Chapter 5 Arrangement of Electrons in Atoms Light








- Slides: 51

Chapter 5 Arrangement of Electrons in Atoms

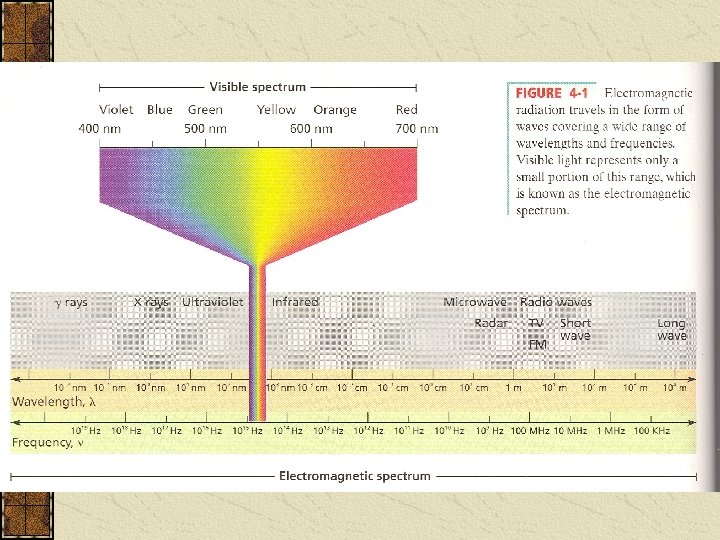
Light Dual Nature of Light: Light can act like_____, and as _____particles. Light is one type of _____ _____which is a form of Energy that has wavelike behaviour Other types of em radiation are: ____________________, and together they all form the ______.


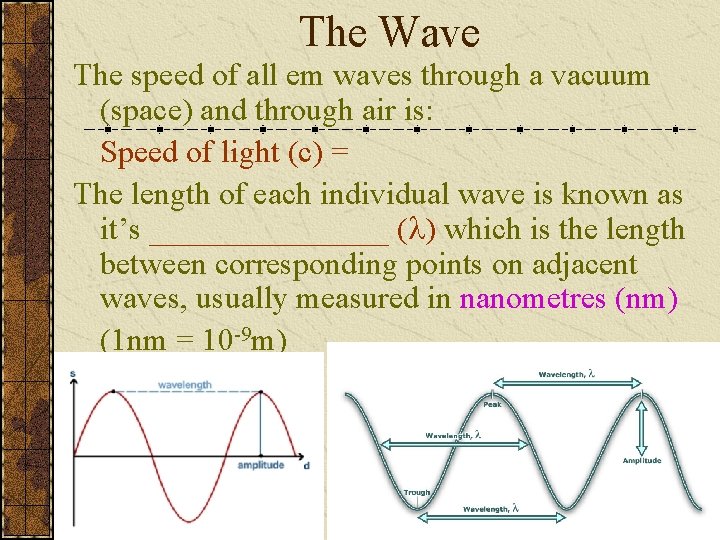
The Wave The speed of all em waves through a vacuum (space) and through air is: Speed of light (c) = The length of each individual wave is known as it’s ________ ( ) which is the length between corresponding points on adjacent waves, usually measured in nanometres (nm) (1 nm = 10 -9 m)

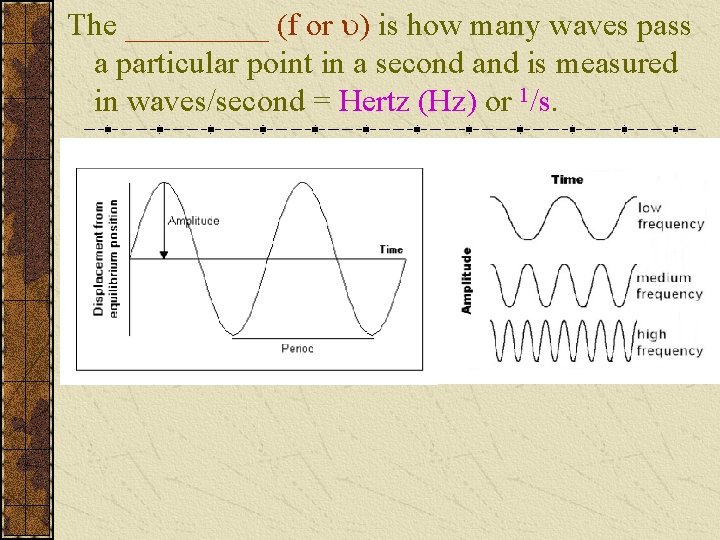
The _____ (f or ) is how many waves pass a particular point in a second and is measured in waves/second = Hertz (Hz) or 1/s.

The relationship between them is as follows: c=f or c= Example: What is the wavelength & colour of light that has a frequency of 6 x 1014 Hz (1/s)?

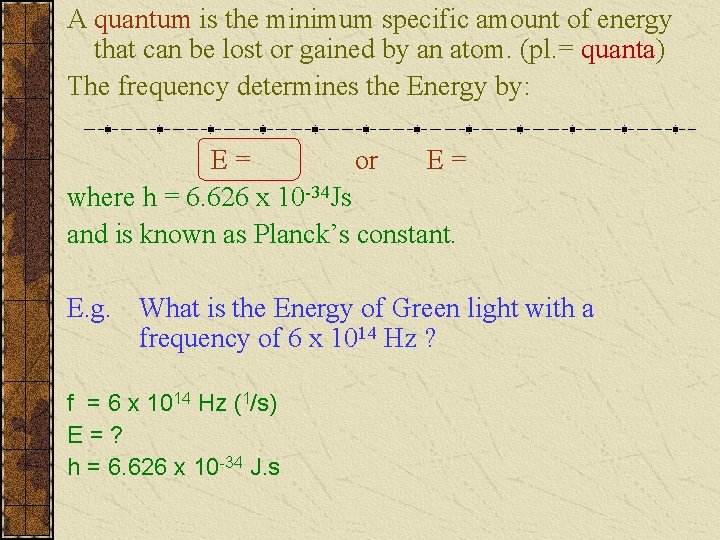
A quantum is the minimum specific amount of energy that can be lost or gained by an atom. (pl. = quanta) The frequency determines the Energy by: E= or E= where h = 6. 626 x 10 -34 Js and is known as Planck’s constant. E. g. What is the Energy of Green light with a frequency of 6 x 1014 Hz ? f = 6 x 1014 Hz (1/s) E=? h = 6. 626 x 10 -34 J. s

If electromagnetic (em) radiation is directed towards a piece of metal, it may have enough energy to knock out electrons. This is known as the. A German physicist: Max Planck, explained the reason why.

It was Einstein that proposed that light acted like a stream of particles called _______. A ______ is a particle of em radiation that has zero mass and carrying a _______ of energy There must be a minimum amount of energy to eject electrons from a metal, and looking at the formula E = h. f, Planck realised that the em radiation providing the energy, must be of a certain ____. Different metals need different minimum amounts of energy, and therefore _____

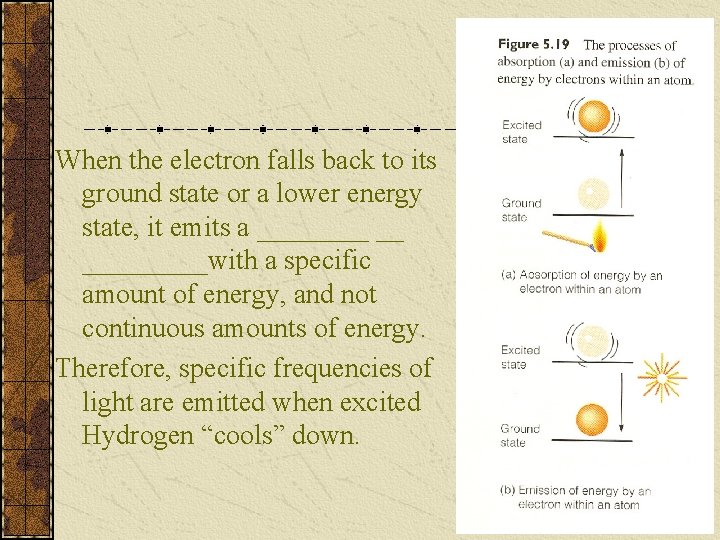
The Hydrogen Atom Consider the H atom with its one electron spinning around its one proton. When the electron is closest to the proton/nucleus which is at its lowest energy state, the atom is in its _______ state. When energy, like heat, is supplied to the atom, the electron jumps to a higher orbit which is at a higher energy state. The atom is now in an ____ state.

When the electron falls back to its ground state or a lower energy state, it emits a ____ __ _____with a specific amount of energy, and not continuous amounts of energy. Therefore, specific frequencies of light are emitted when excited Hydrogen “cools” down.

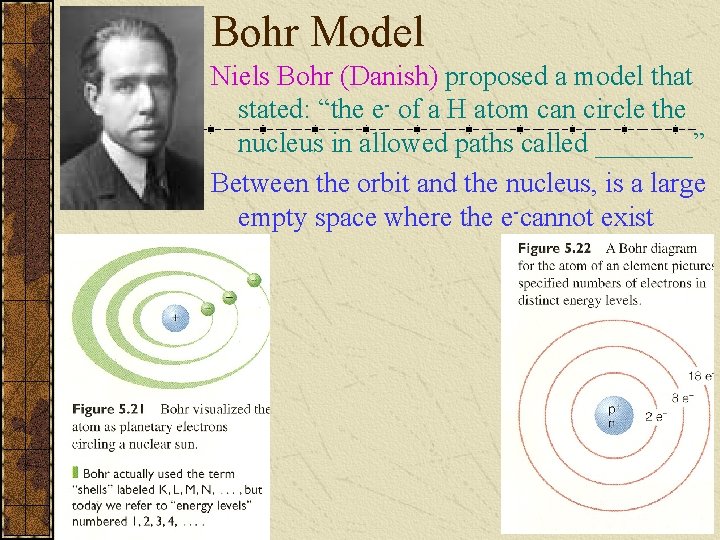
Bohr Model Niels Bohr (Danish) proposed a model that stated: “the e- of a H atom can circle the nucleus in allowed paths called _______” Between the orbit and the nucleus, is a large empty space where the e-cannot exist

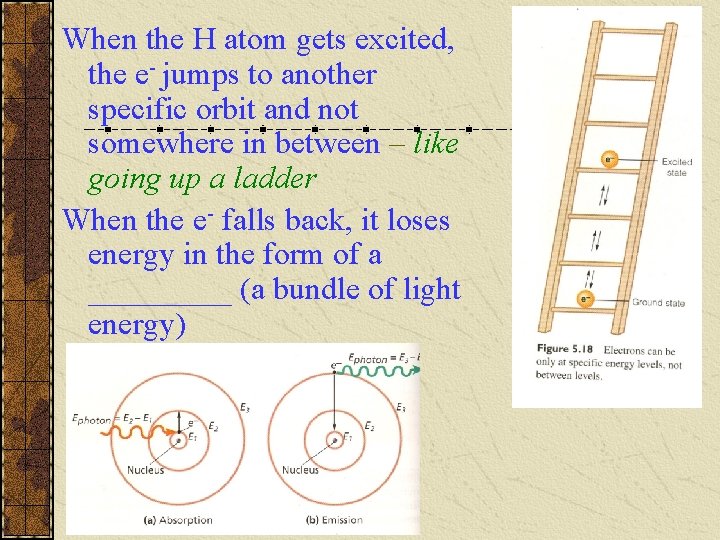
When the H atom gets excited, the e- jumps to another specific orbit and not somewhere in between – like going up a ladder When the e- falls back, it loses energy in the form of a _____ (a bundle of light energy)


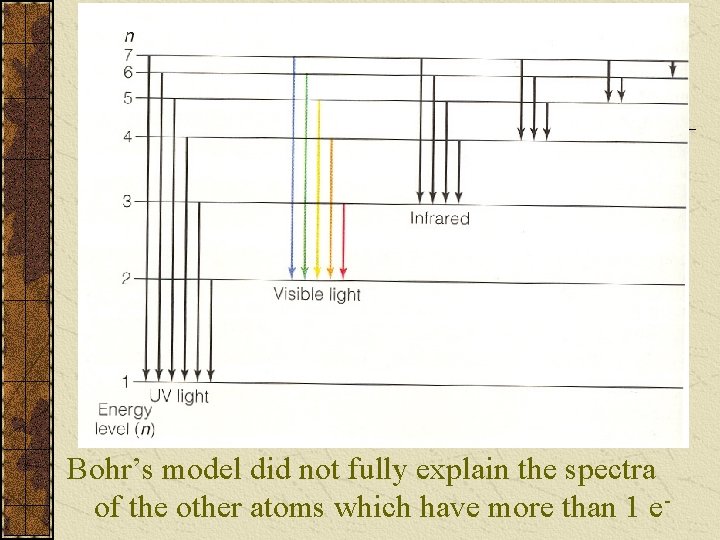
The amount of Energy is equal to the difference in the two Energy (orbit) levels The f of the light is determined by the formula So there are specific frequencies (colours of light) given off when the H e- falls from the higher orbits giving its visible line spectrum (see p. 143 Fig 5. 14 Lyman, Balmer, & Paschen series, or see next slide)

Bohr’s model did not fully explain the spectra of the other atoms which have more than 1 e-

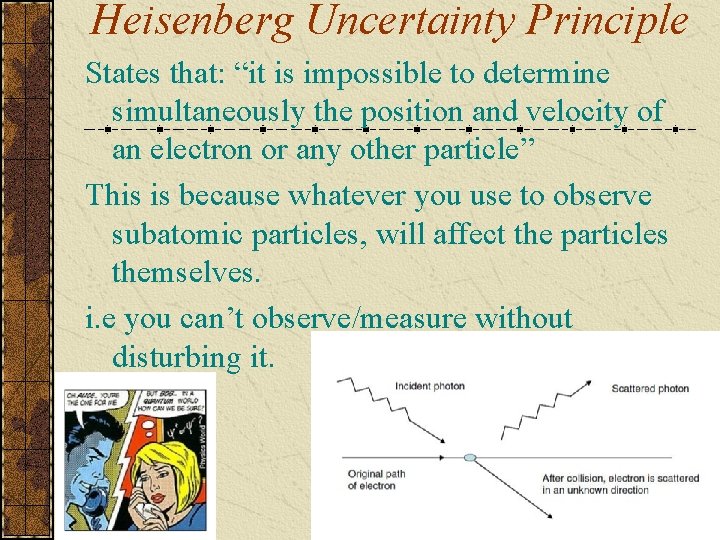
Heisenberg Uncertainty Principle States that: “it is impossible to determine simultaneously the position and velocity of an electron or any other particle” This is because whatever you use to observe subatomic particles, will affect the particles themselves. i. e you can’t observe/measure without disturbing it.

Quantum Model of Atom Question is: Why can’t e-’s be in an orbit between the specific Energy levels? French scientist Louis De. Broglie pointed out that the electron orbits acted like the behaviour of _____. i. e. you can only have a certain amount of waves in a given distance/space – not ½ of a wave If you can only have specific # of waves, then based on the formula c = f. you can only have specific ____, which in turn translates into specific _____.

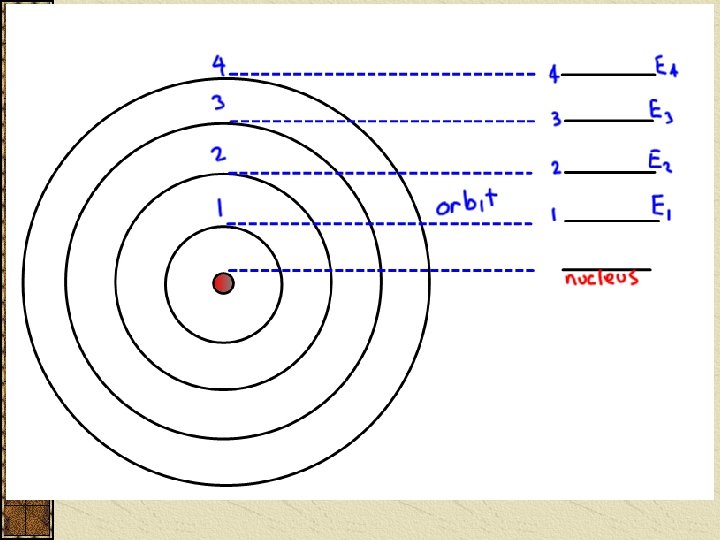
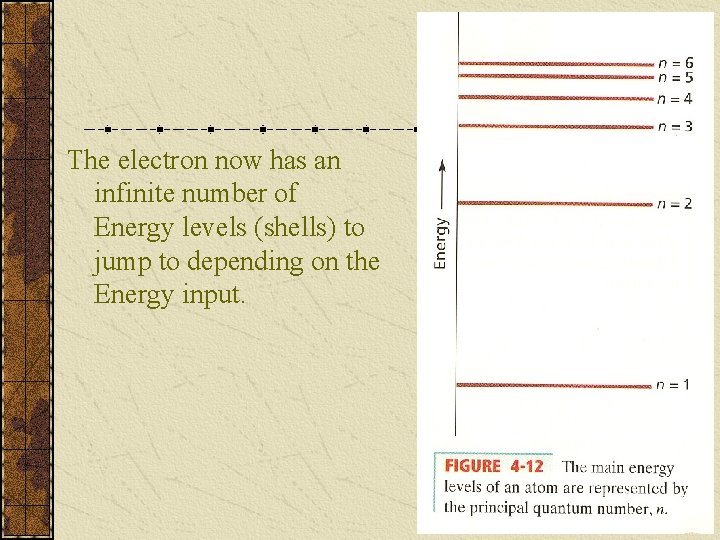
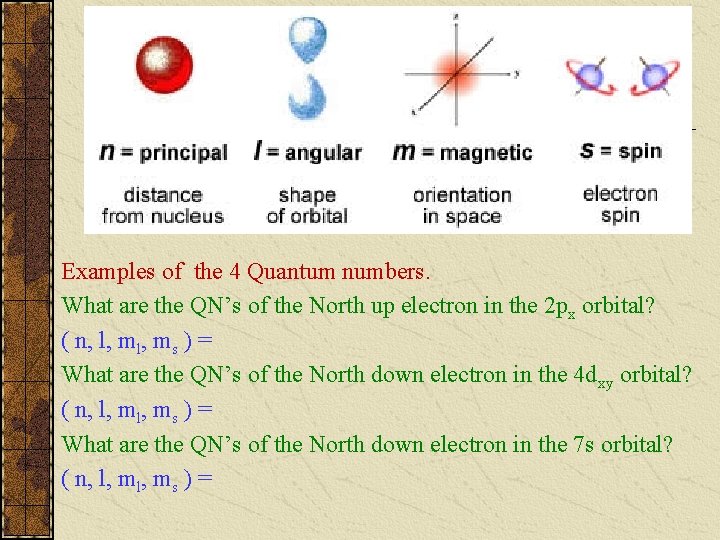
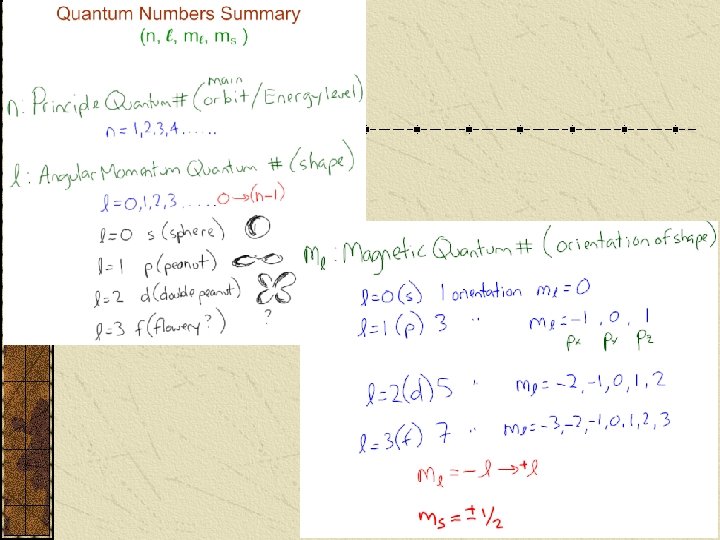
Quantum Numbers Principal Quantum Number (n) indicates the ____ (or shell) occupied by the electron. PQN is also referred to as the Principle Energy Level (En). n = 1, 2, 3, 4… (Bohr originally labeled them K, L, M, N…), where n = 1 is the closest to the nucleus.

The electron now has an infinite number of Energy levels (shells) to jump to depending on the Energy input.

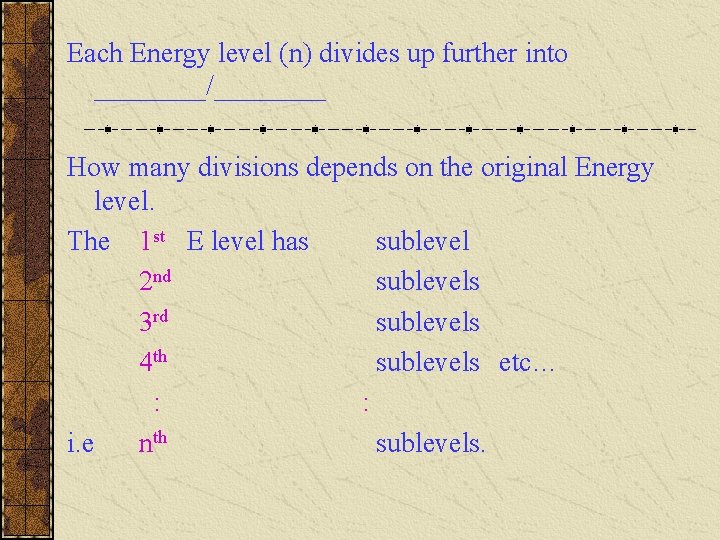
Each Energy level (n) divides up further into ____/____ How many divisions depends on the original Energy level. The 1 st E level has sublevel 2 nd sublevels 3 rd sublevels 4 th sublevels etc… : : i. e nth sublevels.


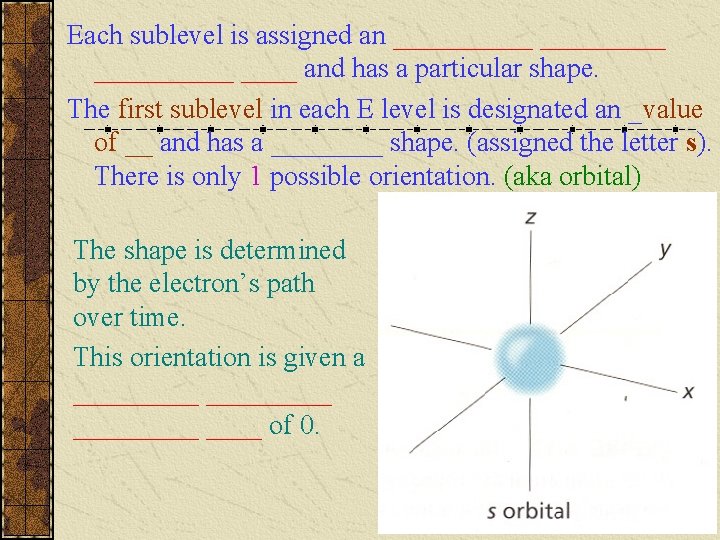
Each sublevel is assigned an __________ and has a particular shape. The first sublevel in each E level is designated an _value of __ and has a ____ shape. (assigned the letter s). There is only 1 possible orientation. (aka orbital) The shape is determined by the electron’s path over time. This orientation is given a _________ ____ of 0.

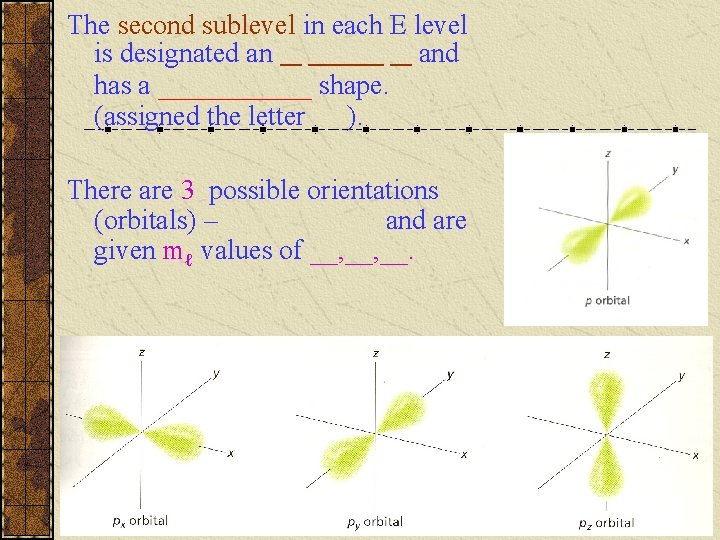
The second sublevel in each E level is designated an __ _______ __ and has a ______ shape. (assigned the letter ). There are 3 possible orientations (orbitals) – and are given mℓ values of __, __.

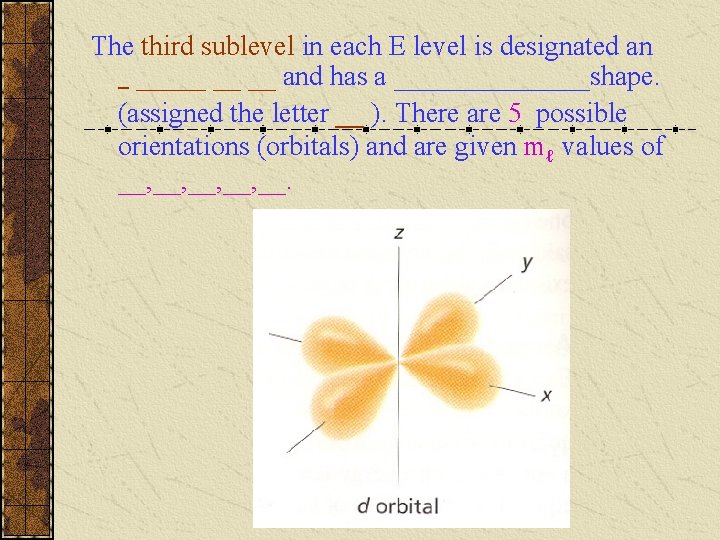
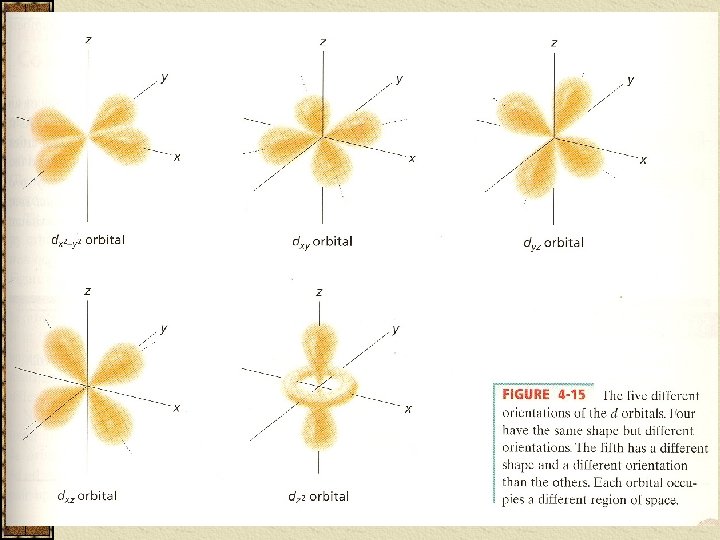
The third sublevel in each E level is designated an _ _____ __ __ and has a _______shape. (assigned the letter __ ). There are 5 possible orientations (orbitals) and are given mℓ values of __, __, __.


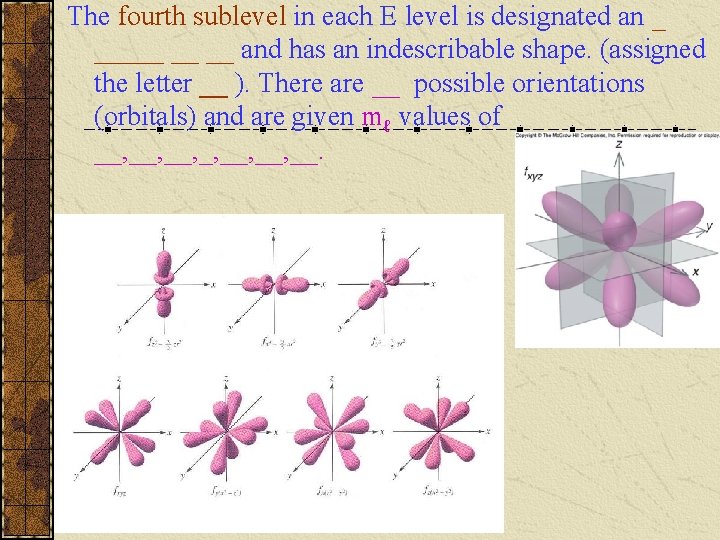
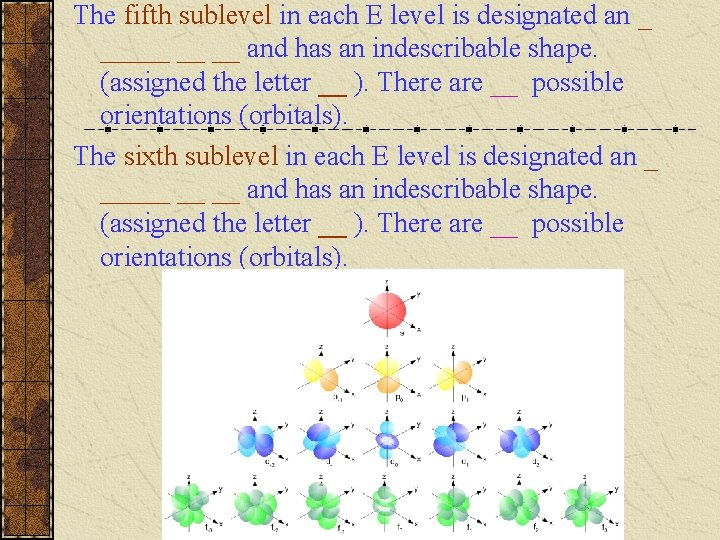
The fourth sublevel in each E level is designated an _ _____ __ __ and has an indescribable shape. (assigned the letter __ ). There are __ possible orientations (orbitals) and are given mℓ values of __, __, __.

The fifth sublevel in each E level is designated an _ _____ __ __ and has an indescribable shape. (assigned the letter __ ). There are __ possible orientations (orbitals). The sixth sublevel in each E level is designated an _ _____ __ __ and has an indescribable shape. (assigned the letter __ ). There are __ possible orientations (orbitals).

Examples of Quantum numbers: What would be the QN’s of an electron residing in the following orbitals? ( n, l, ml ) 2 px 2 py 4 dxy 6 fxyz 7 s

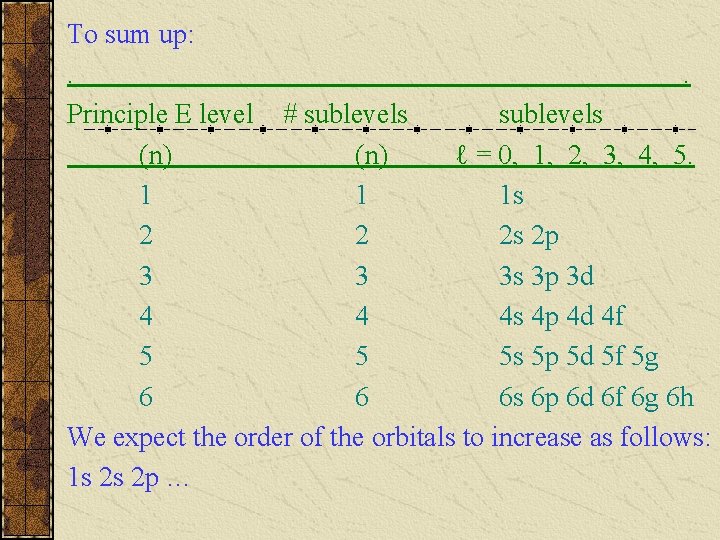
To sum up: . . Principle E level # sublevels (n) ℓ = 0, 1, 2, 3, 4, 5. 1 1 1 s 2 2 2 s 2 p 3 3 3 s 3 p 3 d 4 4 4 s 4 p 4 d 4 f 5 5 5 s 5 p 5 d 5 f 5 g 6 6 6 s 6 p 6 d 6 f 6 g 6 h We expect the order of the orbitals to increase as follows: 1 s 2 s 2 p …

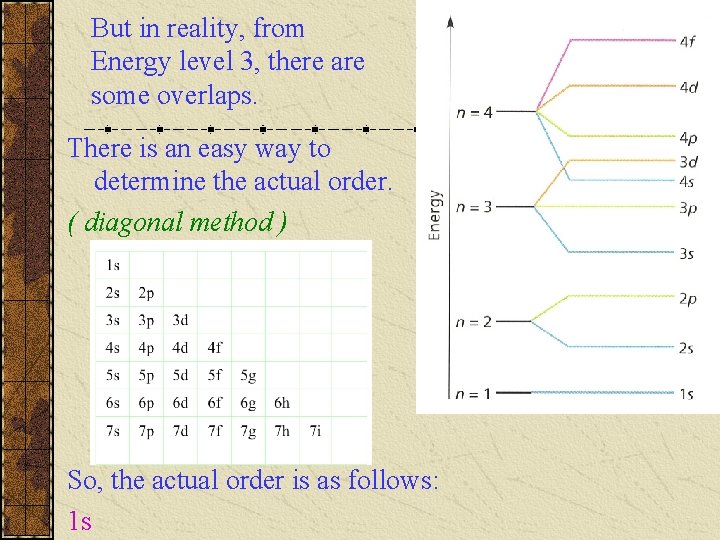
But in reality, from Energy level 3, there are some overlaps. There is an easy way to determine the actual order. ( diagonal method ) So, the actual order is as follows: 1 s

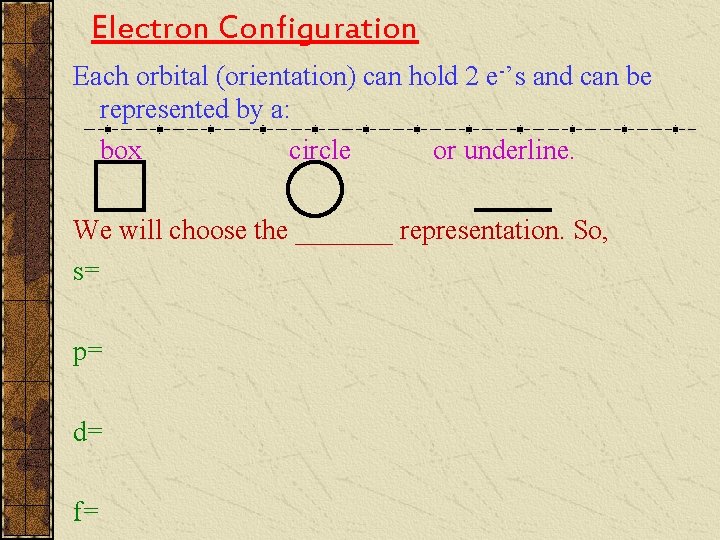
Now each orbital (orientation) can hold 2 electrons. The s sublevel which has 1 orbital can hold 2 e- ’s p d f g h

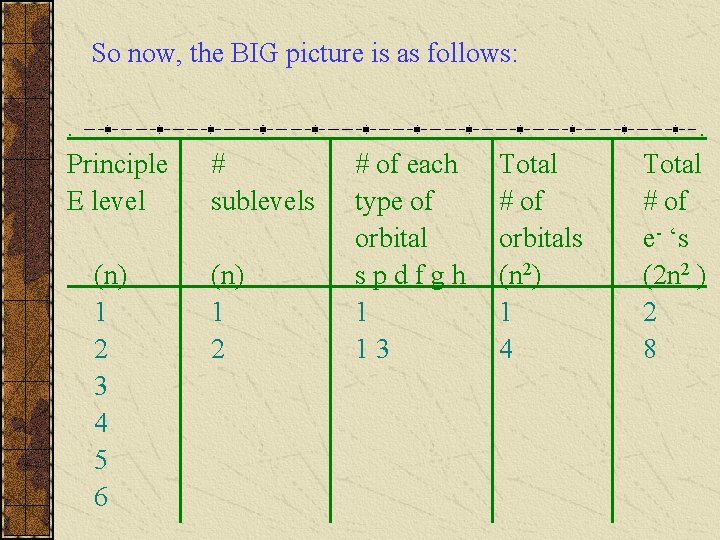
So now, the BIG picture is as follows: . Principle E level (n) 1 2 3 4 5 6 # sublevels (n) 1 2 # of each type of orbital spdfgh 1 13 Total # of orbitals (n 2) 1 4 . Total # of e- ‘s (2 n 2 ) 2 8

Electron Configuration Each orbital (orientation) can hold 2 e-’s and can be represented by a: box circle or underline. We will choose the _______ representation. So, s= p= d= f=

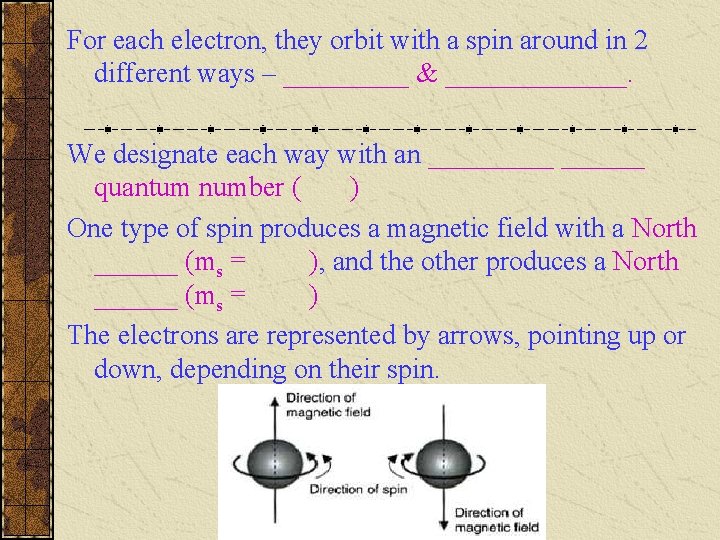
For each electron, they orbit with a spin around in 2 different ways – _____ & _______. We designate each way with an ______ quantum number ( ) One type of spin produces a magnetic field with a North ______ (ms = ), and the other produces a North ______ (ms = ) The electrons are represented by arrows, pointing up or down, depending on their spin.

Examples of the 4 Quantum numbers. What are the QN’s of the North up electron in the 2 px orbital? ( n, l, ms ) = What are the QN’s of the North down electron in the 4 dxy orbital? ( n, l, ms ) = What are the QN’s of the North down electron in the 7 s orbital? ( n, l, ms ) =



According to the: ________ principle, in order for 2 electrons to occupy the same orbital, they must have _______. e. g. Hydrogen has 1 e- that occupies the lowest Energy level – 1 s. Helium has 2 e-’s that also occupies the lowest Energy level – 1 s. Opposing arrows that represent opposite spins.

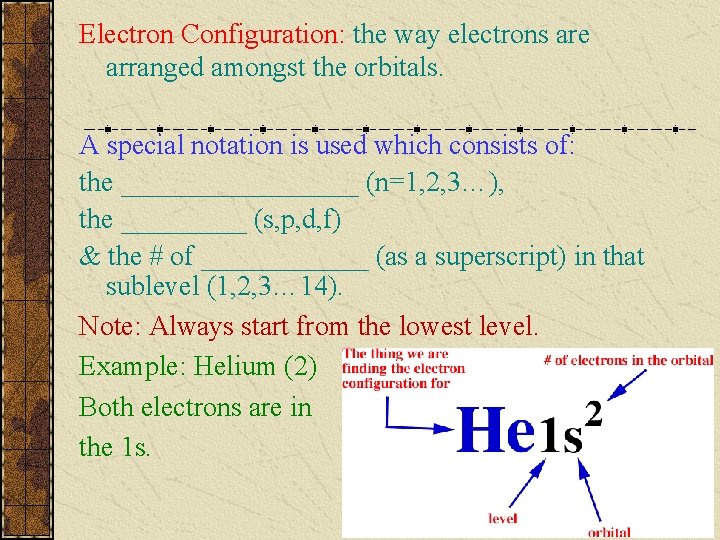
Electron Configuration: the way electrons are arranged amongst the orbitals. A special notation is used which consists of: the _________ (n=1, 2, 3…), the _____ (s, p, d, f) & the # of ______ (as a superscript) in that sublevel (1, 2, 3… 14). Note: Always start from the lowest level. Example: Helium (2) Both electrons are in the 1 s.

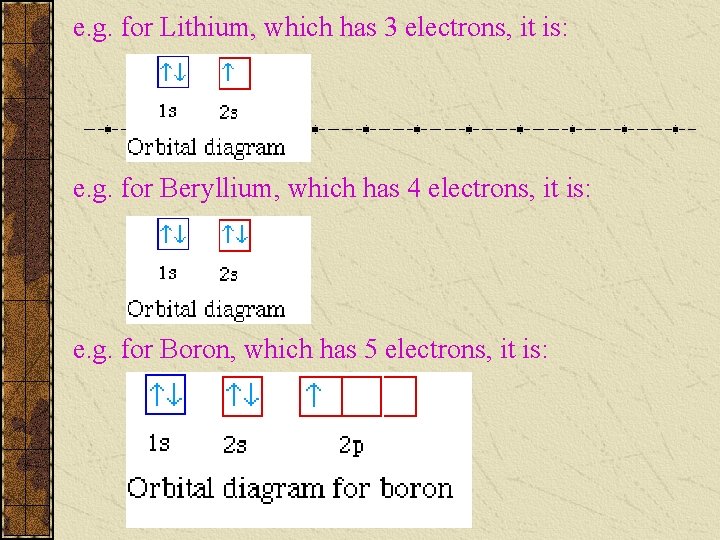
e. g. for Lithium, which has 3 electrons, it is: e. g. for Beryllium, which has 4 electrons, it is: e. g. for Boron, which has 5 electrons, it is:

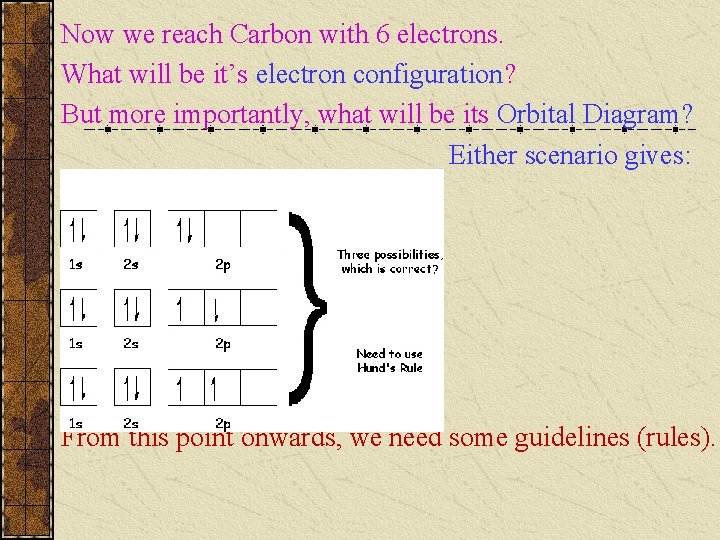
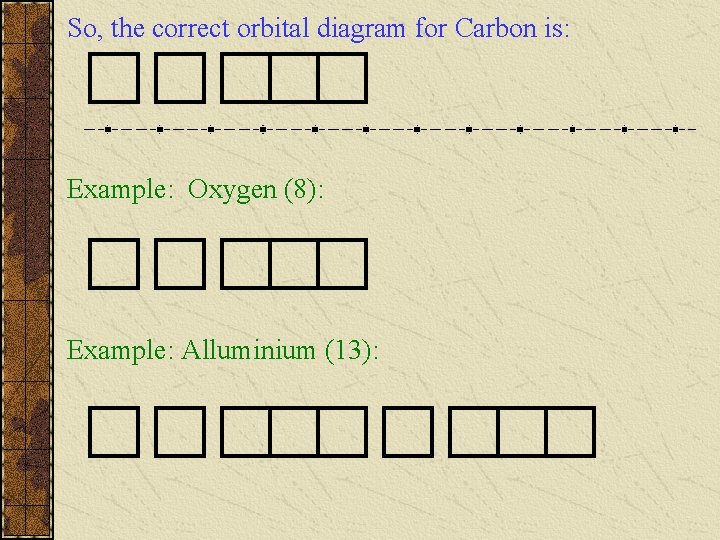
Now we reach Carbon with 6 electrons. What will be it’s electron configuration? But more importantly, what will be its Orbital Diagram? Either scenario gives: From this point onwards, we need some guidelines (rules).

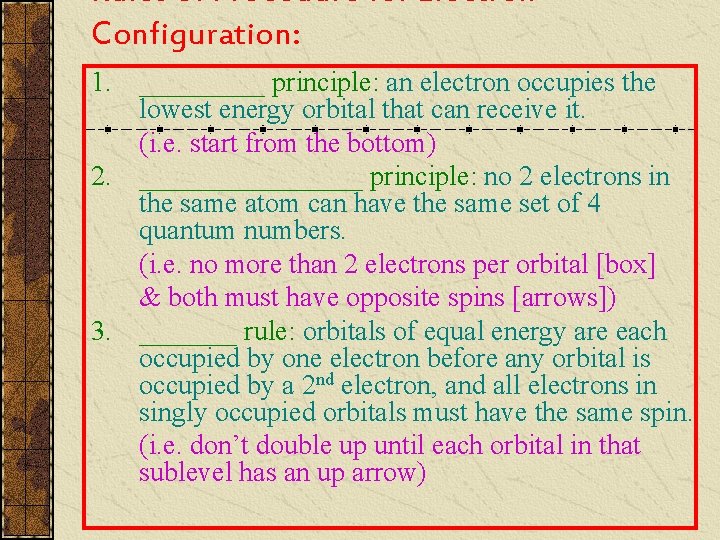
Rules of Procedure for Electron Configuration: 1. _____ principle: an electron occupies the lowest energy orbital that can receive it. (i. e. start from the bottom) 2. ________ principle: no 2 electrons in the same atom can have the same set of 4 quantum numbers. (i. e. no more than 2 electrons per orbital [box] & both must have opposite spins [arrows]) 3. _______ rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a 2 nd electron, and all electrons in singly occupied orbitals must have the same spin. (i. e. don’t double up until each orbital in that sublevel has an up arrow)

So, the correct orbital diagram for Carbon is: Example: Oxygen (8): Example: Alluminium (13):

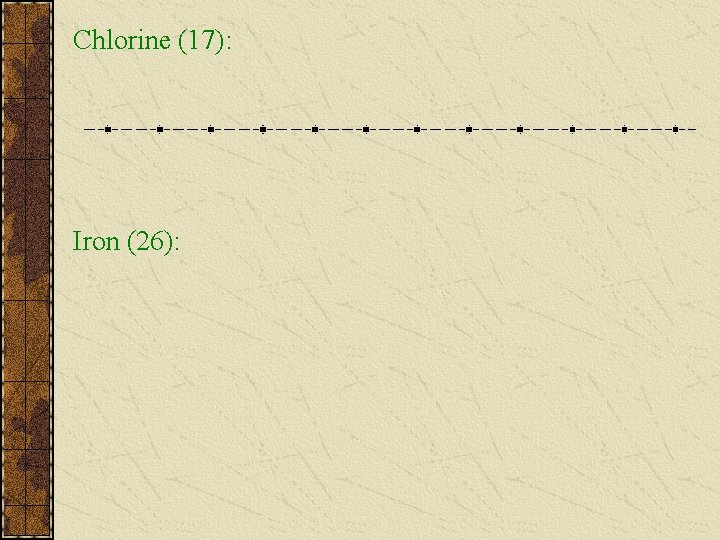
Chlorine (17): Iron (26):

Noble Gas Notation: A shorthand notation of the Electron Configuration. A Noble Gas is used because they are the only group of elements that are We replace as much of the Electron Configuration as possible with a Example: Fe (26)

Silver: Ag (47) Lead: Pb (82) Argon: Ar (18) Noble gases cannot use _____ for NG Notation.

Shell - is the highest occupied Principle Energy level (n = 1, 2, 3. . ), not sublevel (s, p, d, f) electrons: - are the electrons that occupy the _____ shell. : - are the rest of the electrons. Also known as the ________ e. g. : Na (11)

S (16) Fe (26) Br (35)

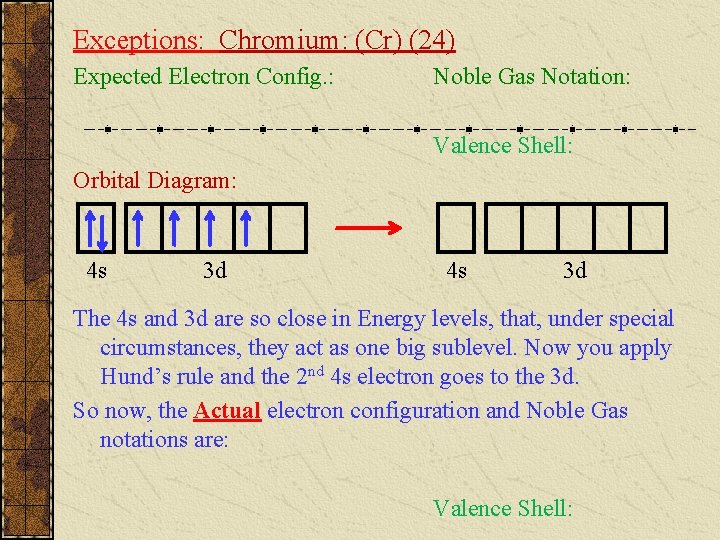
Exceptions: Chromium: (Cr) (24) Expected Electron Config. : Noble Gas Notation: Valence Shell: Orbital Diagram: 4 s 3 d The 4 s and 3 d are so close in Energy levels, that, under special circumstances, they act as one big sublevel. Now you apply Hund’s rule and the 2 nd 4 s electron goes to the 3 d. So now, the Actual electron configuration and Noble Gas notations are: Valence Shell:

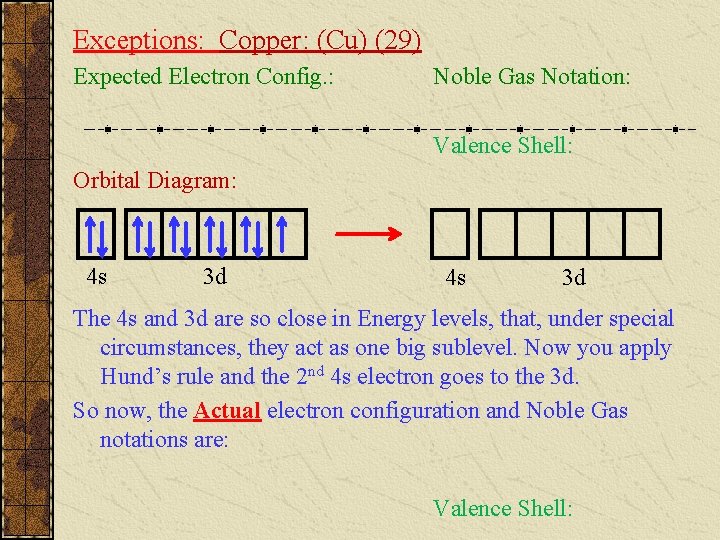
Exceptions: Copper: (Cu) (29) Expected Electron Config. : Noble Gas Notation: Valence Shell: Orbital Diagram: 4 s 3 d The 4 s and 3 d are so close in Energy levels, that, under special circumstances, they act as one big sublevel. Now you apply Hund’s rule and the 2 nd 4 s electron goes to the 3 d. So now, the Actual electron configuration and Noble Gas notations are: Valence Shell: