Electrons Electrons Where are the electrons located in


















- Slides: 36

Electrons

Electrons: • Where are the electrons located in an atom? • Does the arrangement/placement matter?

Valence Electrons: • Electrons in the outer most shell (or outer most level) of an atom • ****Important**** because determine how reactive an element is

What # makes them stable? • Atoms want 8 valence electrons to be “stable”. • There are 2 exceptions to this… what are they?

• Why don’t electrons completely fill the 3 rd principal energy level before they start to fill the forth?


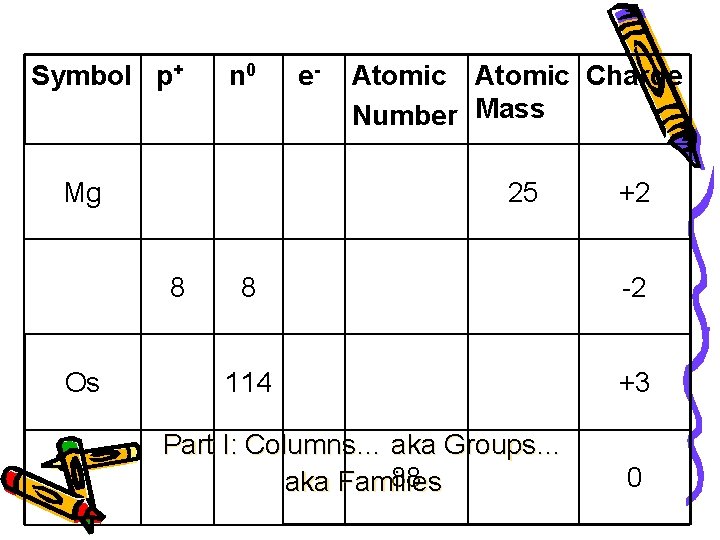
Symbol p+ n 0 Mg Atomic Charge Number Mass 25 8 Os e- +2 8 -2 114 +3 Part I: Columns… aka Groups… 88 aka Families 0

DECIPHERING THE PERIODIC TABLE Part I: Columns… aka Groups… aka Families

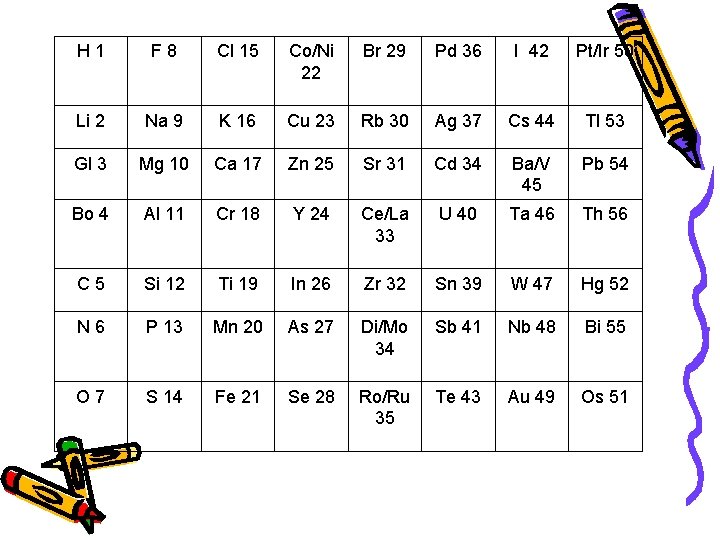
John Newlands • 1865 – arranged the known elements according to their properties and in order of increasing atomic mass – Noticed all of the elements in a given row had similar chemical and physical properties. • Law of Octaves- properties repeated every 8 elements

H 1 F 8 Cl 15 Co/Ni 22 Br 29 Pd 36 I 42 Pt/Ir 50 Li 2 Na 9 K 16 Cu 23 Rb 30 Ag 37 Cs 44 Tl 53 Gl 3 Mg 10 Ca 17 Zn 25 Sr 31 Cd 34 Ba/V 45 Pb 54 Bo 4 Al 11 Cr 18 Y 24 Ce/La 33 U 40 Ta 46 Th 56 C 5 Si 12 Ti 19 In 26 Zr 32 Sn 39 W 47 Hg 52 N 6 P 13 Mn 20 As 27 Di/Mo 34 Sb 41 Nb 48 Bi 55 O 7 S 14 Fe 21 Se 28 Ro/Ru 35 Te 43 Au 49 Os 51

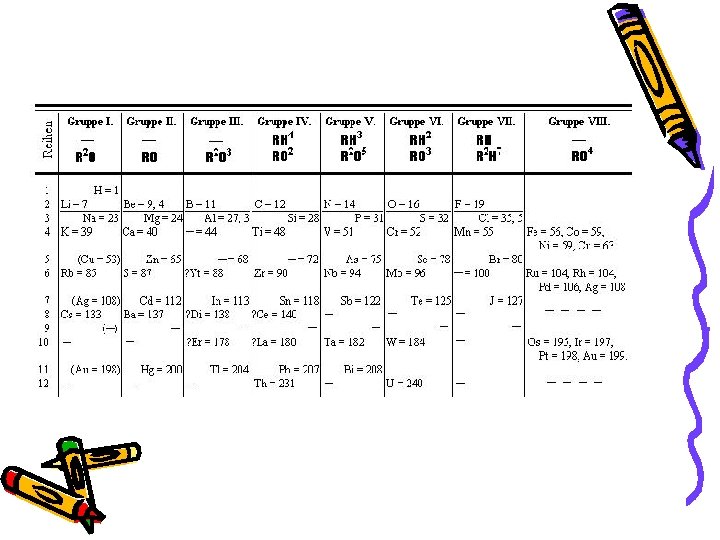
Dmitri Mendeleev (1869) • Used Newlands observations • Listed elements in columns according to atomic mass…. then arranged columns so elements of similar properties were side by side

Two Interesting Observations: 1) Mendeleev left gaps in the table and predicted particular properties those elements should have • Those elements were discovered and his predictions were true 2) Switched the order of Tellurium (Te) and Iodine (I)


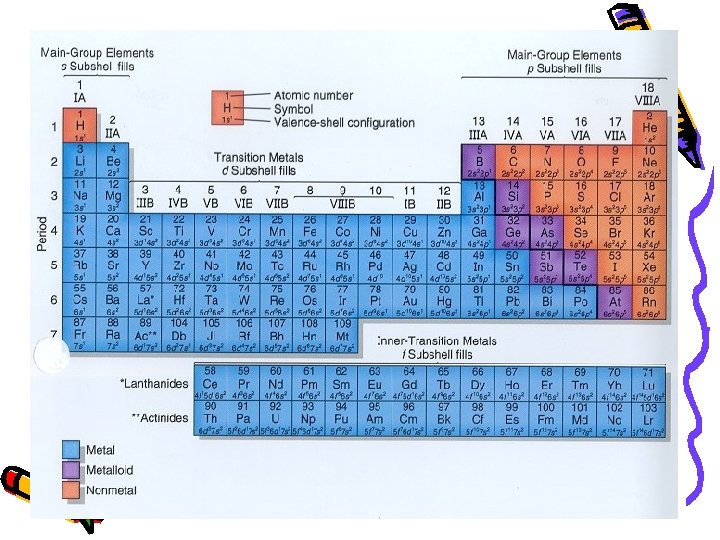
Henry Moseley (1887 -1915) • 1913 - made his own periodic table • Arranged elements by atomic number British Physicist… bookworm…

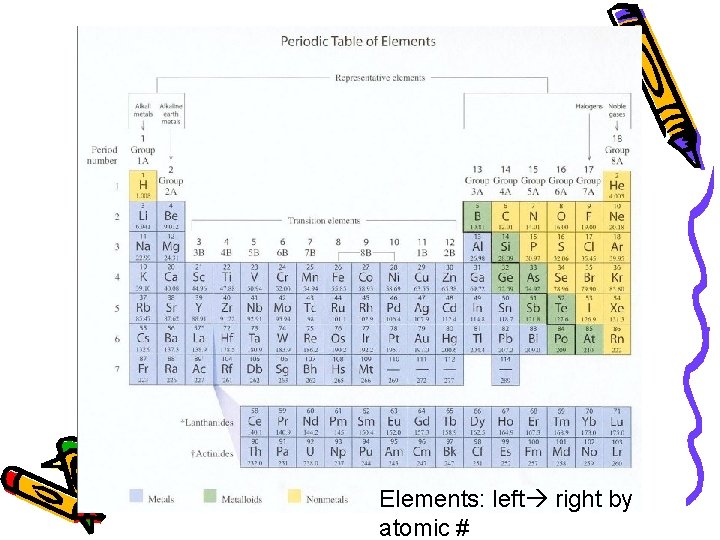
Elements: left right by atomic #

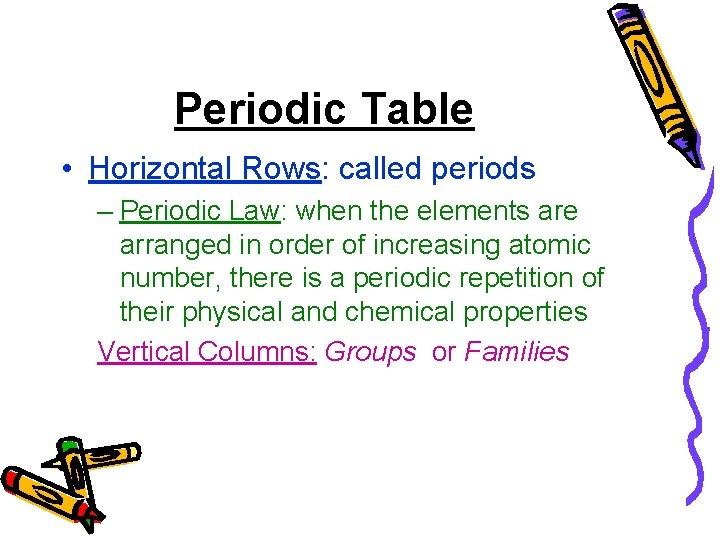
Periodic Table • Horizontal Rows: called periods – Periodic Law: when the elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties Vertical Columns: Groups or Families

Metals: • ~80% of all elements are metals • High electrical conductivity and have a high luster when cleaned • Ductile (can be made into wires) and Malleable • Are solid at room temperature except for one… which one is it? • Hint: Think “Barometer”

• Group 1: Alkali Metals – Except Hydrogen, all react vigorously with water • Group 2: call Alkaline Earth Metals

Non-Metals: • Generally nonlustrous… which means not shiny • Generally poor conductors of electricity

Specific Group Names: • Group 17 - Halogens • Like halogen lamps • Group 18 - Noble Gases • Sometimes called inert gases • Undergo few chemical rxns

Metalloids • Intermediate Properties • Semi-Conductors

Moving into Bonding…

Bonding: • How do atoms bond together to form molecules? – Electrons… specifically the valence electrons • What are valence electrons again?

First Things First: • First we need to learn how to draw the appropriate symbols. • Lewis Dot Structure- consists of the chemical symbol for the element plus a dot for each valence electron

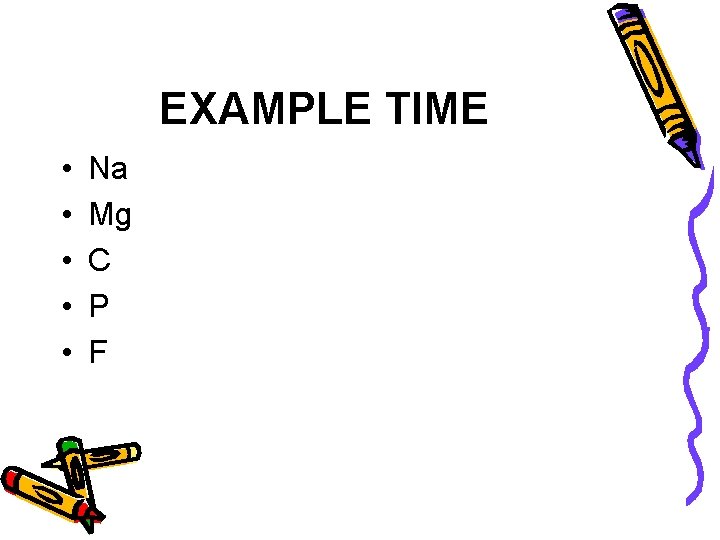
EXAMPLE TIME • • • Na Mg C P F

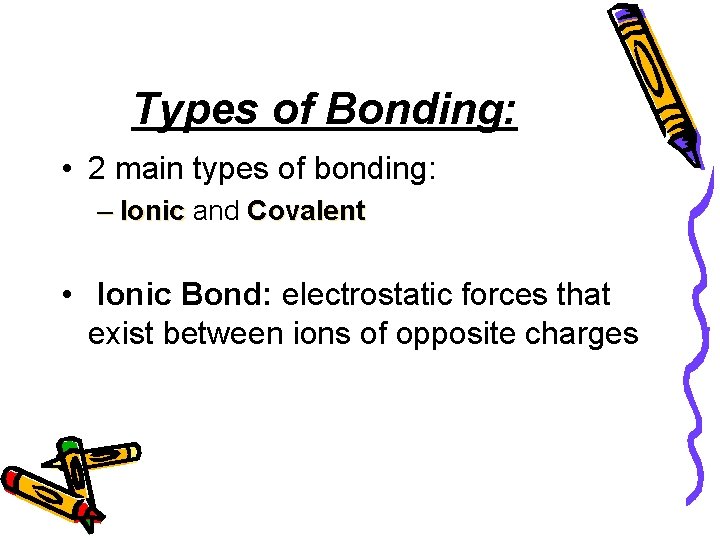
Types of Bonding: • 2 main types of bonding: – Ionic and Covalent • Ionic Bond: electrostatic forces that exist between ions of opposite charges

Ionic Bond • “Bond. Ionic Bond. Taken, not shared. ” • Happens between metals and nonmetals. • One atom “takes” the electron from another – forming ions

Ions • Cations- positively charged ions • Anions – negatively charged ions

Let’s Back Track A Little: • Ions and Ionic Compounds – In general, metal atoms tend to lose electrons to form cations (positive ions), whereas nonmetal atoms tend to gain electrons to form anions (negative ions)

Predicting Ions: • Look at the periodic table… what charge do you think Group I ions are likely to have? Group 2? Group 13? Group 15? Group 16? Group 17? – Group 1 – likely to lose 1 electron… so (+1) – Group 2 – (+2) – Group 13 – (+3) – Group 15 – (-3) – Group 16 – (-2) – Group 17 – (-1)

Chemical Formula: • Textbook Definition: A notation that uses chemical symbols with numerical subscripts to convey the relative proportions of atoms of the different elements in a substance • Tells you what something is made of using the element’s symbols

Nomenclature (aka, Naming): • Chemists like to name things… we’ve talked about those annoying chemists… so how do we know that Na. Cl is called sodium chloride instead of N-a - C-l ? • Let’s name Cation, Anions, and then Ionic Compounds…

Cations (positive ions): • Cations formed from metal atoms have the same name as the metal ion – Na+ (sodium ion) – Zn+2 (zinc ion) – Al+3 (aluminum ion)

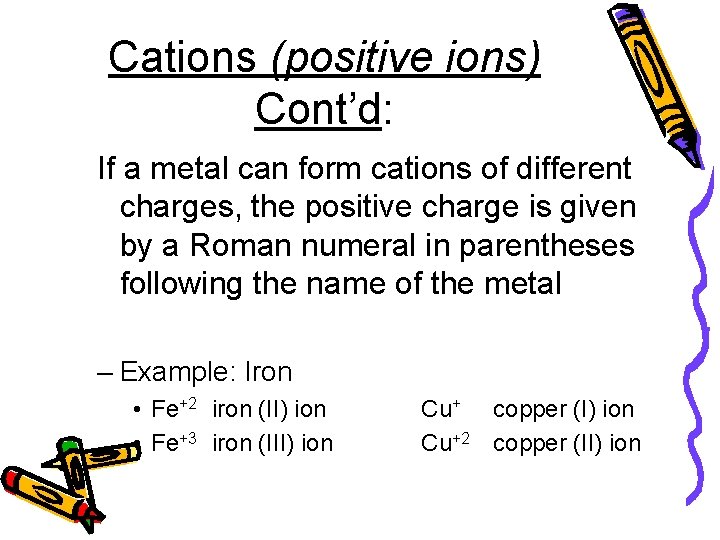
Cations (positive ions) Cont’d: If a metal can form cations of different charges, the positive charge is given by a Roman numeral in parentheses following the name of the metal – Example: Iron • Fe+2 iron (II) ion • Fe+3 iron (III) ion Cu+ copper (I) ion Cu+2 copper (II) ion

Anions (negative ions): • Monatomic anions have names formed by replacing the ending of the name of the element with “-ide” – H- Hydride – O-2 Oxide N-3 Nitride

Ionic Compounds: • Consist of cation name followed by the anion name… – Basically name the first part, then the second part – Ca. Cl 2 – Al(NO 3)3 calcium chloride aluminum nitrate