Paramagnetism and Diamagnetism l Atoms with unpaired electrons

- Slides: 17

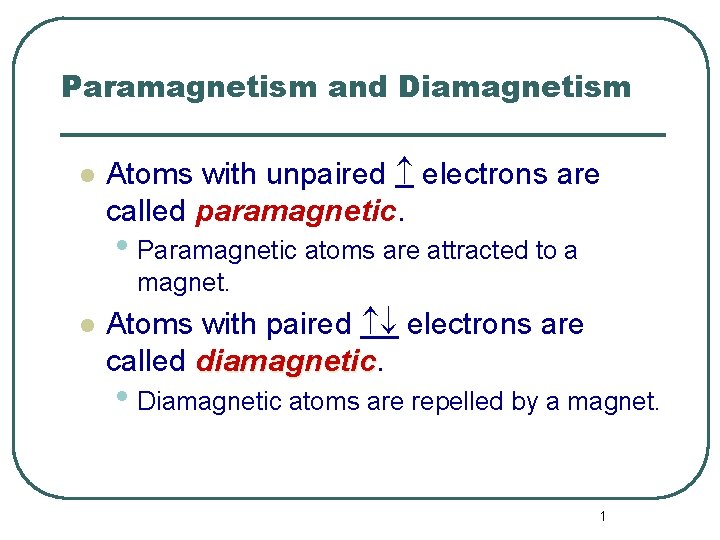
Paramagnetism and Diamagnetism l Atoms with unpaired electrons are called paramagnetic. • Paramagnetic atoms are attracted to a magnet. l Atoms with paired electrons are called diamagnetic • Diamagnetic atoms are repelled by a magnet. 1

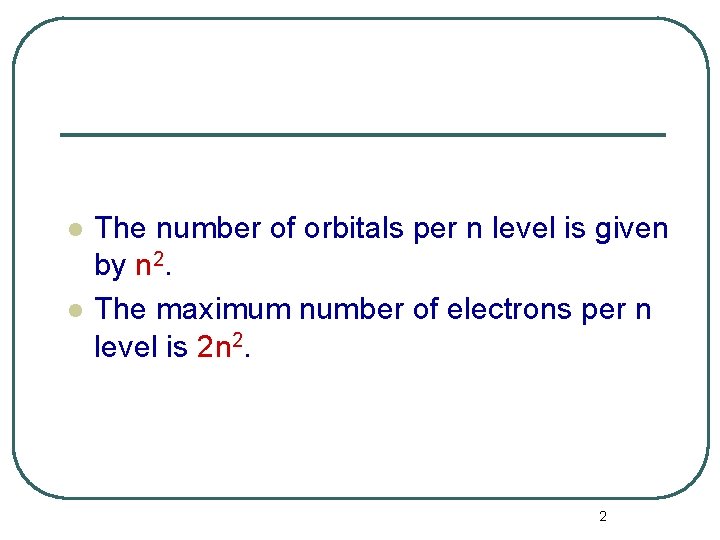
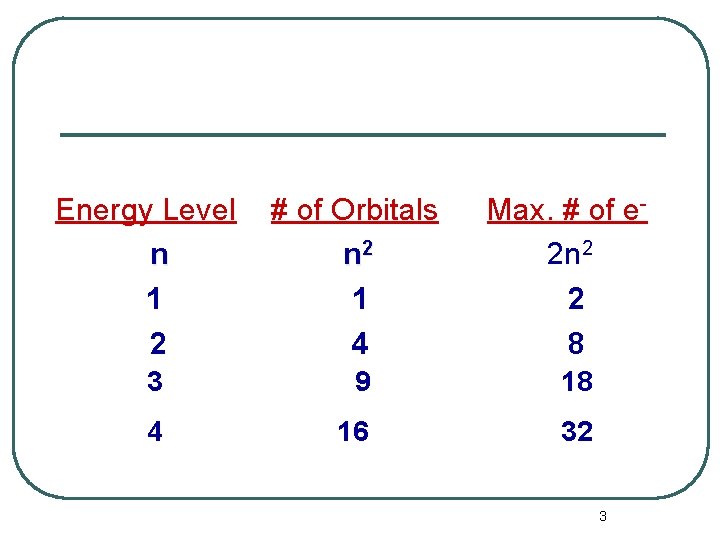
l l The number of orbitals per n level is given by n 2. The maximum number of electrons per n level is 2 n 2. 2

Energy Level n 1 2 # of Orbitals n 2 1 4 Max. # of e 2 n 2 2 8 3 9 18 4 16 32 3

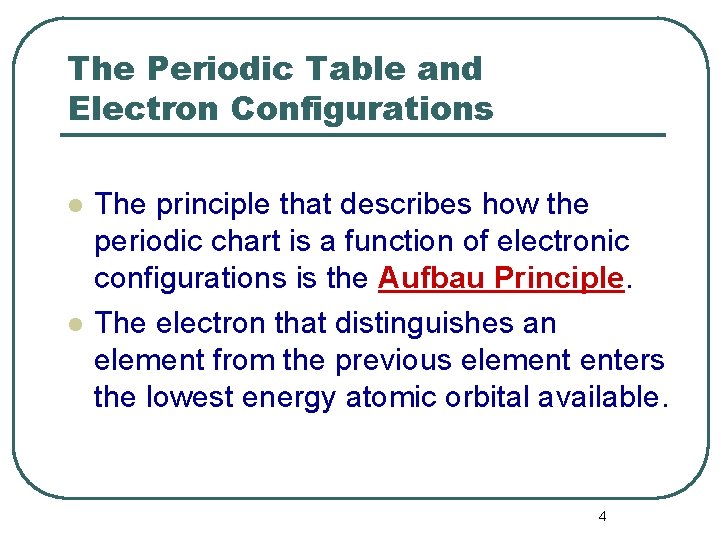
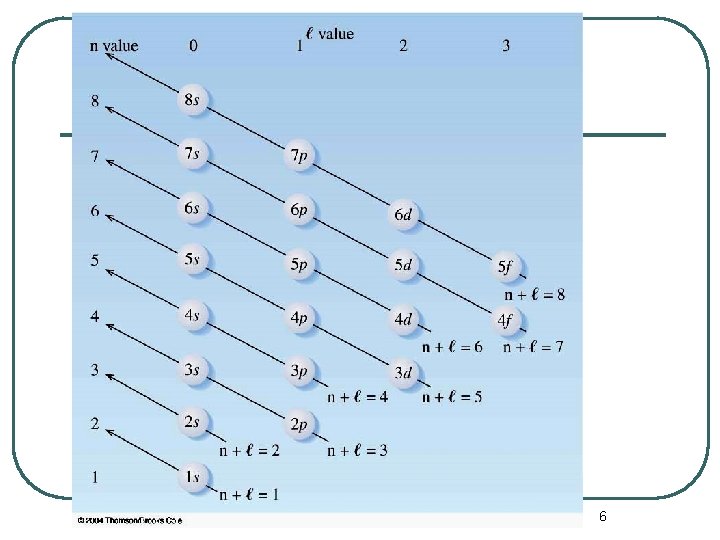
The Periodic Table and Electron Configurations l l The principle that describes how the periodic chart is a function of electronic configurations is the Aufbau Principle. The electron that distinguishes an element from the previous element enters the lowest energy atomic orbital available. 4

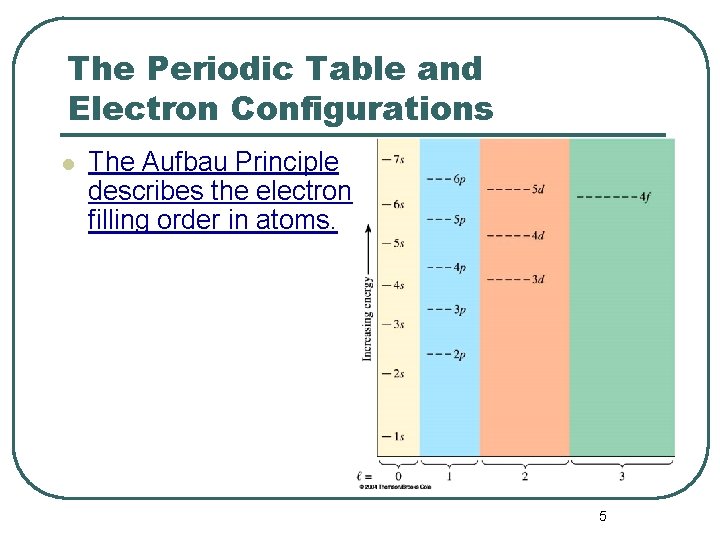
The Periodic Table and Electron Configurations l The Aufbau Principle describes the electron filling order in atoms. 5

6

Hund’s rule • Hund’s rule tells us that the electrons will fill the p orbitals by placing electrons in each orbital singly and with same spin until halffilled. Then the electrons will pair to finish the p orbitals. 7

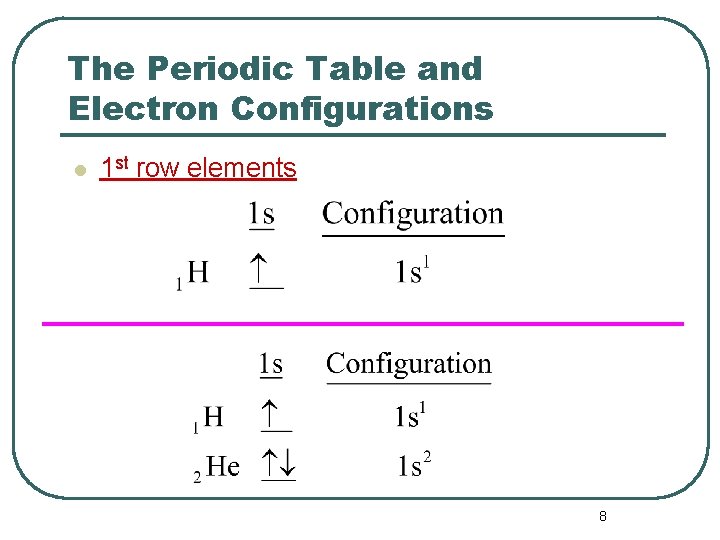
The Periodic Table and Electron Configurations l 1 st row elements 8

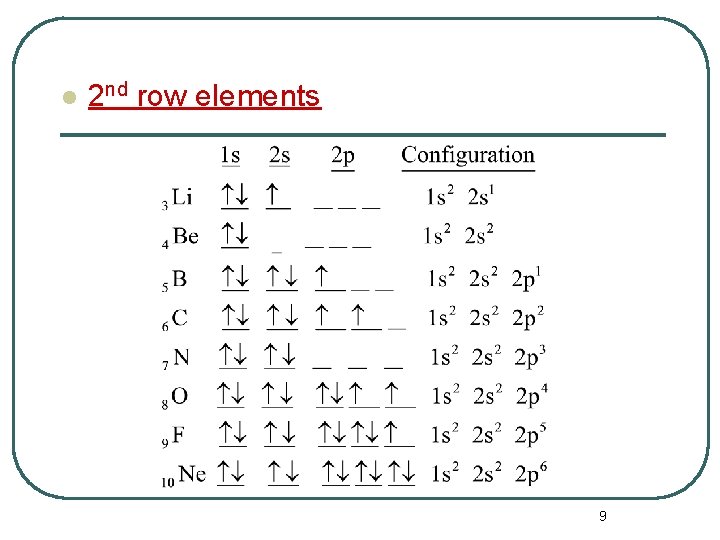
l 2 nd row elements 9

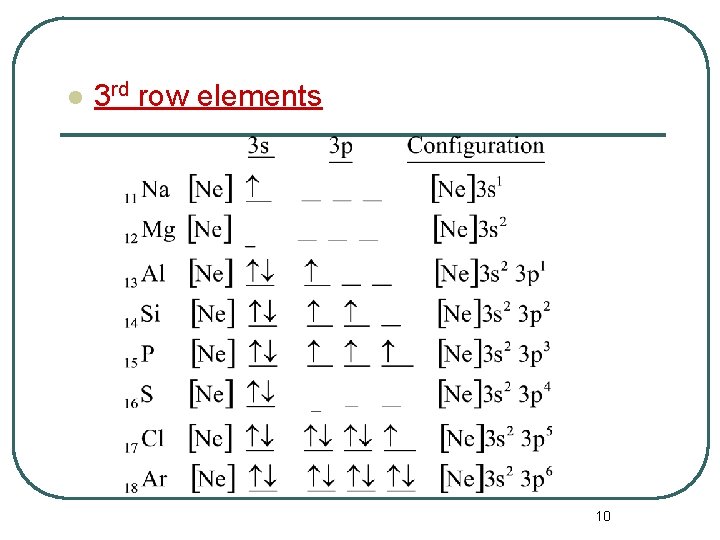
l 3 rd row elements 10

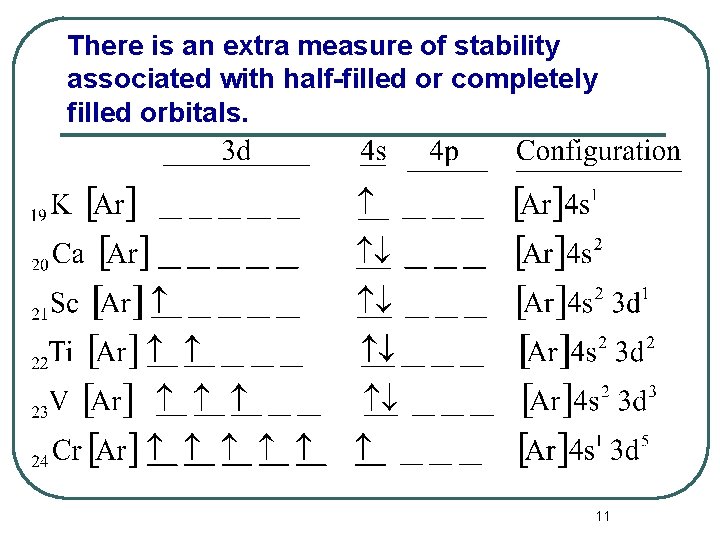
There is an extra measure of stability associated with half-filled or completely filled orbitals. 11

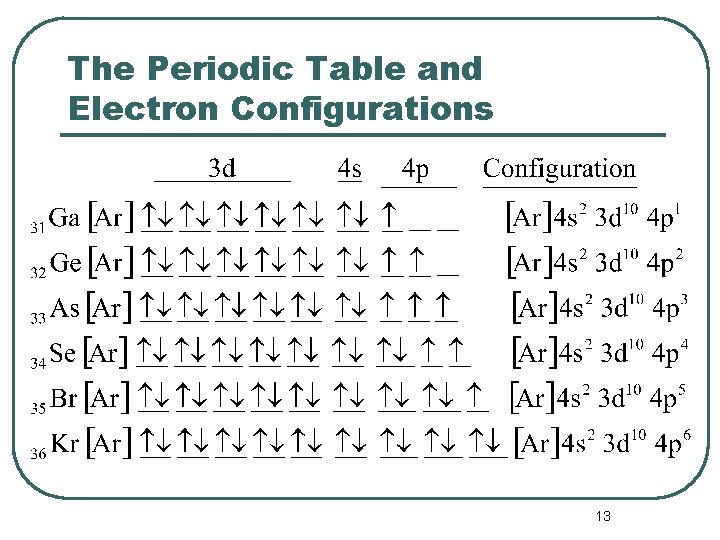
The Periodic Table and Electron Configurations 12

The Periodic Table and Electron Configurations 13

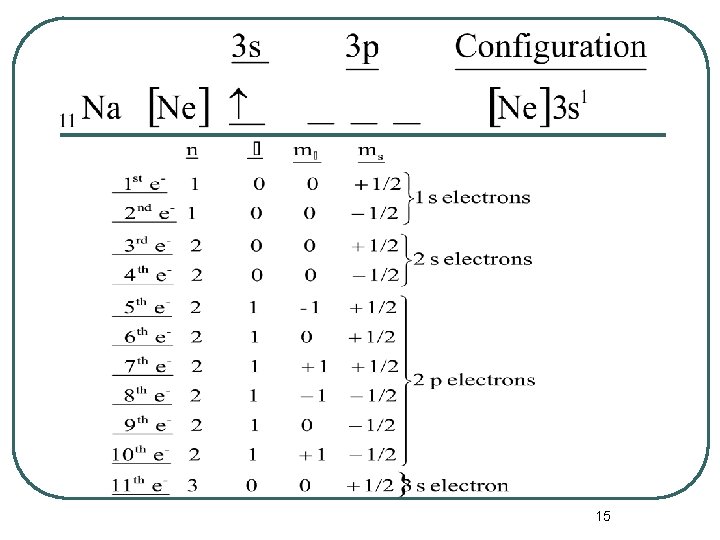
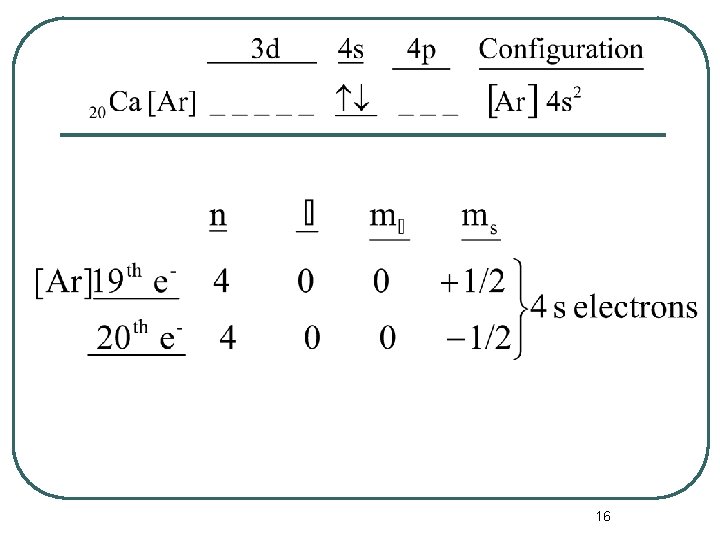
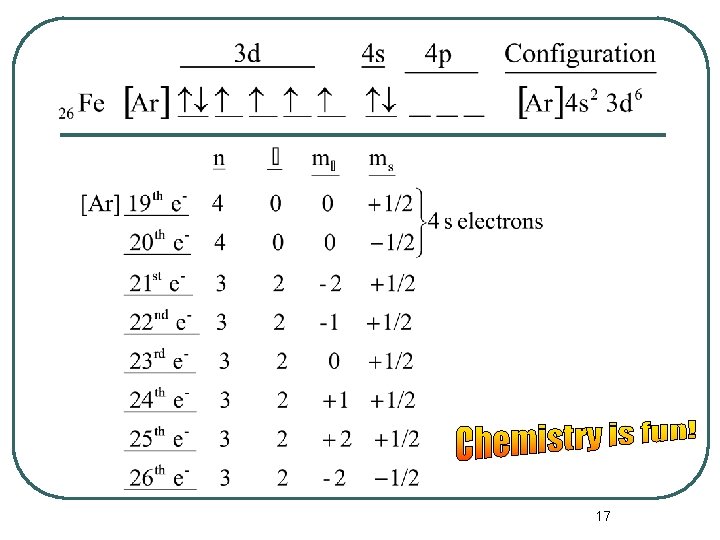
The Periodic Table and Electron Configurations l Now we can write a complete set of quantum numbers for all of the electrons in these three elements as examples. • Na • Ca • Fe 14

15

16

17
 Atoms with unpaired electrons are called diamagnetic.
Atoms with unpaired electrons are called diamagnetic. Paramagnetic unpaired electrons
Paramagnetic unpaired electrons Valence electrons of gallium
Valence electrons of gallium How many unpaired electrons does selenium have
How many unpaired electrons does selenium have Selenium unpaired electrons
Selenium unpaired electrons Paramagnetism
Paramagnetism Langevin paramagnetic equation
Langevin paramagnetic equation Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy The lowest allowable energy state of an atom
The lowest allowable energy state of an atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Periodic table regents
Periodic table regents Atoms with 4 valence electrons
Atoms with 4 valence electrons Proton neutron electron
Proton neutron electron Arrangement of electrons in atoms chapter 4 test
Arrangement of electrons in atoms chapter 4 test Chapter 5 electrons in atoms
Chapter 5 electrons in atoms Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons What is the oxidation number of lithium
What is the oxidation number of lithium