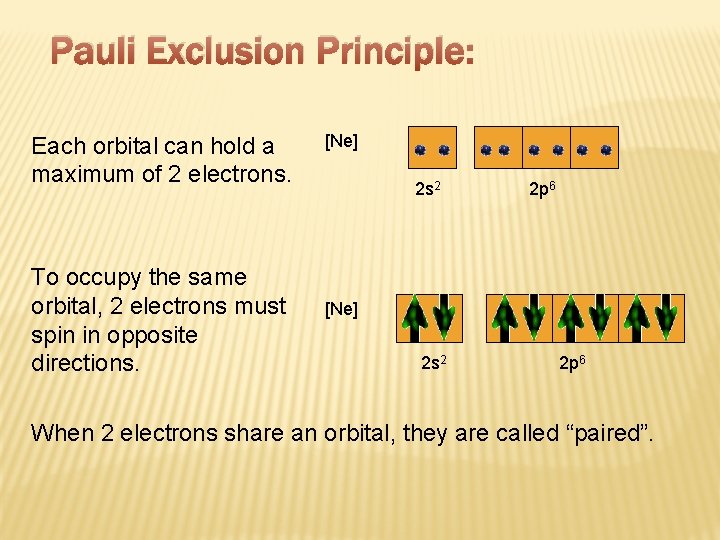
Pauli Exclusion Principle: Each orbital can hold a maximum of 2 electrons. To occupy the same orbital, 2 electrons must spin in opposite directions. [Ne] 2 s 2 2 p 6 When 2 electrons share an orbital, they are called “paired”.
 Chapter 4 arrangement of electrons in atoms test
Chapter 4 arrangement of electrons in atoms test Chapter 5 review arrangement of electrons in atoms
Chapter 5 review arrangement of electrons in atoms Ccechs
Ccechs Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Atoms with 4 valence electrons
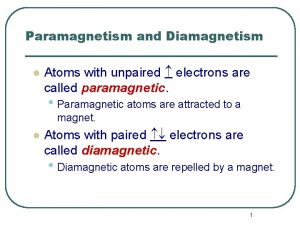
Atoms with 4 valence electrons Atoms with unpaired electrons are called diamagnetic.
Atoms with unpaired electrons are called diamagnetic. Periodic table protons neutrons electrons
Periodic table protons neutrons electrons Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Quantum mechanical model
Quantum mechanical model Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Aluminum chloride charge
Aluminum chloride charge How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? Metallic bond formula
Metallic bond formula Electrons in atoms section 3 electron configuration
Electrons in atoms section 3 electron configuration 5 electrons in atoms
5 electrons in atoms Unstable arrangement of atoms
Unstable arrangement of atoms Arrangement of electrons
Arrangement of electrons Configuracion electronica
Configuracion electronica Complete ground state electron configuration
Complete ground state electron configuration Chapter 4 section 2 the structure of atoms answer key
Chapter 4 section 2 the structure of atoms answer key Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers