Chapter 7 Electronic Structure of Atoms Considering only






![The element with the electronic configuration [Ar]4 s 13 d 5 is: 1. 2. The element with the electronic configuration [Ar]4 s 13 d 5 is: 1. 2.](https://slidetodoc.com/presentation_image/541e099e28360602c98e4564f3b84c7d/image-36.jpg)


- Slides: 41

Chapter 7 Electronic Structure of Atoms

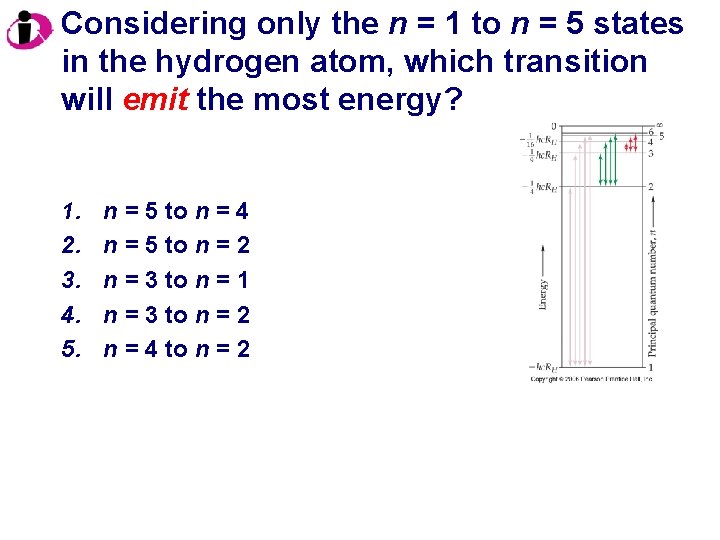
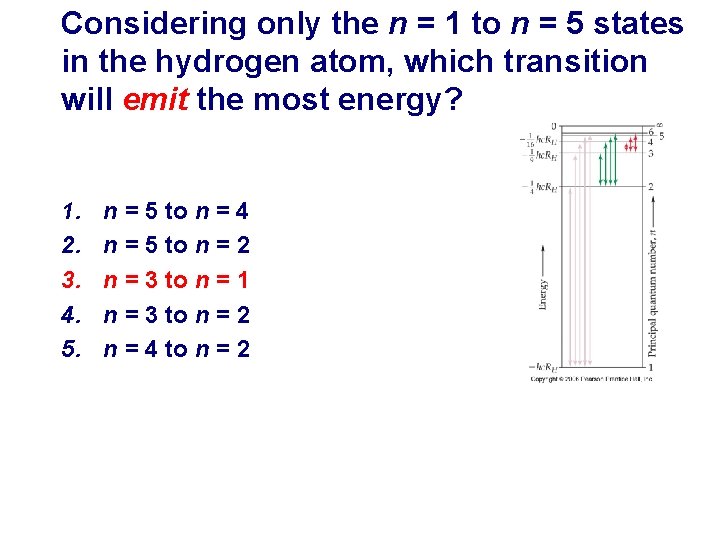
Considering only the n = 1 to n = 5 states in the hydrogen atom, which transition will emit the most energy? 1. 2. 3. 4. 5. n = 5 to n = 4 n = 5 to n = 2 n = 3 to n = 1 n = 3 to n = 2 n = 4 to n = 2

Considering only the n = 1 to n = 5 states in the hydrogen atom, which transition will emit the most energy? 1. 2. 3. 4. 5. n = 5 to n = 4 n = 5 to n = 2 n = 3 to n = 1 n = 3 to n = 2 n = 4 to n = 2

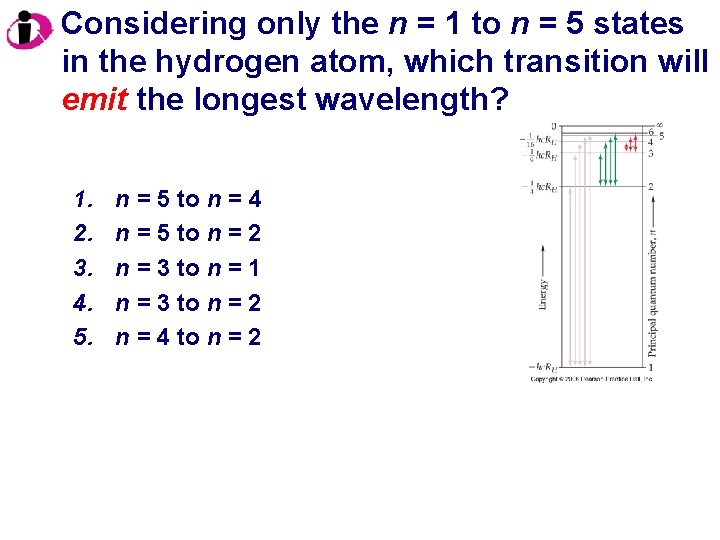
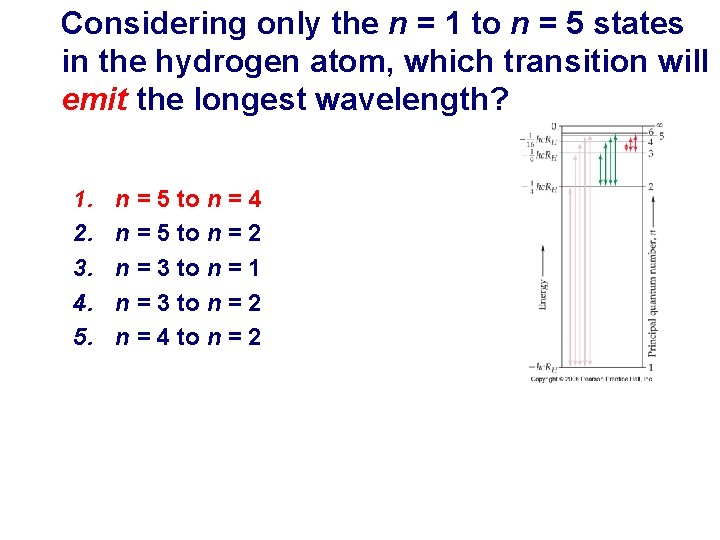
Considering only the n = 1 to n = 5 states in the hydrogen atom, which transition will emit the longest wavelength? 1. 2. 3. 4. 5. n = 5 to n = 4 n = 5 to n = 2 n = 3 to n = 1 n = 3 to n = 2 n = 4 to n = 2

Considering only the n = 1 to n = 5 states in the hydrogen atom, which transition will emit the longest wavelength? 1. 2. 3. 4. 5. n = 5 to n = 4 n = 5 to n = 2 n = 3 to n = 1 n = 3 to n = 2 n = 4 to n = 2

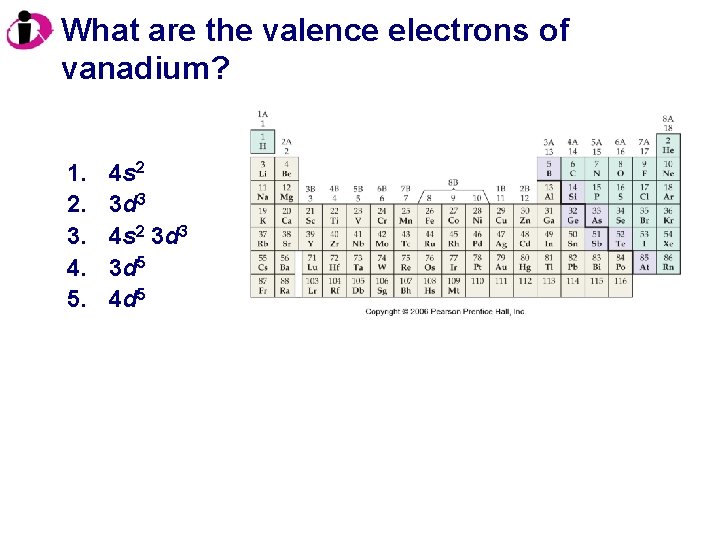
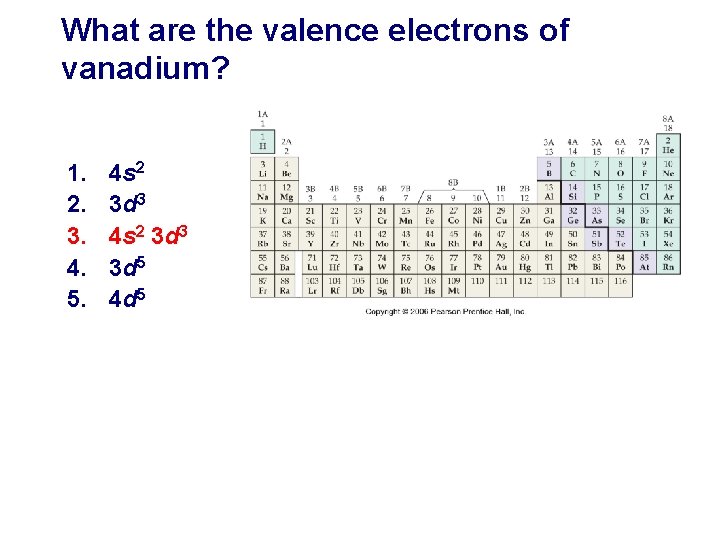
What are the valence electrons of vanadium? 1. 2. 3. 4. 5. 4 s 2 3 d 3 3 d 5 4 d 5

What are the valence electrons of vanadium? 1. 2. 3. 4. 5. 4 s 2 3 d 3 3 d 5 4 d 5

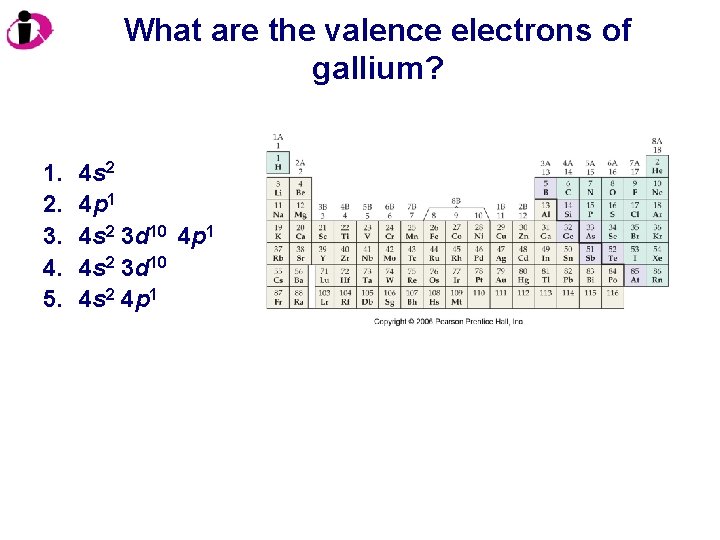
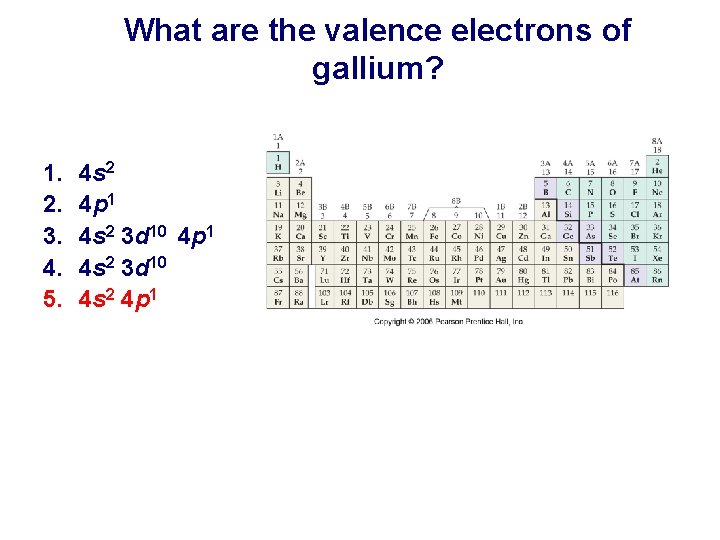
What are the valence electrons of gallium? 1. 2. 3. 4. 5. 4 s 2 4 p 1 4 s 2 3 d 10 4 s 2 4 p 1

What are the valence electrons of gallium? 1. 2. 3. 4. 5. 4 s 2 4 p 1 4 s 2 3 d 10 4 s 2 4 p 1

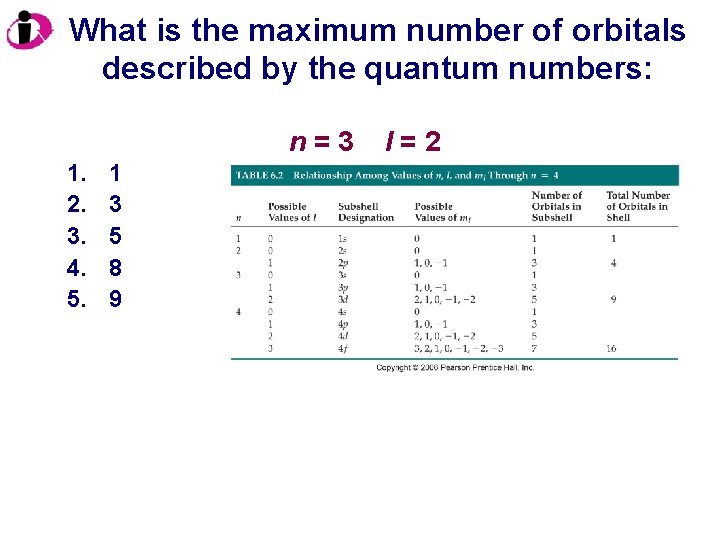
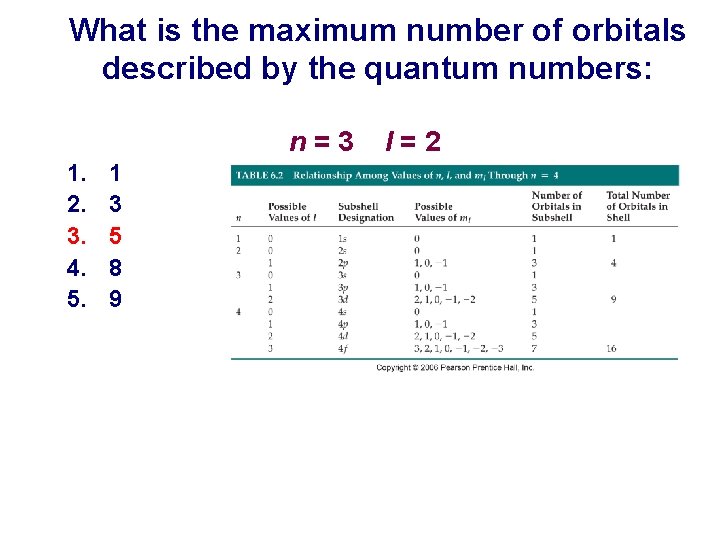
What is the maximum number of orbitals described by the quantum numbers: n=3 1. 2. 3. 4. 5. 1 3 5 8 9 l=2

What is the maximum number of orbitals described by the quantum numbers: n=3 1. 2. 3. 4. 5. 1 3 5 8 9 l=2

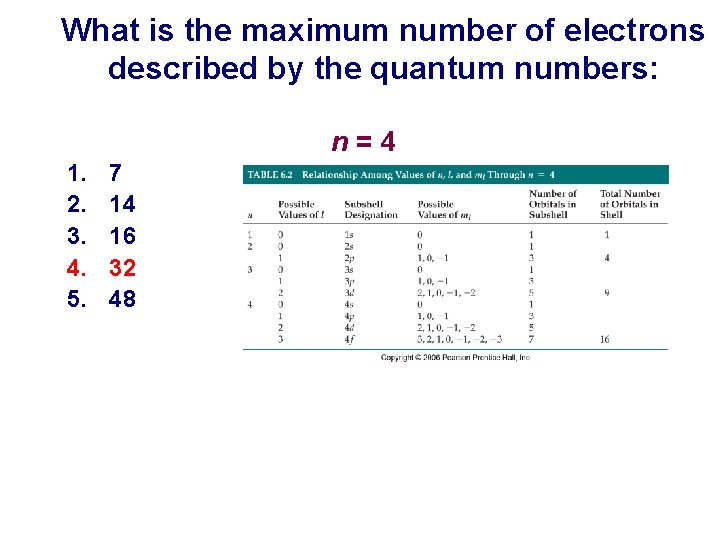
What is the maximum number of electrons described by the quantum numbers: n=4 1. 2. 3. 4. 5. 7 14 16 32 48

What is the maximum number of electrons described by the quantum numbers: n=4 1. 2. 3. 4. 5. 7 14 16 32 48

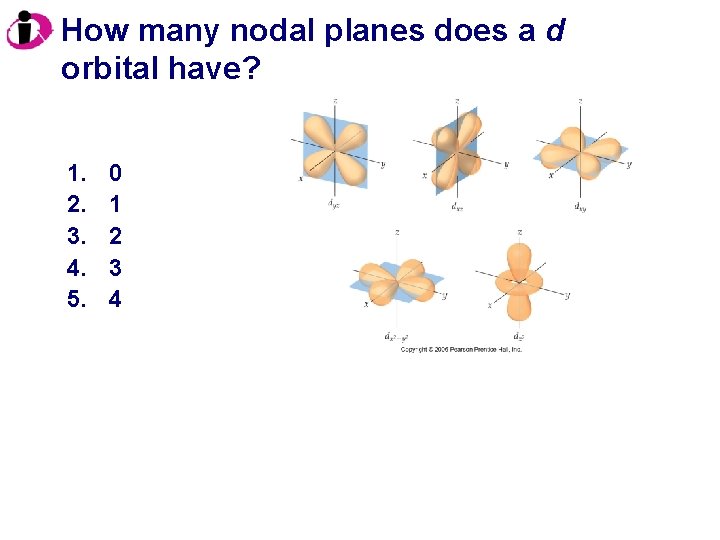
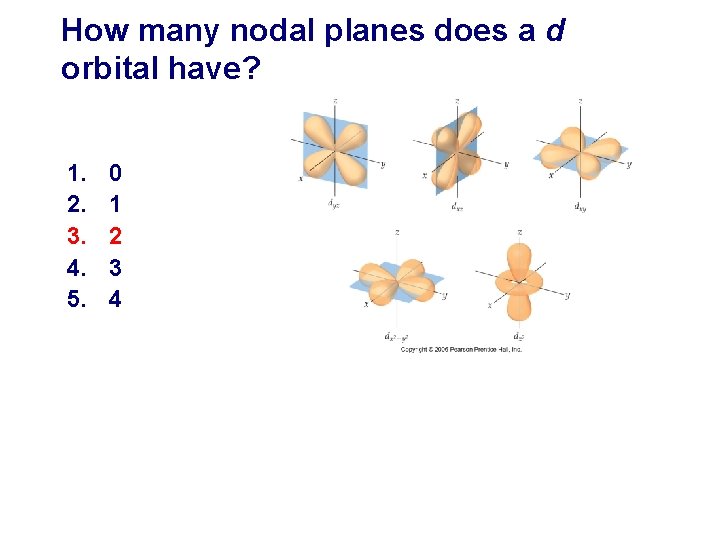
How many nodal planes does a d orbital have? 1. 2. 3. 4. 5. 0 1 2 3 4

How many nodal planes does a d orbital have? 1. 2. 3. 4. 5. 0 1 2 3 4

How many unpaired electrons does selenium have? 1. 2. 3. 4. 5. 0 2 4 6 8

How many unpaired electrons does selenium have? 1. 2. 3. 4. 5. 0 2 4 6 8

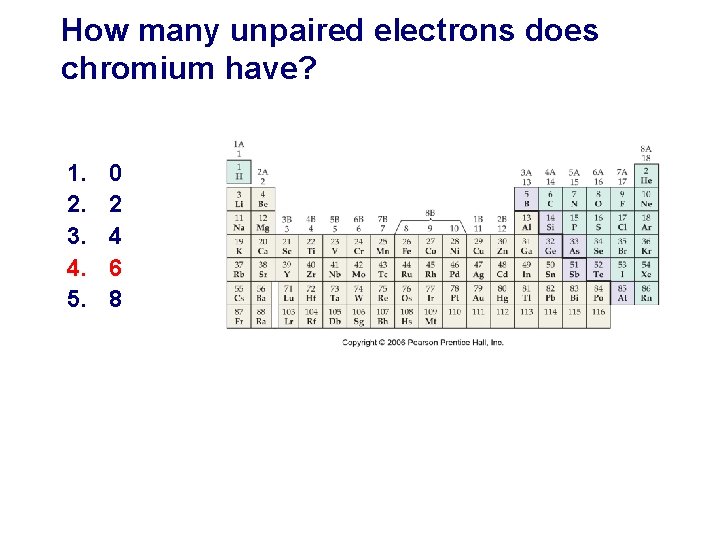
How many unpaired electrons does chromium have? 1. 2. 3. 4. 5. 0 2 4 6 8

How many unpaired electrons does chromium have? 1. 2. 3. 4. 5. 0 2 4 6 8

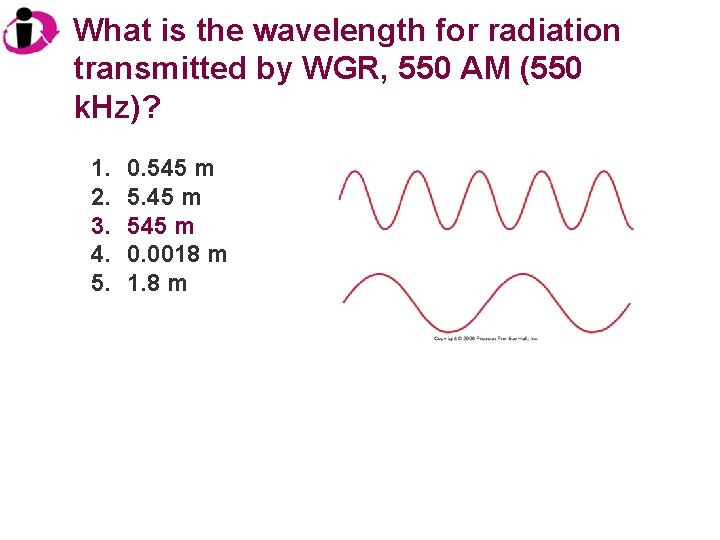
What is the wavelength for radiation transmitted by WGR, 550 AM (550 k. Hz)? 1. 2. 3. 4. 5. 0. 545 m 0. 0018 m 1. 8 m

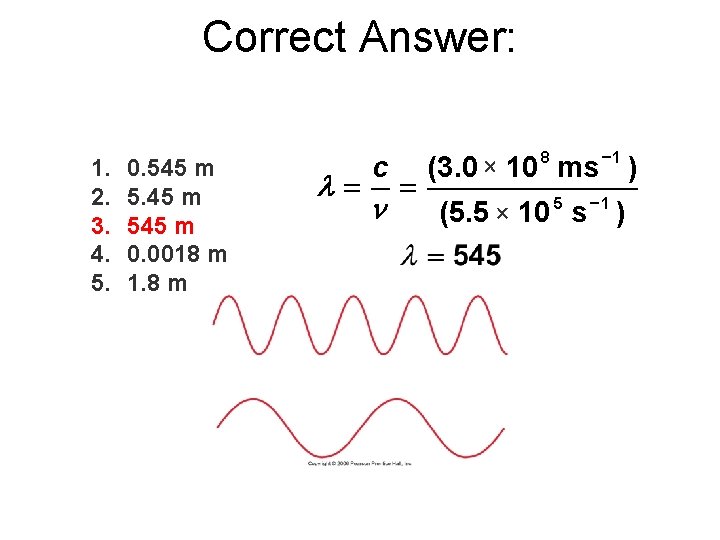
Correct Answer: 1. 2. 3. 4. 5. 0. 545 m 0. 0018 m 1. 8 m l= c n = (3. 0 1 8 10 ms ) (5. 5 5 1 10 s )

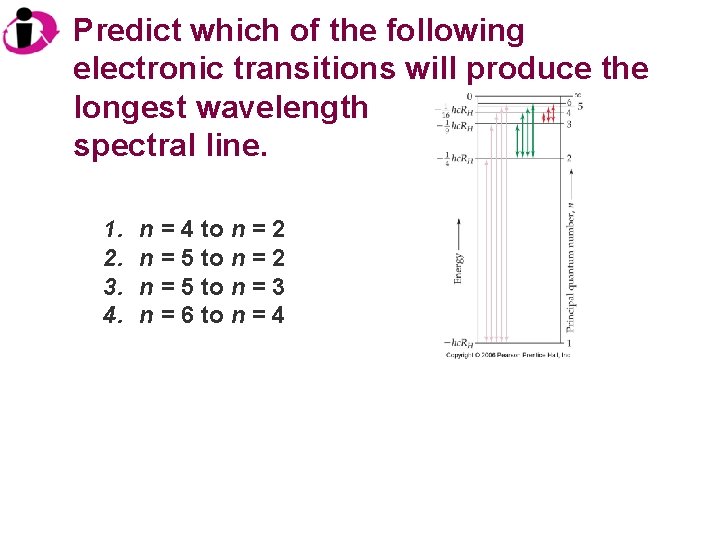
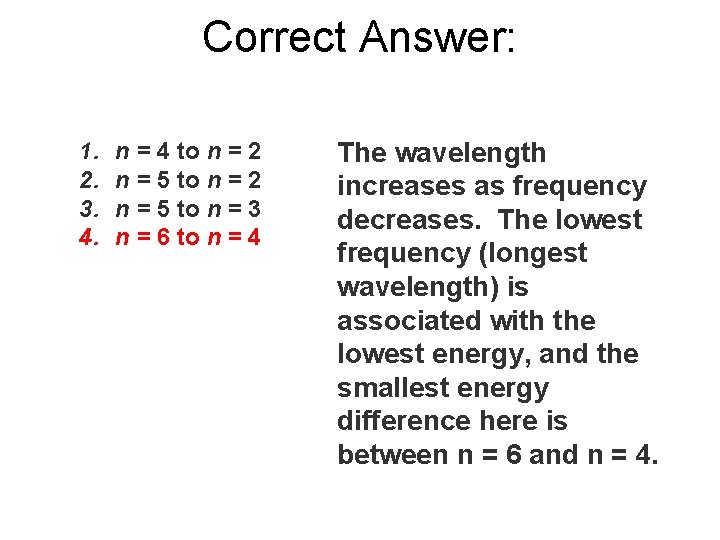
Predict which of the following electronic transitions will produce the longest wavelength spectral line. 1. 2. 3. 4. n = 4 to n = 2 n = 5 to n = 3 n = 6 to n = 4

Correct Answer: 1. 2. 3. 4. n = 4 to n = 2 n = 5 to n = 3 n = 6 to n = 4 The wavelength increases as frequency decreases. The lowest frequency (longest wavelength) is associated with the lowest energy, and the smallest energy difference here is between n = 6 and n = 4.

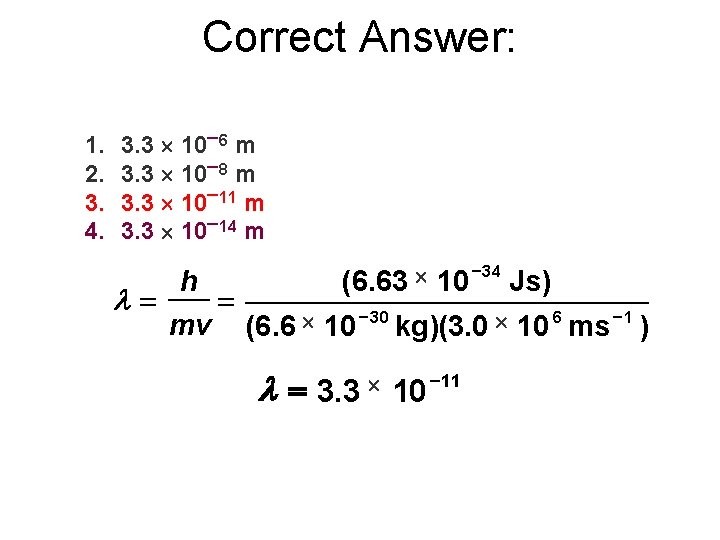
What is the wavelength of a helium 4 ion moving in a cyclotron at 1. 0% the speed of light? Mass of helium 4 ion = 6. 6 10 27 g. 1. 2. 3. 4. 3. 3 10 6 m 3. 3 10 8 m 3. 3 10 11 m 3. 3 10 14 m

Correct Answer: 1. 2. 3. 4. 3. 3 10 6 m 3. 3 10 8 m 3. 3 10 11 m 3. 3 10 14 m l= h mv = (6. 63 (6. 6 10 30 10 34 kg)(3. 0 l = 3. 3 10 11 Js) 6 1 10 ms )

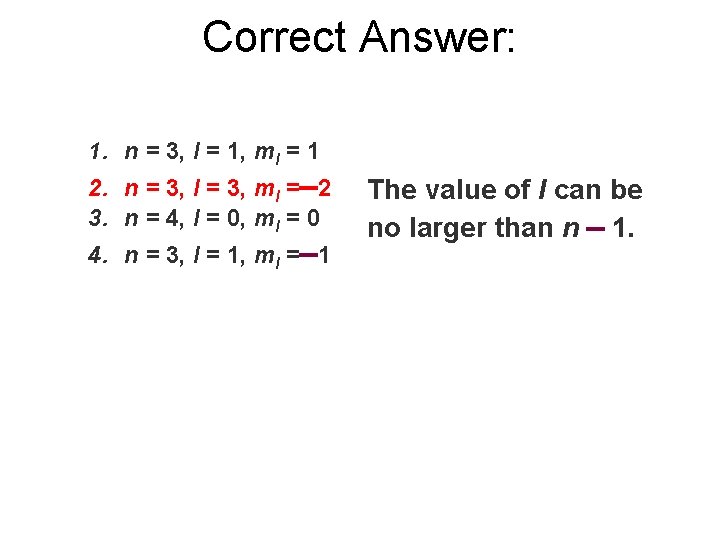
Which of the following is not an allowed set of quantum numbers? 1. n = 3, l = 1, ml = 1 2. n = 3, l = 3, ml = 2 3. n = 4, l = 0, ml = 0 4. n = 3, l = 1, ml = 1

Correct Answer: 1. n = 3, l = 1, ml = 1 2. n = 3, l = 3, ml = 2 3. n = 4, l = 0, ml = 0 4. n = 3, l = 1, ml = 1 The value of l can be no larger than n 1.

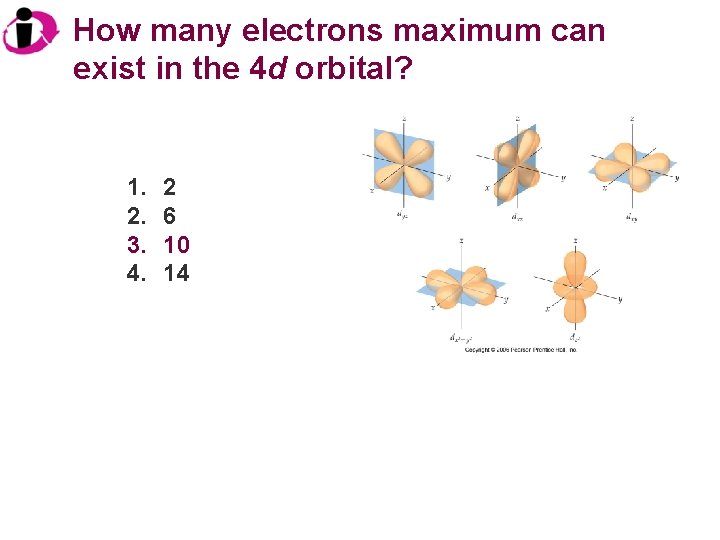
How many electrons maximum can exist in the 4 d orbital? 1. 2. 3. 4. 2 6 10 14

Correct Answer: 1. 2. 3. 4. 2 6 10 14 There are five 4 d orbitals, each of which can contain up to 2 electrons each for a total of 10 maximum.

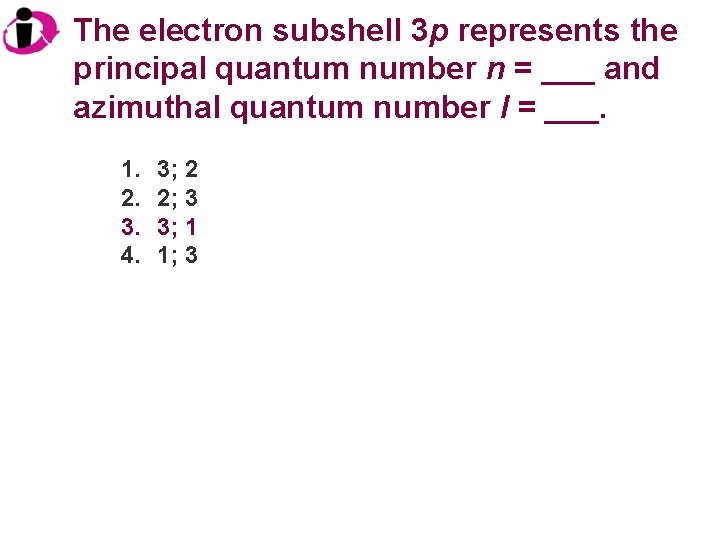
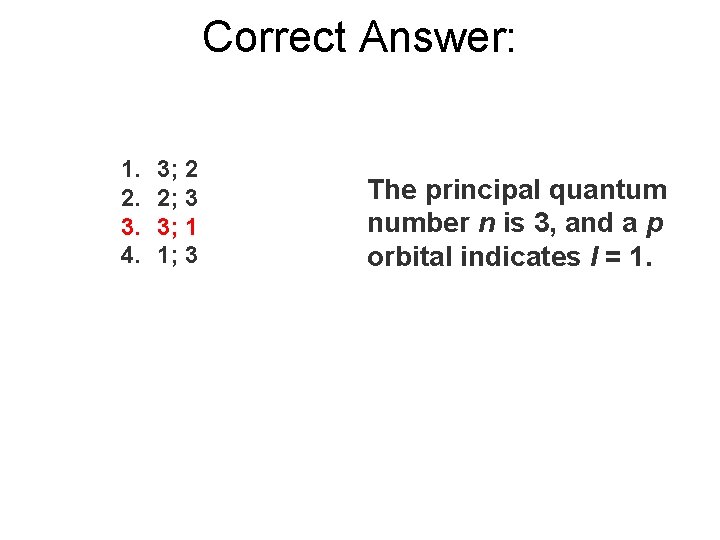
The electron subshell 3 p represents the principal quantum number n = ___ and azimuthal quantum number l = ___. 1. 2. 3. 4. 3; 2 2; 3 3; 1 1; 3

Correct Answer: 1. 2. 3. 4. 3; 2 2; 3 3; 1 1; 3 The principal quantum number n is 3, and a p orbital indicates l = 1.

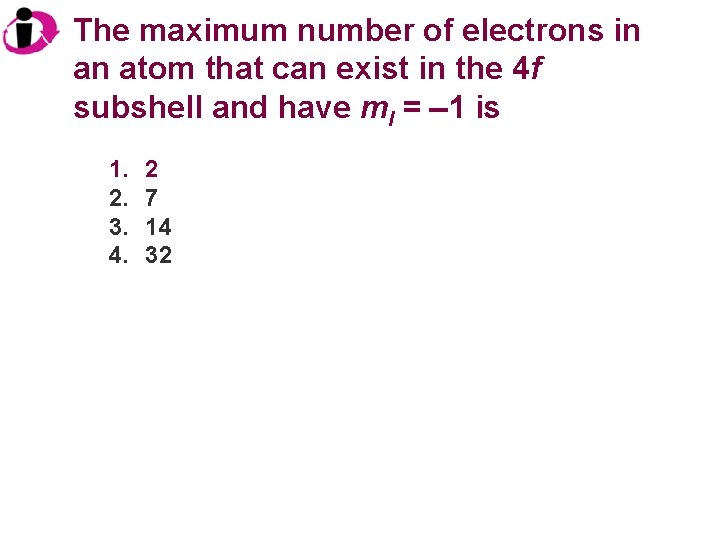
The maximum number of electrons in an atom that can exist in the 4 f subshell and have ml = 1 is 1. 2. 3. 4. 2 7 14 32

Correct Answer: 1. 2. 3. 4. 2 7 14 32 For any value of ml, the maximum number of electrons is 2.

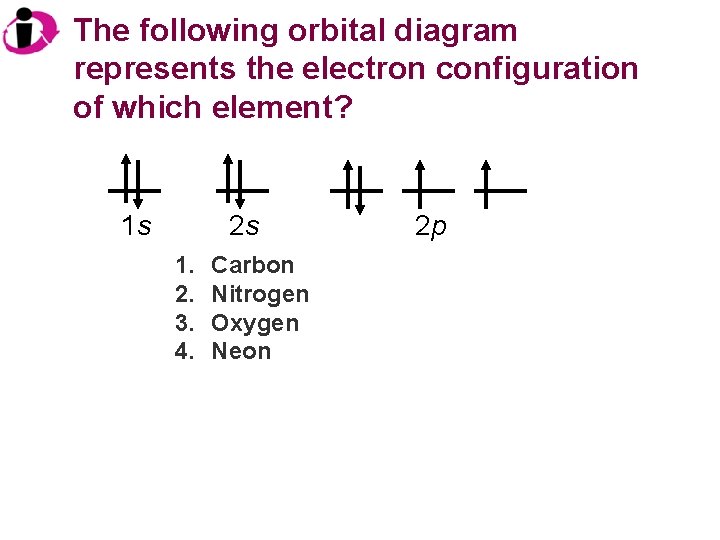
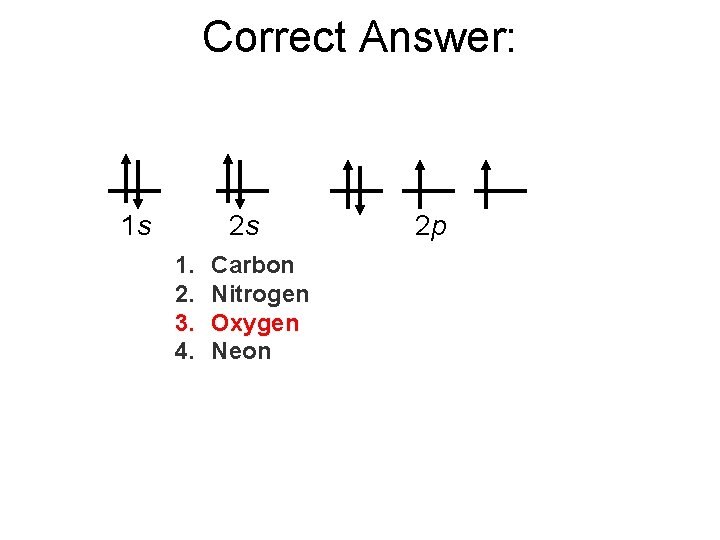
The following orbital diagram represents the electron configuration of which element? 1 s 2 s 1. 2. 3. 4. Carbon Nitrogen Oxygen Neon 2 p

Correct Answer: 1 s 2 s 1. 2. 3. 4. Carbon Nitrogen Oxygen Neon 2 p
![The element with the electronic configuration Ar4 s 13 d 5 is 1 2 The element with the electronic configuration [Ar]4 s 13 d 5 is: 1. 2.](https://slidetodoc.com/presentation_image/541e099e28360602c98e4564f3b84c7d/image-36.jpg)
The element with the electronic configuration [Ar]4 s 13 d 5 is: 1. 2. 3. 4. Manganese Chromium Magnesium Iron

Correct Answer: 1. 2. 3. 4. Manganese Chromium Magnesium Iron

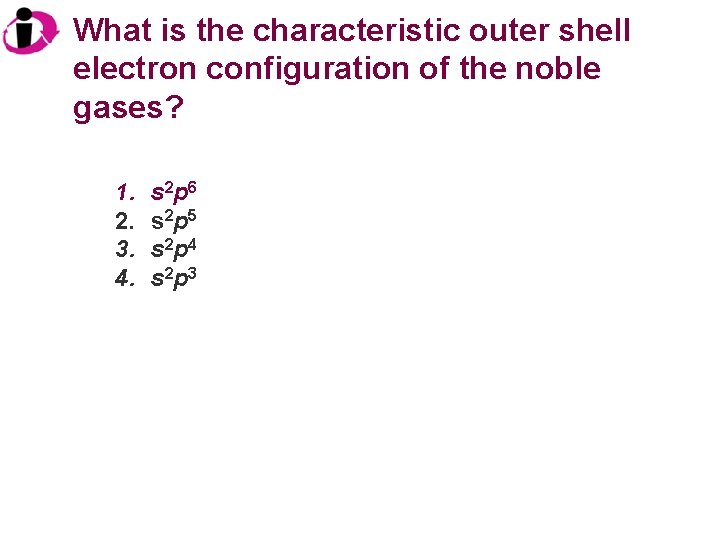
What is the characteristic outer shell electron configuration of the noble gases? 1. 2. 3. 4. s 2 p 6 s 2 p 5 s 2 p 4 s 2 p 3

Correct Answer: 1. 2. 3. 4. s 2 p 6 s 2 p 5 s 2 p 4 s 2 p 3 Noble gases have completely filled s and p orbitals, hence their lack of reactivity.

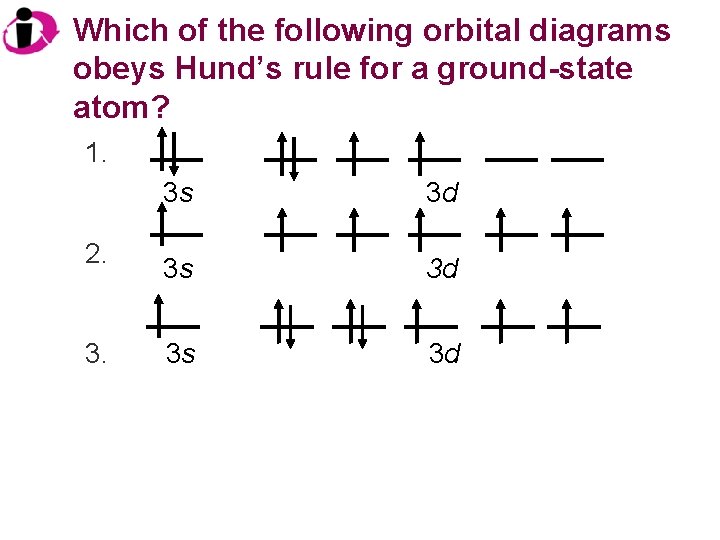
Which of the following orbital diagrams obeys Hund’s rule for a ground-state atom? 1. 2. 3. 3 s 3 d

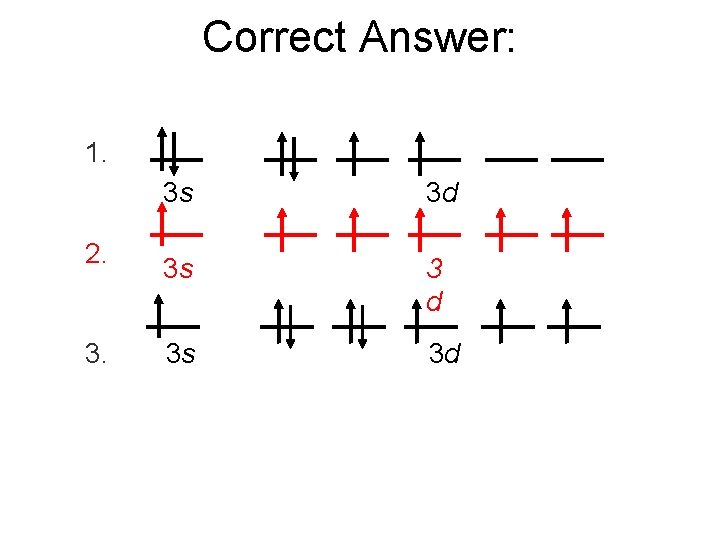
Correct Answer: 1. 2. 3. 3 s 3 d 3 s 3 d