The Nature of Electrons Split Personality Electrons Electrons
















- Slides: 37

The Nature of Electrons Split Personality

Electrons • Electrons are negatively charged particles that move around the positive nucleus. • Why don’t they get “sucked in” by the positive charge? • The energy associated with their motion as they move around the nucleus!

Electrons Act As Both: • energy created when the e“wiggles” making waves as it moves • particles which can absorb little bundles of energy called photons

• De. Broglie called this Wave-Particle Duality since e- can move in a wavelike motion and act as particles capable of absorbing photons! e- orbit forms a wave around nucleus as it moves 1892 -1987

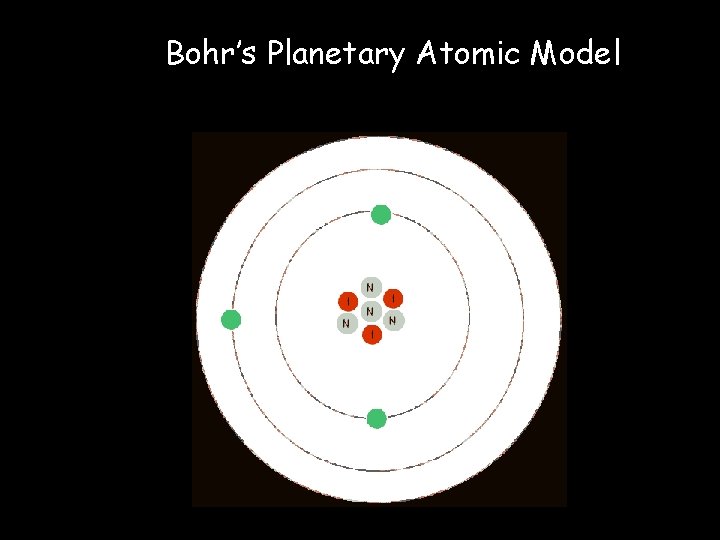
What about the PARTICLE aspect of the Wave-Particle Duality of an e-? • Remember: Rutherford’s Nuclear Atomic Model organized the nucleus. • Scientists began to wonder how the eoutside the nucleus are arranged. • Bohr came up with the Planetary Atomic Model which was: • e- move around the nucleus in circular orbits (paths) much like planets orbit the sun

Bohr’s Planetary Atomic Model

Heisenberg Who? Heisenberg’s Uncertainty Principle It is imposible to know the exact position and velocity of an electron as it moves around the nucleus. We can know it is in a certain energy level or moving to a higher or lower one, but not where it is EXACTLY or how fast it is moving.

There is a GOOD probability of finding an electron in an atomic orbital. An atomic orbital is a 3 -D region around the nucleus (it’s not an orbit – contrary to what Bohr thought).

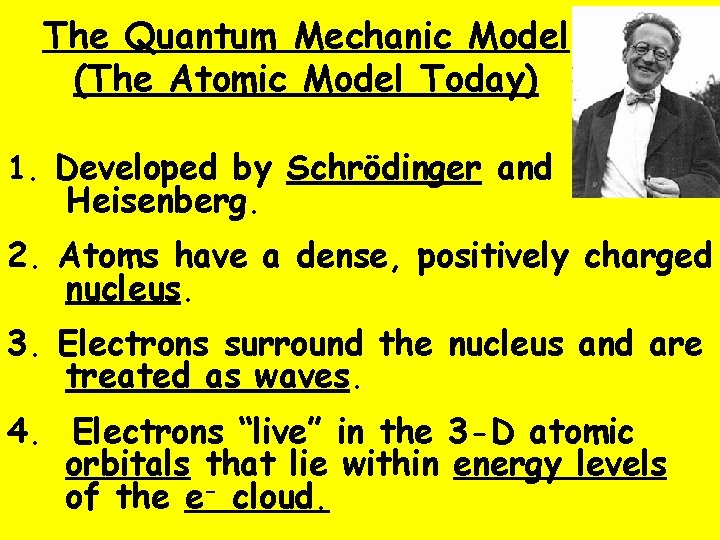
The Quantum Mechanic Model (The Atomic Model Today) 1. Developed by Schrödinger and Heisenberg. 2. Atoms have a dense, positively charged nucleus. 3. Electrons surround the nucleus and are treated as waves. 4. Electrons “live” in the 3 -D atomic orbitals that lie within energy levels of the e- cloud.

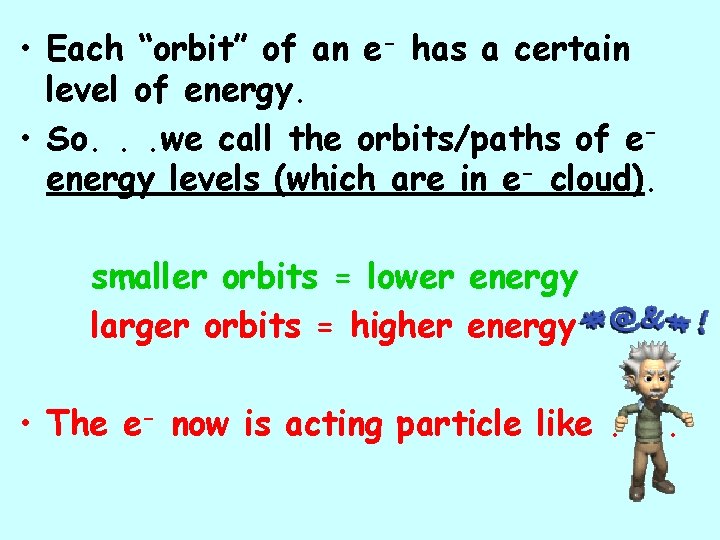
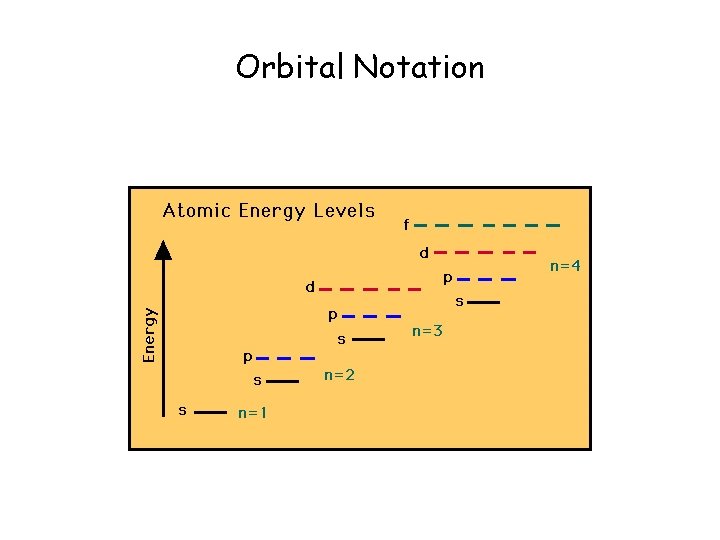
• Each “orbit” of an e- has a certain level of energy. • So. . . we call the orbits/paths of eenergy levels (which are in e- cloud). smaller orbits = lower energy larger orbits = higher energy • The e- now is acting particle like. . .

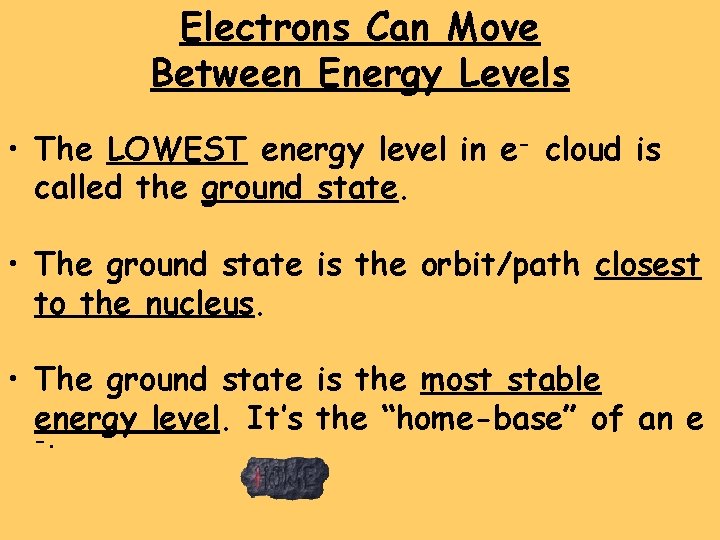
Electrons Can Move Between Energy Levels • The LOWEST energy level in e- cloud is called the ground state. • The ground state is the orbit/path closest to the nucleus. • The ground state is the most stable energy level. It’s the “home-base” of an e -.

nucleus ground state higher energy level

• Electrons are able to change energy levels (like climbing the rungs of a ladder). • An e- can change energy levels by absorbing little bundles of energy called photons

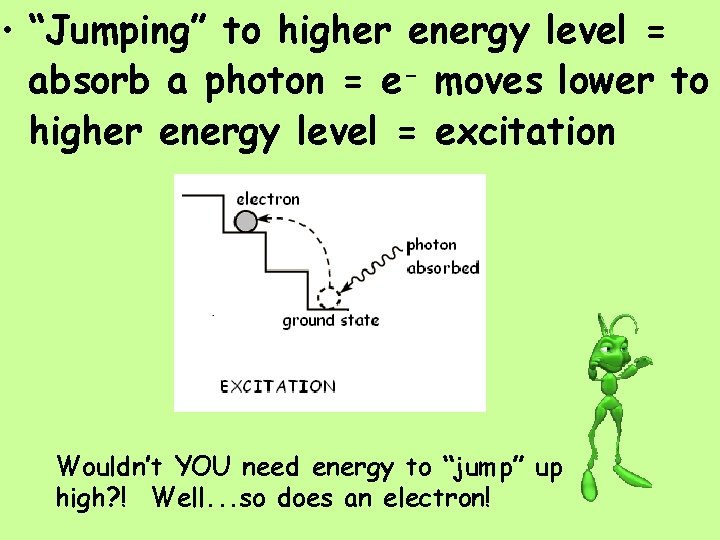
• “Jumping” to higher energy level = absorb a photon = e- moves lower to higher energy level = excitation Wouldn’t YOU need energy to “jump” up high? ! Well. . . so does an electron!

• Once an electron is “excited” and in a higher energy level, it can’t stay there because it’s not the “home” orbit.

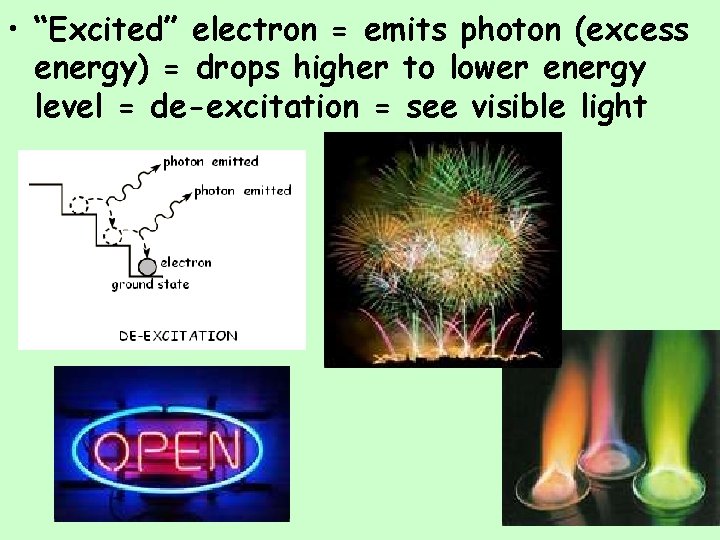
• “Excited” electron = emits photon (excess energy) = drops higher to lower energy level = de-excitation = see visible light

Have you ever noticed. . . • Neon lights give off a red-orange color? • Some fireworks are purple? Others white? And some all different colors?

Have you ever wondered. . . • How we know what elements are in the sun and stars? • How we know what elements are in comets? This is all because of the excitation and deexcitation of e- as they absorb and release photons (energy)!

• How did scientists figure all this out? • Scientists noticed when certain elements are burned, they emit visible colored light! Why? • e- absorbed the heat energy (photons) from the flame. • The heat energy causes the e- to excite. • The e- then emits a photon during de -excitation. • The photon emitted corresponds with the same energy as a wavelength of a certain color.


Schrödinger figured this out with his wave equation (below). Let’s Practice! Just kidding!

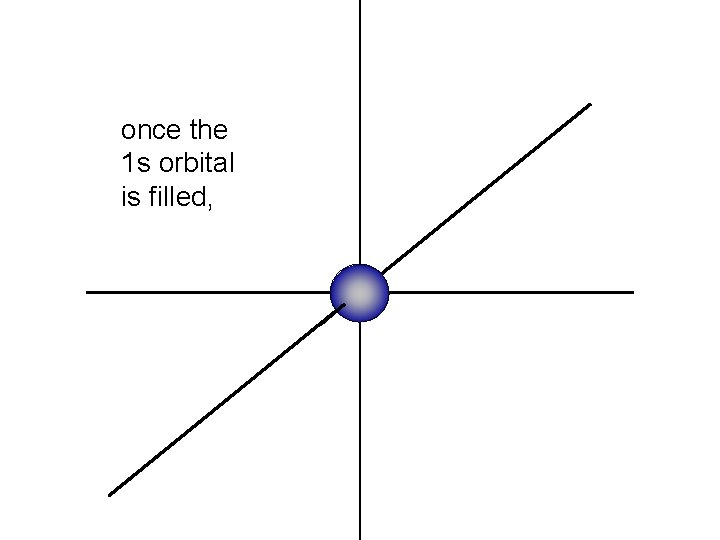
once the 1 s orbital is filled,

the 2 s orbital begins to fill around the 1 s orbital

once the 2 s orbital is filled,

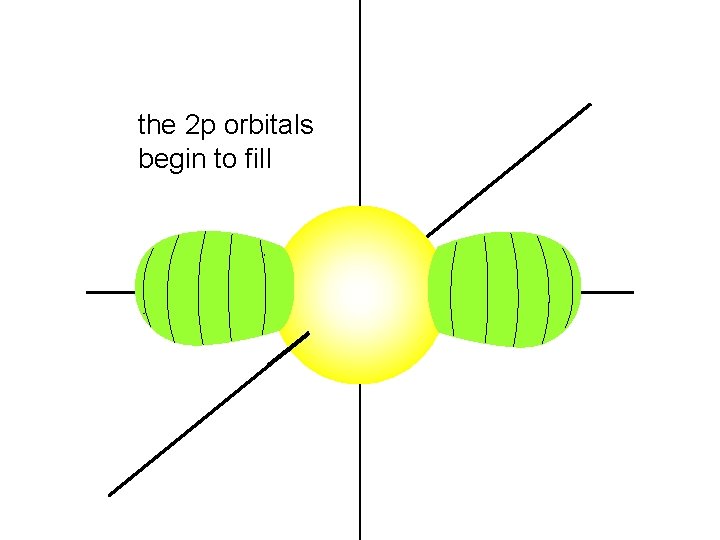
the 2 p orbitals begin to fill

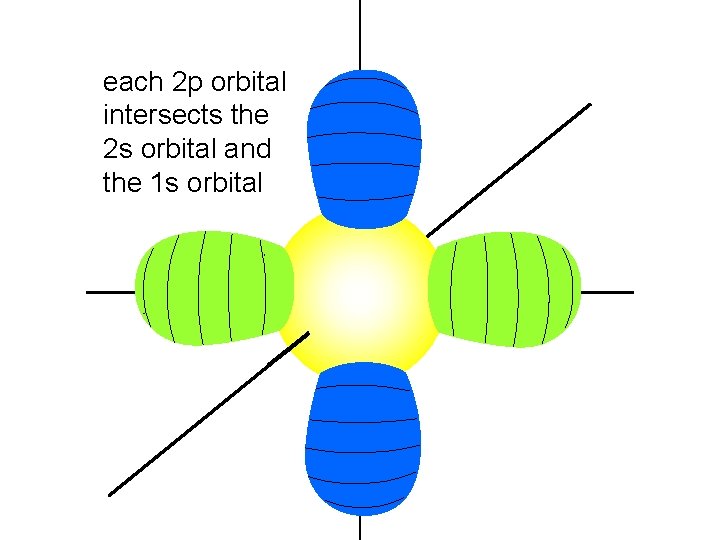
each 2 p orbital intersects the 2 s orbital and the 1 s orbital

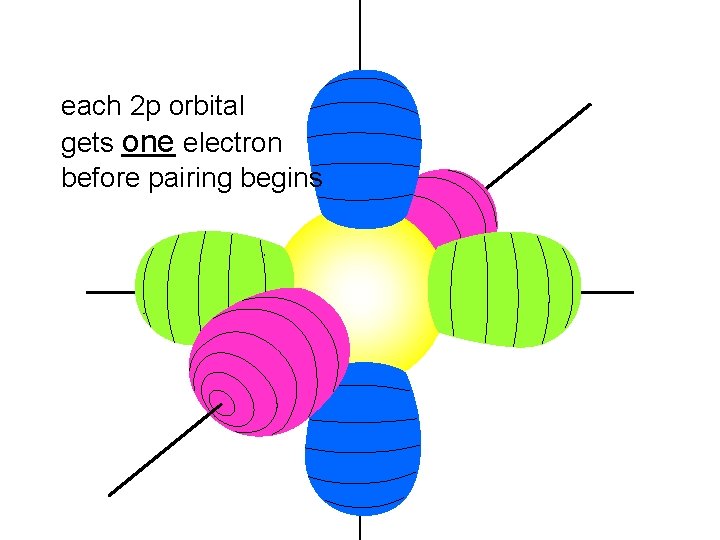
each 2 p orbital gets one electron before pairing begins

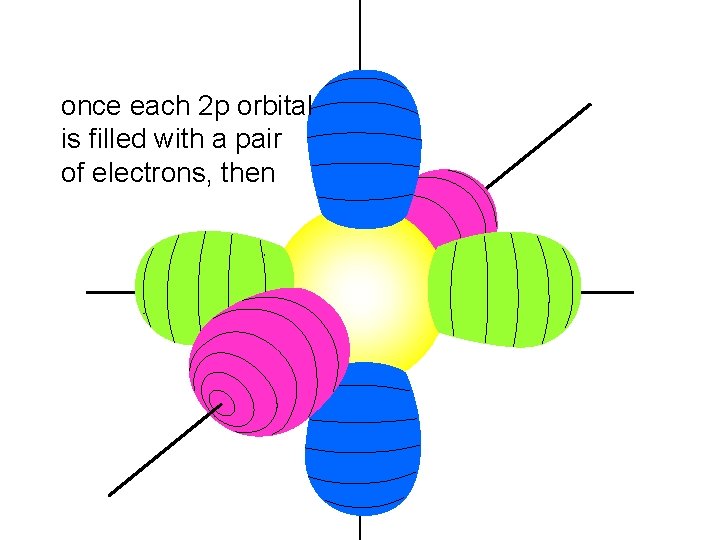
once each 2 p orbital is filled with a pair of electrons, then

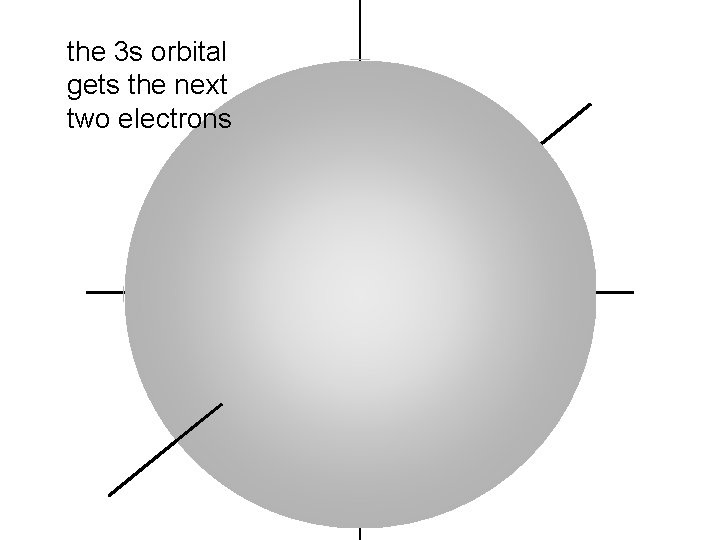
the 3 s orbital gets the next two electrons

the 3 s electrons have a higher energy than 1 s, 2 s, or 2 p electrons,

so 3 s electrons are generally found further from the nucleus than 1 s, 2 s, or 2 p electrons

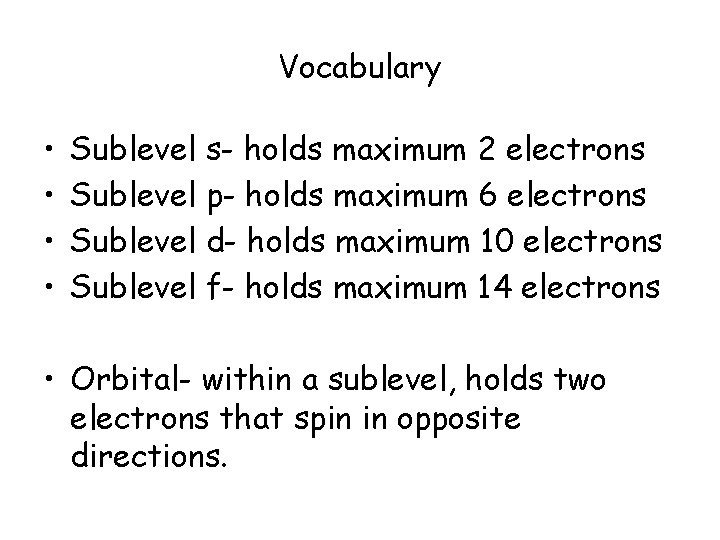
Vocabulary • Quantum- the minimum amount of energy that can be gained or lost by an atom • Quantum Number (n)- assigned to the energy level (1 -7). • Sublevel- the shaped cloud the electrons enter. (s, p, d, f)

Vocabulary • • Sublevel s- holds maximum 2 electrons Sublevel p- holds maximum 6 electrons Sublevel d- holds maximum 10 electrons Sublevel f- holds maximum 14 electrons • Orbital- within a sublevel, holds two electrons that spin in opposite directions.

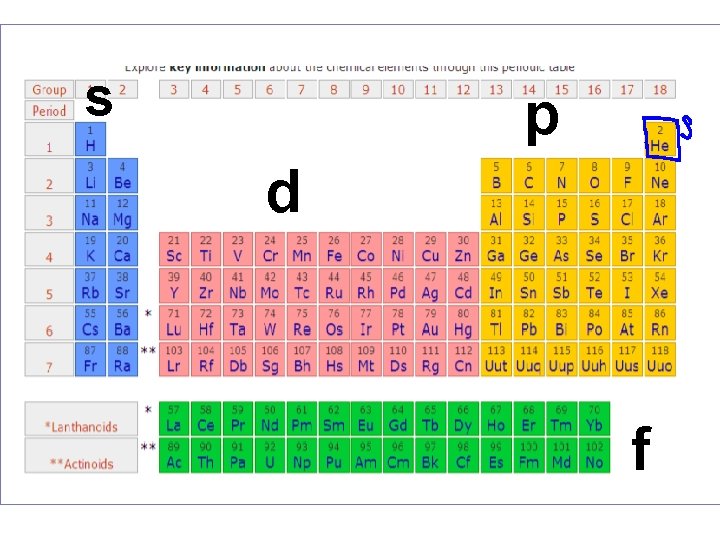
s p d f

Three Principles • Three Principles govern the filling of orbitals by electrons – AUFBAU- electrons enter orbitals of lowest energy first – PAULI EXCLUSION PRINCIPLE- an atomic orbital contains a maximum of two electrons – HUND’S RULE- when electrons occupy orbitals of equal energy, one electron enters each orbital until all the orbitals contain one electron which spins parallel

Orbital Notation

Orbital Notation
 Split and split-less injectors
Split and split-less injectors Twenty rules for writing detective stories
Twenty rules for writing detective stories Define personality psychology
Define personality psychology Reciprocal determinism definition psychology
Reciprocal determinism definition psychology Personality in consumer behavior
Personality in consumer behavior Nature and nature's law lay hid in night
Nature and nature's law lay hid in night Nature nature controversy
Nature nature controversy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sơ đồ cơ thể người
Sơ đồ cơ thể người ưu thế lai là gì
ưu thế lai là gì Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Tư thế ngồi viết
Tư thế ngồi viết Cái miệng nó xinh thế
Cái miệng nó xinh thế Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Tư thế ngồi viết
Tư thế ngồi viết Chó sói
Chó sói Thẻ vin
Thẻ vin Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Ng-html
Ng-html V. c c
V. c c Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau 101012 bằng
101012 bằng Lời thề hippocrates
Lời thề hippocrates Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công của trọng lực
Công của trọng lực Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dot
Dot Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Phản ứng thế ankan
Phản ứng thế ankan