Electron Configuration and Periodic Trends Essential Question How

- Slides: 16

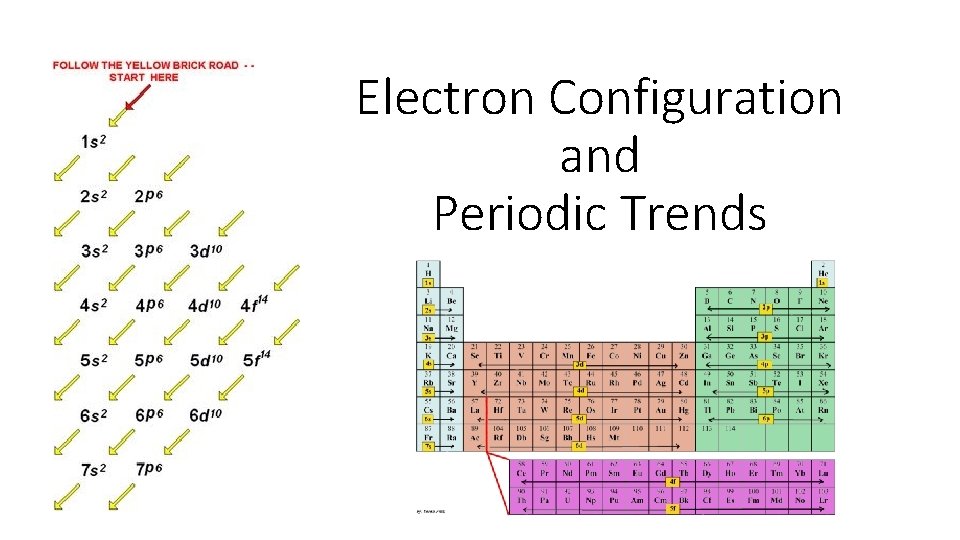
Electron Configuration and Periodic Trends

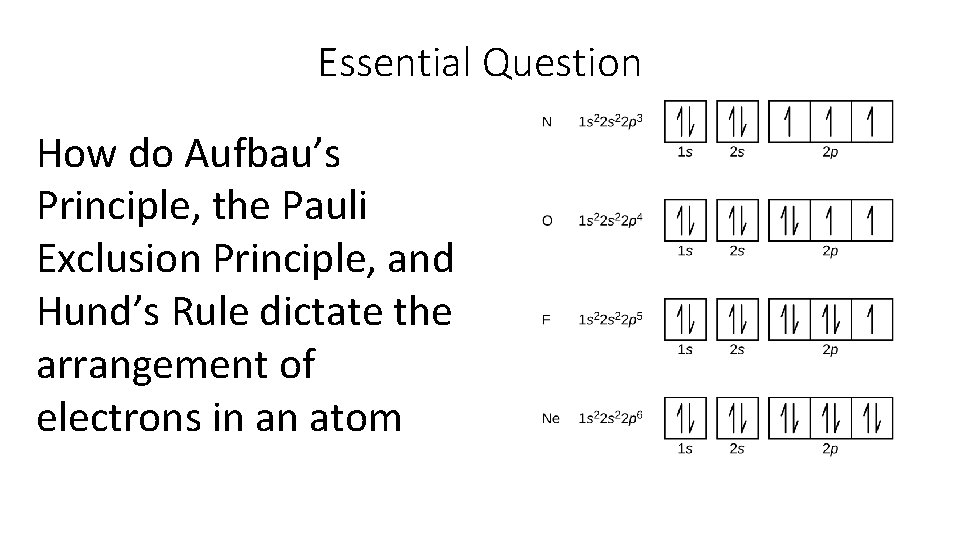
Essential Question How do Aufbau’s Principle, the Pauli Exclusion Principle, and Hund’s Rule dictate the arrangement of electrons in an atom

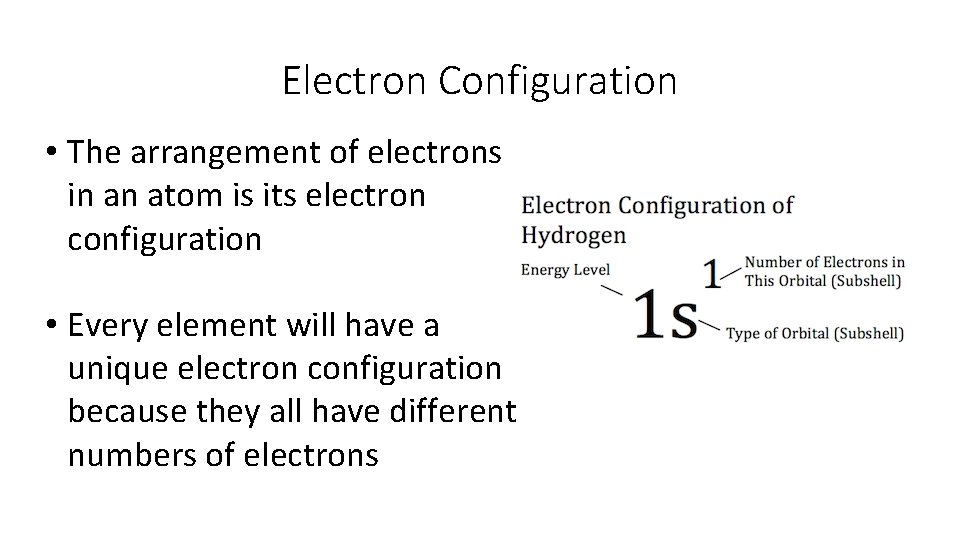
Electron Configuration • The arrangement of electrons in an atom is its electron configuration • Every element will have a unique electron configuration because they all have different numbers of electrons

Ground State Electron Configuration Electrons in an atom tend to assume arrangements that have the lowest possible energies. This is called the electrons ground state electron configuration.

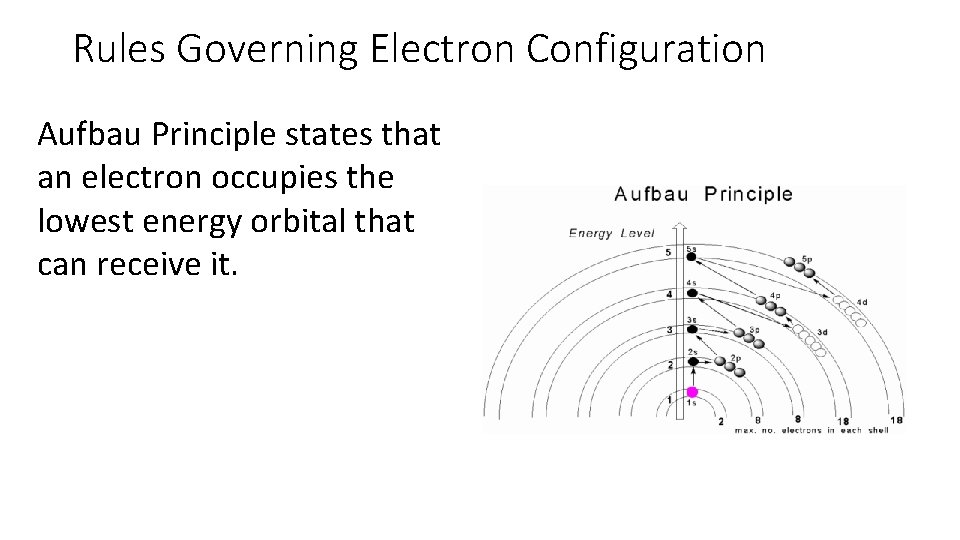
Rules Governing Electron Configuration Aufbau Principle states that an electron occupies the lowest energy orbital that can receive it.

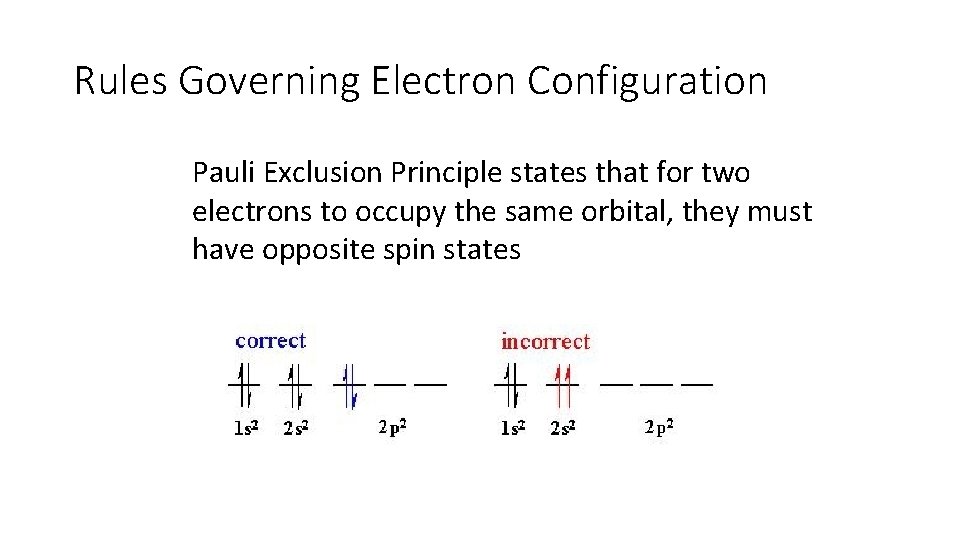
Rules Governing Electron Configuration Pauli Exclusion Principle states that for two electrons to occupy the same orbital, they must have opposite spin states

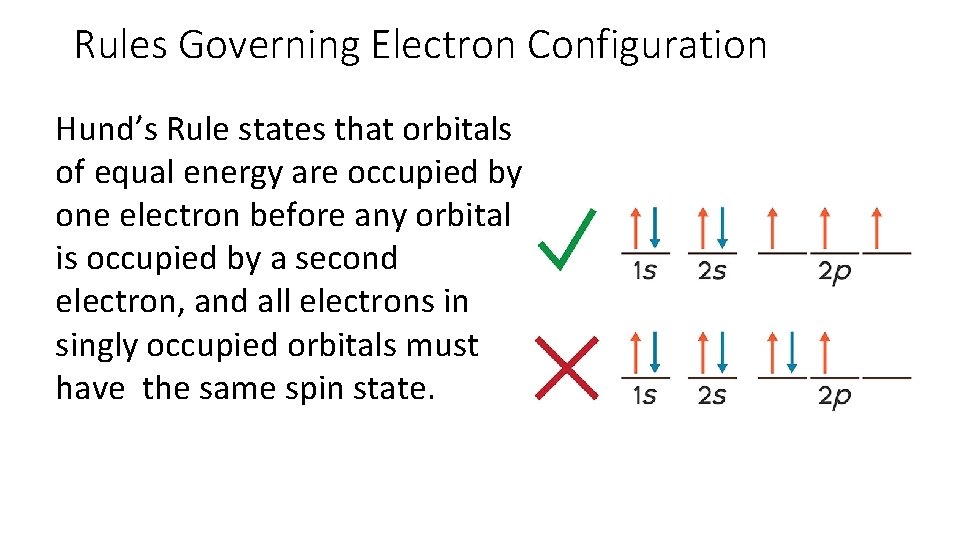
Rules Governing Electron Configuration Hund’s Rule states that orbitals of equal energy are occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin state.

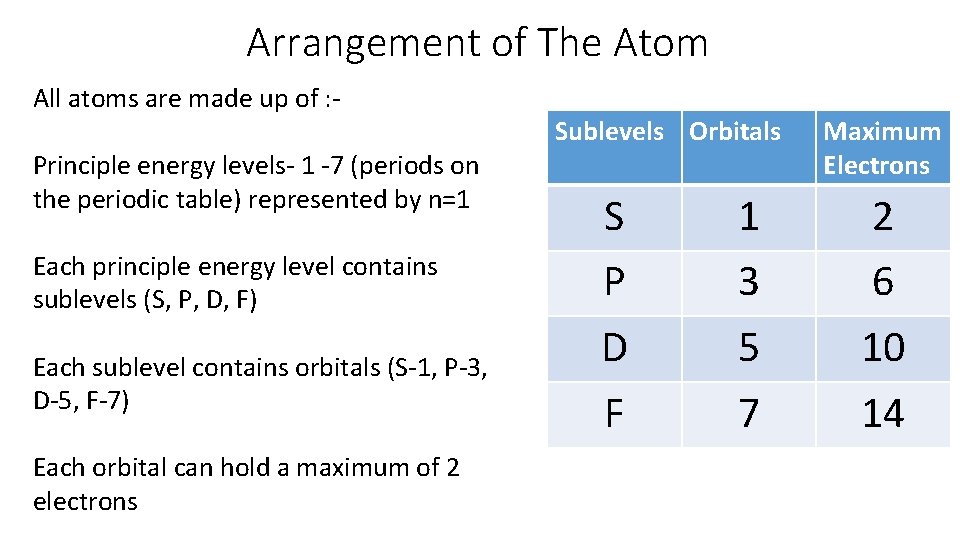
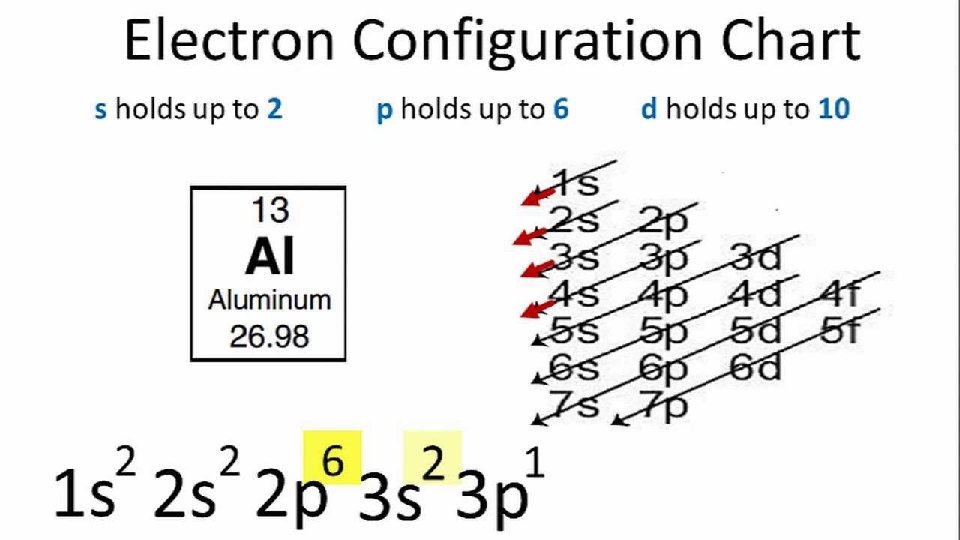
Arrangement of The Atom All atoms are made up of : Principle energy levels- 1 -7 (periods on the periodic table) represented by n=1 Each principle energy level contains sublevels (S, P, D, F) Each sublevel contains orbitals (S-1, P-3, D-5, F-7) Each orbital can hold a maximum of 2 electrons Sublevels Orbitals S P D F 1 3 5 7 Maximum Electrons 2 6 10 14

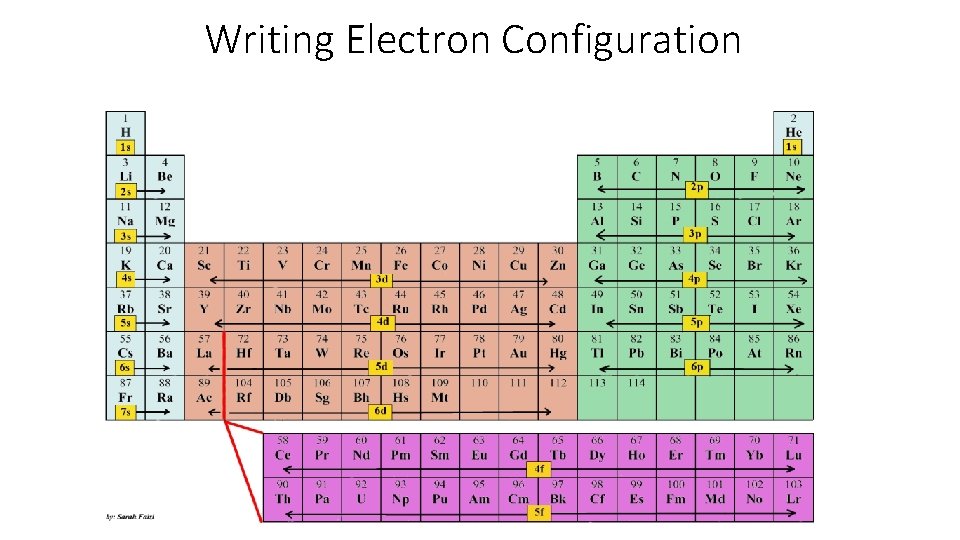
Writing Electron Configuration

Writing Electron Configuration Draw the electron configuration chart and use it to write the unabbreviated electron configurations for the following elements. Lithium Aluminum iron

Writing Electron Configuration Draw the electron configuration chart and use it to write the abbreviated electron configurations for the following elements using the noble gas notation. Lithium Aluminum iron

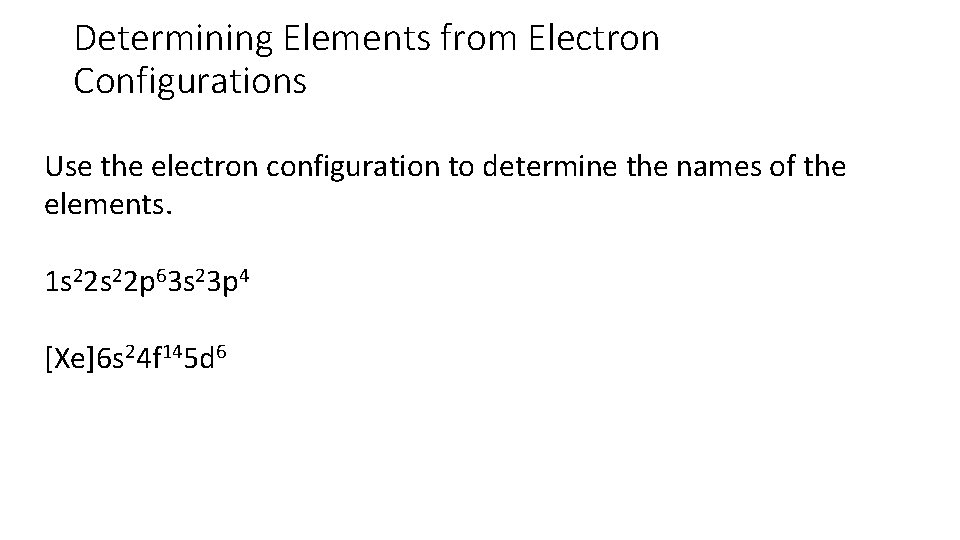
Determining Elements from Electron Configurations Use the electron configuration to determine the names of the elements. 1 s 22 p 63 s 23 p 4 [Xe]6 s 24 f 145 d 6

Writing Electron Configuration

Writing Electron Configuration Use the periodic electron configuration chart and use it to write the unabbreviated electron configurations for the following elements. Lithium Aluminum iron

Writing Electron Configuration Use the periodic electron configuration chart and use it to write the abbreviated electron configurations for the following elements using the noble gas notation. Lithium Aluminum iron
