EPR Spectroscopy Spying on unpaired electrons What information







![EPR Oxegen Mapping (EPROM) of tubes with 15 N-PDT Amplitude Map [Oxygen] 0. 50 EPR Oxegen Mapping (EPROM) of tubes with 15 N-PDT Amplitude Map [Oxygen] 0. 50](https://slidetodoc.com/presentation_image_h/de9db04337cbf17767a43cab27d64fda/image-27.jpg)






- Slides: 45

EPR Spectroscopy Spying on unpaired electrons - What information can we get? Periannan Kuppusamy, Ph. D Center for Biomedical EPR Spectroscopy & Imaging Davis Heart & Lung Research Institute Ohio State University, Columbus, OH E-mail: Kuppusamy. 1@osu. edu Sunrise Free Radical School Oxygen-2002 : : Nov 21, 2002

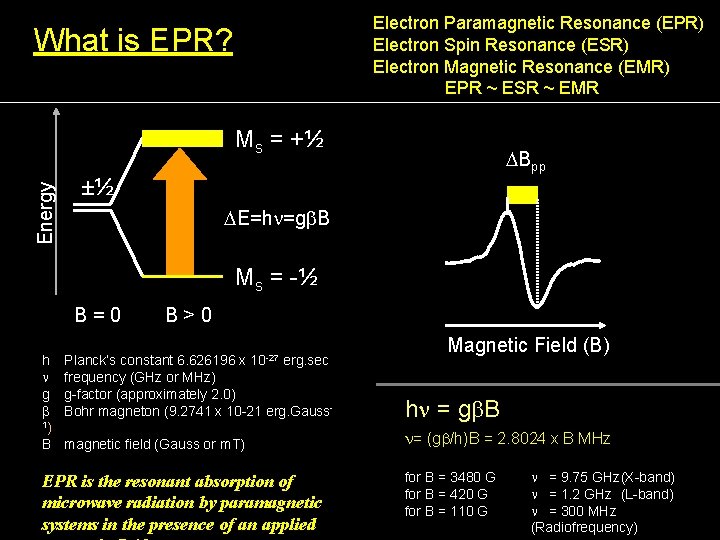
Electron Paramagnetic Resonance (EPR) Electron Spin Resonance (ESR) Electron Magnetic Resonance (EMR) EPR ~ ESR ~ EMR What is EPR? Energy Ms = +½ DBpp ±½ DE=hn=gb. B Ms = -½ B=0 h n g b 1) B B>0 10 -27 Magnetic Field (B) Planck’s constant 6. 626196 x erg. sec frequency (GHz or MHz) g-factor (approximately 2. 0) Bohr magneton (9. 2741 x 10 -21 erg. Gauss- hn = gb. B magnetic field (Gauss or m. T) n= (gb/h)B = 2. 8024 x B MHz EPR is the resonant absorption of microwave radiation by paramagnetic systems in the presence of an applied for B = 3480 G for B = 420 G for B = 110 G n = 9. 75 GHz(X-band) n = 1. 2 GHz (L-band) n = 300 MHz (Radiofrequency)

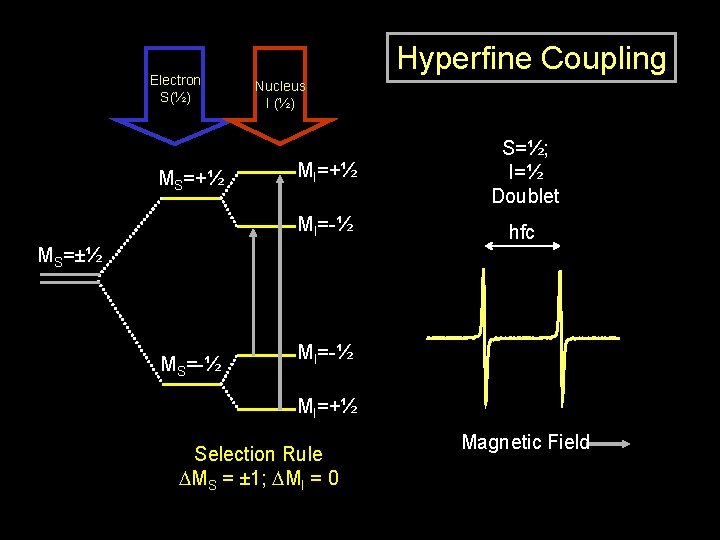
Electron S(½) MS=+½ Hyperfine Coupling Nucleus I (½) MI=+½ S=½; I=½ Doublet MI=-½ hfc MS=±½ MS=-½ MI=+½ Selection Rule DMS = ± 1; DMI = 0 Magnetic Field

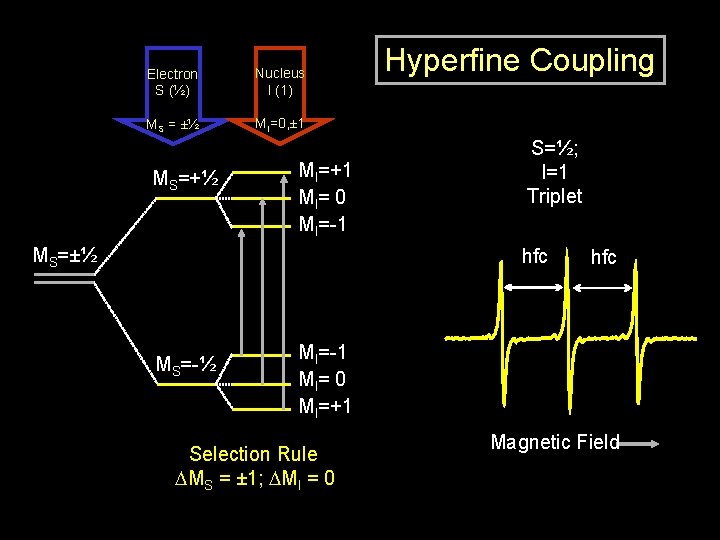
Electron S (½) Nucleus I (1) MS = ±½ MI=0, ± 1 MS=+½ MI=+1 MI= 0 MI=-1 MS=±½ Hyperfine Coupling S=½; I=1 Triplet hfc MS=-½ hfc MI=-1 MI= 0 MI=+1 Selection Rule DMS = ± 1; DMI = 0 Magnetic Field

What do we do with EPR? We can detect & measure free radicals and paramagnetic species • High sensitivity (nanomolar concentrations) • No background • Definitive & quantitative Direct detection e. g. : semiquinones, nitroxides, trityls Indirect detection Spin-trapping Species: superoxide, hydroxyl, alkyl, NO Spin-traps: DMPO, PBN, DEPMPO, Fe-DTCs Chemical modifications Spin-formation : hydroxylamines (Dikhalov et al) Spin-change : nitronylnitroxides ( Kalyanaraman et al) Spin-loss : trityl radicals

Can we use EPR to measure free radicals from biological systems (in vivo or ex vivo)? What do we do with it? Yes! Intact tissues, organs or whole-body can be measured. But there is a catch! Biological samples contain large proportion of water. They are aqueous and highly dielectric. Conventional EPR spectrometers operate at X-band ((9 -10 GHz) frequencies, which result in (i) ‘non-resonant’ absorption (heating) of energy and (ii) poor penetration of samples. Hence the frequency of the instrumentation needs to be reduced. What is the optimum frequency? - depends on sample size Frequency ~300 MHz ~750 MHz 1 -2 GHz ~3 GHz 9 -10 GHz Penetration Depth > 10 cm 6 -8 cm 1 -1. 5 cm 1 -3 mm 1 mm Objects Mouse, rat heart Mouse tail Topical (skin) In vitro samples (~100 u. L vol. ) Pioneers Halpern et al Krishna et al Zweier et al Hyde et al Swartz et al Zweier et al Hyde et al Zweier et al

What else can we do with EPR? We can use free radicals as “spying probes” to obtain information from biological systems • A known free radical probes is infused or injected into the animal • The change in the EPR line-shape profile, which is correlated to some physiological function, is then monitored as a function of time or any other parameter. • The measurements can be performed in realtime and in vivo to obtain ‘functional parameters’.

Functional parameters from an EPR spectrum In vivo EPR spectroscopy is capable of providing useful physiologic and metabolic (functional) information from tissues Oxygen, p. O 2 Redox status Oxygen, p. O 2 Acidosis, p. H Viscosity Molecular motion Acidosis, p. H Thiols (GSH) Cell viability Viscosity Tissue perfusion Molecular width Redox status Cell viability Tissue perfusion amplitude motion Splittin g

Can we image free radicals in biological systems? Spatially-resolved information (mapping) can be obtained using EPR imaging (EPRI) techniques

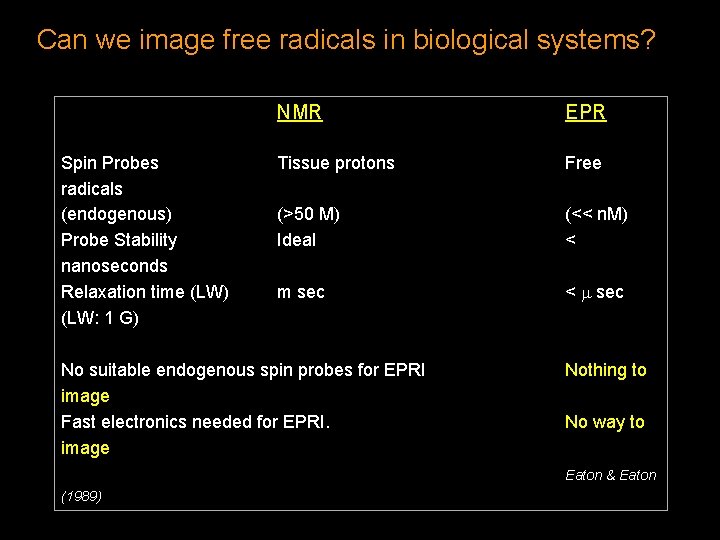
Can we image free radicals in biological systems? Spin Probes radicals (endogenous) Probe Stability nanoseconds Relaxation time (LW) (LW: 1 G) NMR EPR Tissue protons Free (>50 M) Ideal (<< n. M) < m sec No suitable endogenous spin probes for EPRI image Fast electronics needed for EPRI. image Nothing to No way to Eaton & Eaton (1989)

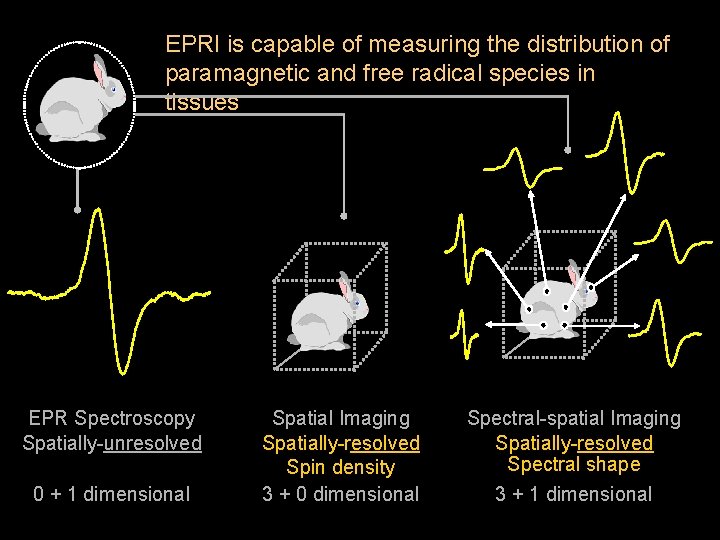
EPRI is capable of measuring the distribution of paramagnetic and free radical species in tissues EPR Spectroscopy Spatially-unresolved 0 + 1 dimensional Spatial Imaging Spatially-resolved Spin density 3 + 0 dimensional Spectral-spatial Imaging Spatially-resolved Spectral shape 3 + 1 dimensional

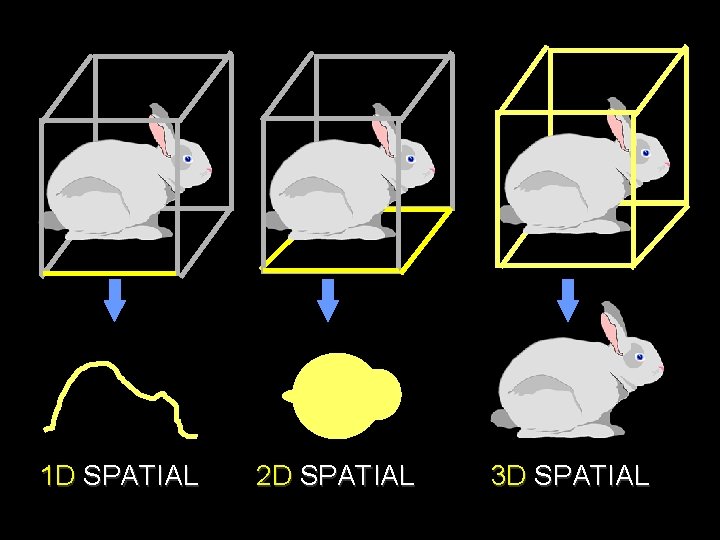
1 D SPATIAL 2 D SPATIAL 3 D SPATIAL

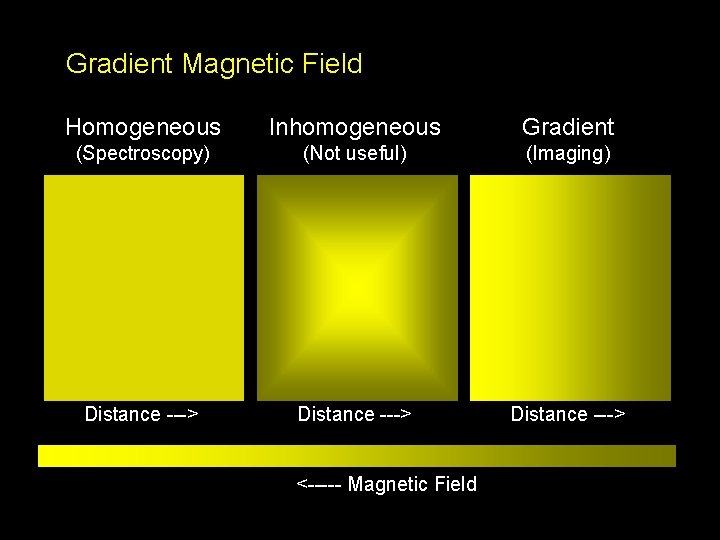
Gradient Magnetic Field Homogeneous Inhomogeneous Gradient (Spectroscopy) (Not useful) (Imaging) Distance ---> <----- Magnetic Field

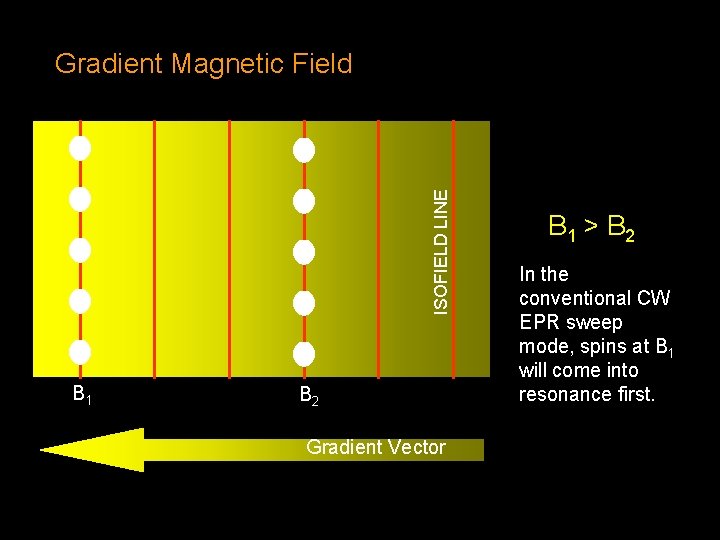
ISOFIELD LINE Gradient Magnetic Field B 1 B 2 Gradient Vector B 1 > B 2 In the conventional CW EPR sweep mode, spins at B 1 will come into resonance first.

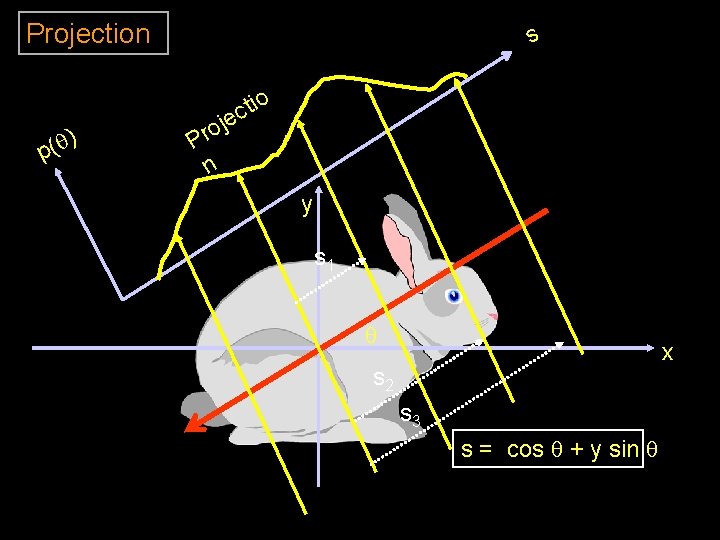
Projection ) q ( p s o Pr n tio c je y s 1 q x s 2 s 3 s = cos q + y sin q

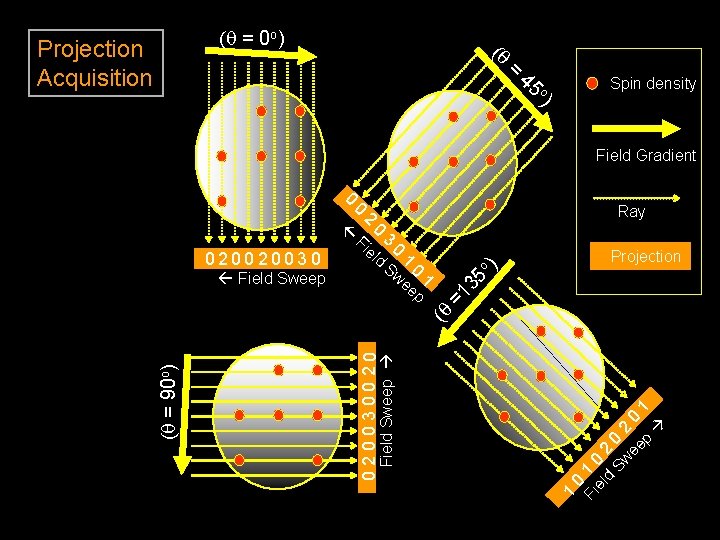
(q = 0 o) (q Projection Acquisition = o 45 Spin density ) Field Gradient Fi 0 el d 3 0 1 Sw 0 ee 1 p Projection 0 Fi 1 0 el d 20 Sw 2 ee 0 1 p 1 Field Sweep 020030020 (q = 90 o) (q Field Sweep Ray ) 020020030 2 o 0 =1 35 0

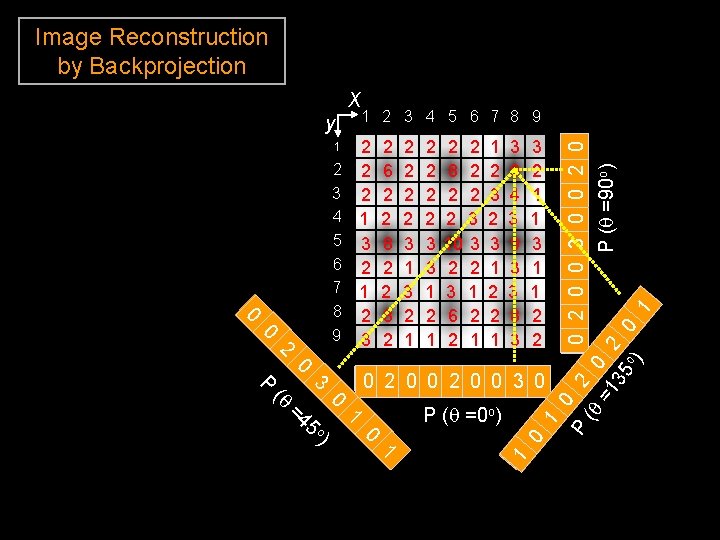
Image Reconstruction by Backprojection 1 2 3 4 5 6 7 8 9 2 2 2 1 3 2 1 2 3 1 0 2 3 4 5 6 7 8 9 0 2 6 2 2 8 2 2 2 3 1 3 2 1 2 2 3 3 1 2 2 8 2 2 3 10 3 2 2 3 1 6 2 2 1 1 2 3 1 2 2 1 3 9 4 3 9 3 3 8 3 3 2 1 1 3 1 1 2 2 2 0 0 3 0 0 1 ) 0 o 5 =4 0 (q P (q =0 o) 1 1 3 P 1 0 P (q 2 0 0 3 0 0 2 0 =1 o) P (q =90 2 35 o 0 ) 1 y x

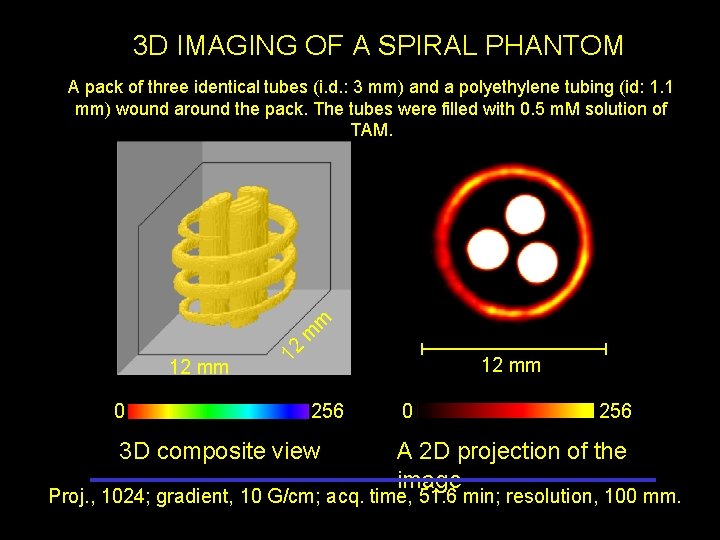
3 D IMAGING OF A SPIRAL PHANTOM 12 mm 0 12 m m A pack of three identical tubes (i. d. : 3 mm) and a polyethylene tubing (id: 1. 1 mm) wound around the pack. The tubes were filled with 0. 5 m. M solution of TAM. 12 mm 256 3 D composite view 0 256 A 2 D projection of the image Proj. , 1024; gradient, 10 G/cm; acq. time, 51. 6 min; resolution, 100 mm.

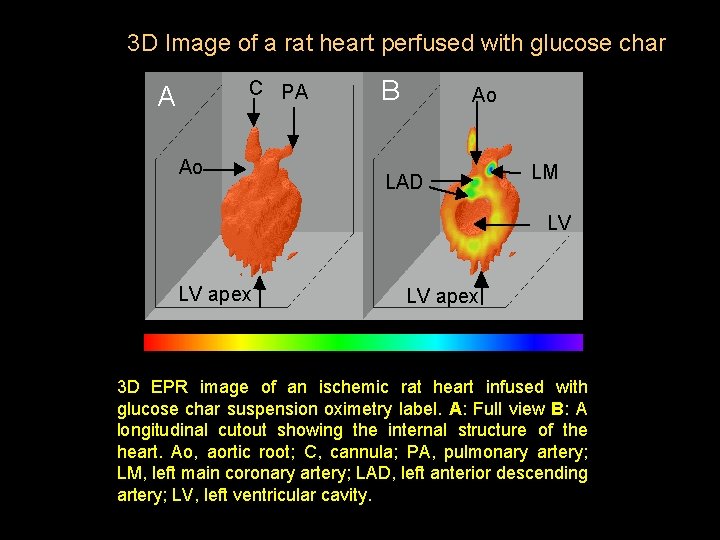
3 D Image of a rat heart perfused with glucose char C PA A Ao B Ao LAD LM LV LV apex 3 D EPR image of an ischemic rat heart infused with glucose char suspension oximetry label. A: Full view B: A longitudinal cutout showing the internal structure of the heart. Ao, aortic root; C, cannula; PA, pulmonary artery; LM, left main coronary artery; LAD, left anterior descending artery; LV, left ventricular cavity.

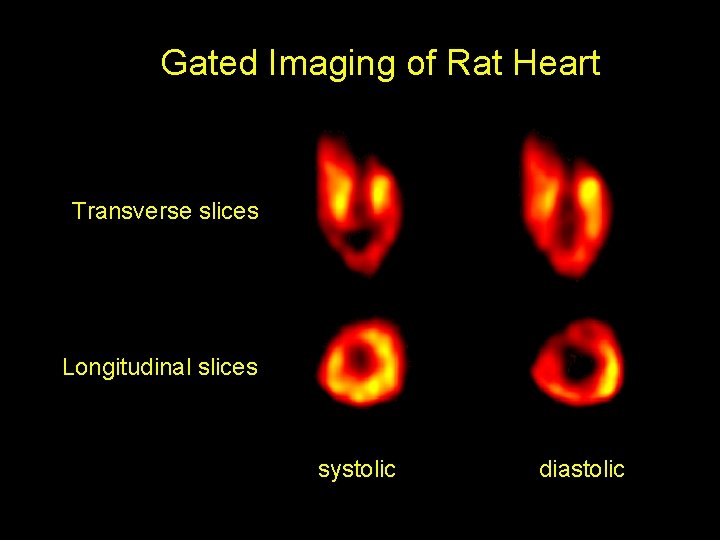
Gated Imaging of Rat Heart Transverse slices Longitudinal slices systolic diastolic

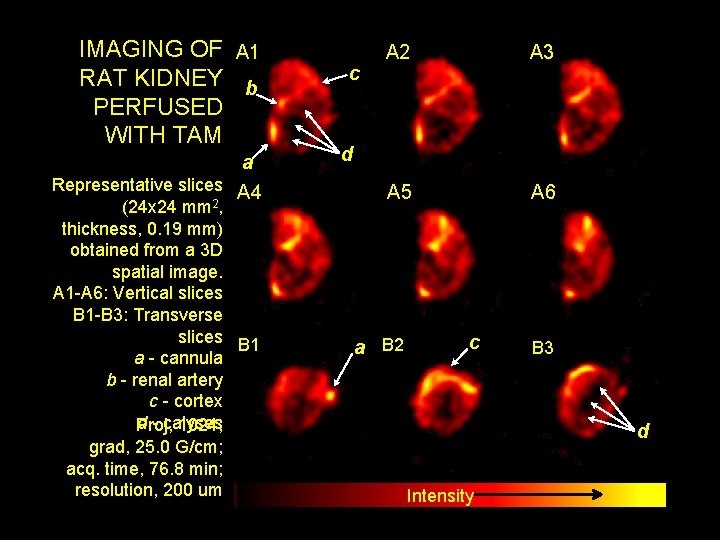
IMAGING OF A 1 RAT KIDNEY b PERFUSED WITH TAM a Representative slices A 4 (24 x 24 mm 2, thickness, 0. 19 mm) obtained from a 3 D spatial image. A 1 -A 6: Vertical slices B 1 -B 3: Transverse slices B 1 a - cannula b - renal artery c - cortex d - calyses Proj, 1024; grad, 25. 0 G/cm; acq. time, 76. 8 min; resolution, 200 um c A 2 A 3 A 5 A 6 d a B 2 c B 3 d Intensity

3 D IMAGING OF NITROXIDE DISTRIBUTION IN TUMORS SCCVII RIF-1 NORMAL SLICES (0. 3 MM) FROM 3 D IMAGES OF MURINE TUMORS (3 -CP; 100 mg/kg) Intensity --> C 3 H mice; gradient 20 G/cm; 144 projections; 10 x 10 mm 2 Kuppusamy, P. , et al. Cancer Research, 58, 1562 -1568

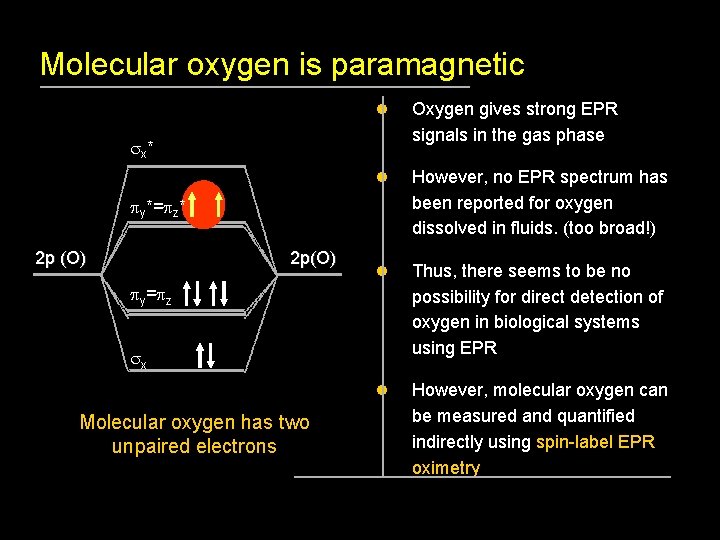
Molecular oxygen is paramagnetic l Oxygen gives strong EPR signals in the gas phase l However, no EPR spectrum has been reported for oxygen dissolved in fluids. (too broad!) l Thus, there seems to be no possibility for direct detection of oxygen in biological systems using EPR l However, molecular oxygen can be measured and quantified indirectly using spin-label EPR oximetry sx * py*=pz* 2 p (O) 2 p(O) py=pz sx Molecular oxygen has two unpaired electrons

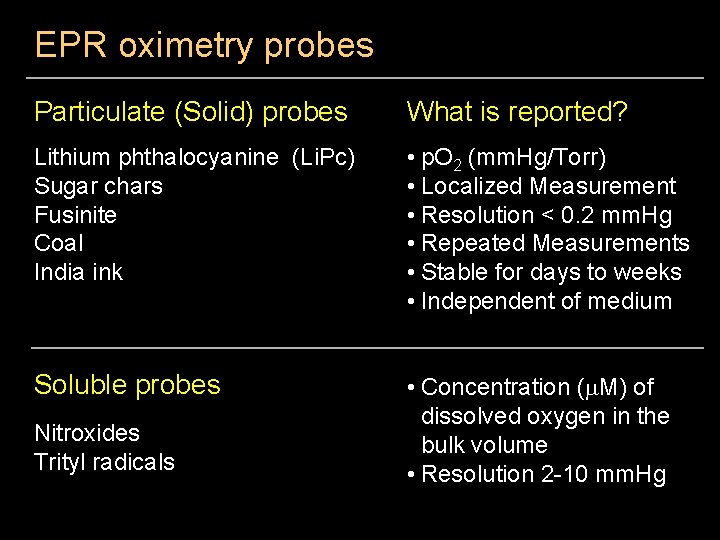
EPR oximetry probes Particulate (Solid) probes What is reported? Lithium phthalocyanine (Li. Pc) Sugar chars Fusinite Coal India ink • p. O 2 (mm. Hg/Torr) • Localized Measurement • Resolution < 0. 2 mm. Hg • Repeated Measurements • Stable for days to weeks • Independent of medium Soluble probes • Concentration (m. M) of dissolved oxygen in the bulk volume • Resolution 2 -10 mm. Hg Nitroxides Trityl radicals

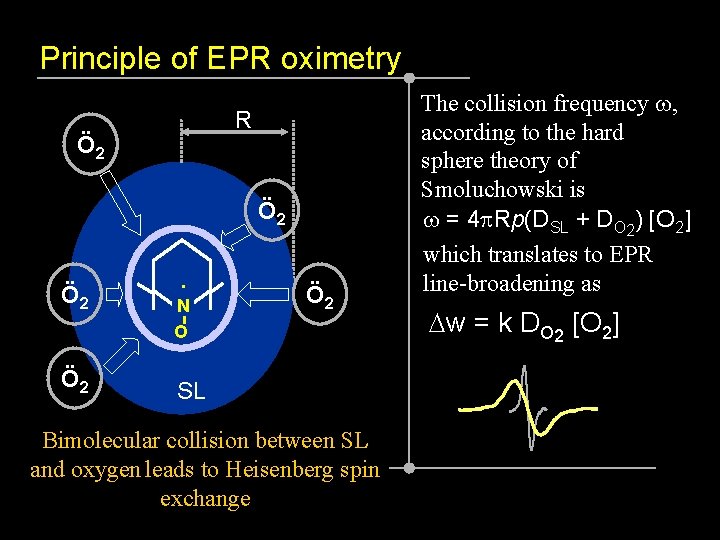
Principle of EPR oximetry R Ö 2 Ö 2 . N O Ö 2 SL Bimolecular collision between SL and oxygen leads to Heisenberg spin exchange The collision frequency w, according to the hard sphere theory of Smoluchowski is w = 4 p. Rp(DSL + DO 2) [O 2] which translates to EPR line-broadening as Dw = k DO 2 [O 2]

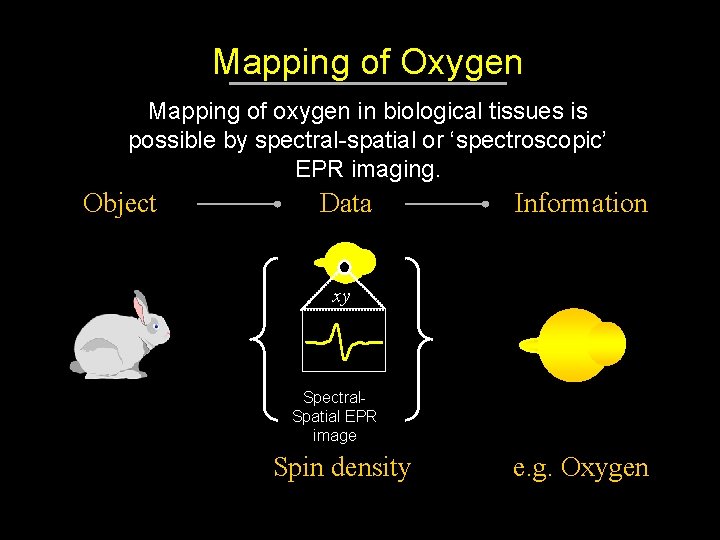
Mapping of Oxygen Mapping of oxygen in biological tissues is possible by spectral-spatial or ‘spectroscopic’ EPR imaging. Object Data Information xy Spectral. Spatial EPR image Spin density e. g. Oxygen
![EPR Oxegen Mapping EPROM of tubes with 15 NPDT Amplitude Map Oxygen 0 50 EPR Oxegen Mapping (EPROM) of tubes with 15 N-PDT Amplitude Map [Oxygen] 0. 50](https://slidetodoc.com/presentation_image_h/de9db04337cbf17767a43cab27d64fda/image-27.jpg)
EPR Oxegen Mapping (EPROM) of tubes with 15 N-PDT Amplitude Map [Oxygen] 0. 50 0. 25 38% 0. 25 0. 50 21% Spin Density Map Oxygen Map 40% 30% 20% 10% 0% S. Sendhil Velan, R. G. S. Spencer, J. L. Zweier & P. Kuppusamy, Magn. Reson. Med. 43, 804 -809 (2000) Oxygen Phantom [PDT, m. M]

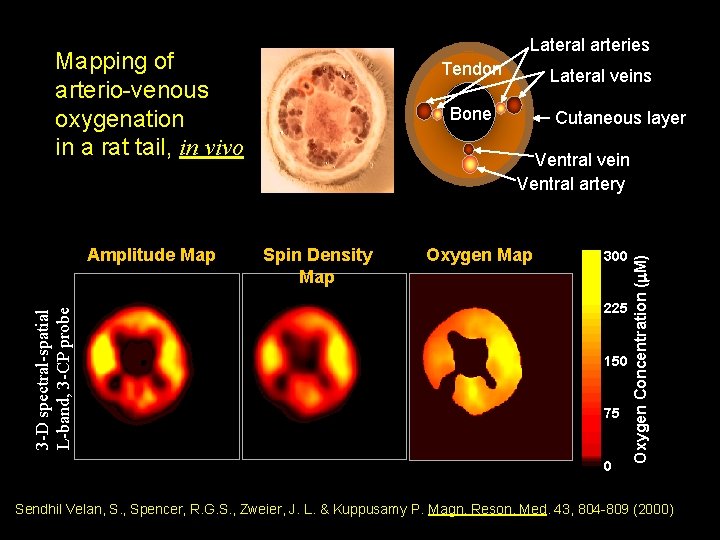
Lateral arteries Mapping of arterio-venous oxygenation in a rat tail, in vivo Lateral veins Bone Cutaneous layer Ventral vein Ventral artery Spin Density Map Oxygen Map 300 225 150 75 0 Oxygen Concentration (m. M) 3 -D spectral-spatial L-band, 3 -CP probe Amplitude Map Tendon Sendhil Velan, S. , Spencer, R. G. S. , Zweier, J. L. & Kuppusamy P. Magn. Reson. Med. 43, 804 -809 (2000)

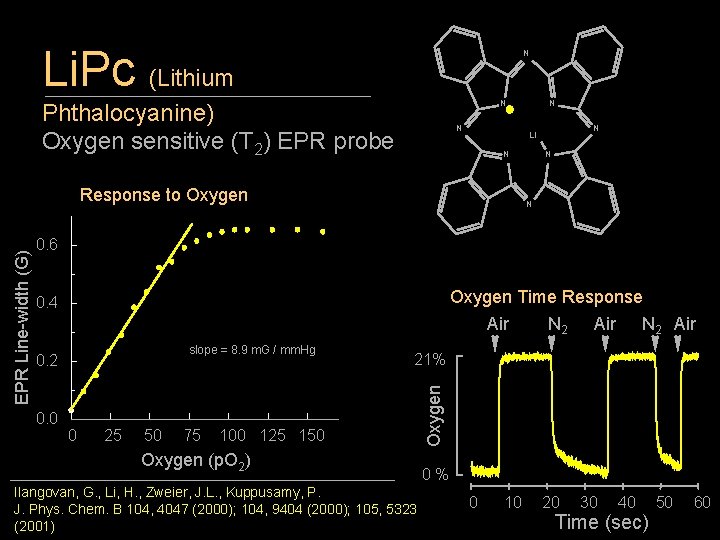
Li. Pc (Lithium N N Phthalocyanine) Oxygen sensitive (T 2) EPR probe N N Li N Response to Oxygen N N 0. 6 Oxygen Time Response Air N 2 Air 0. 4 slope = 8. 9 m. G / mm. Hg 0. 2 0. 0 0 25 50 75 21% 100 125 150 Oxygen (p. O 2) Ilangovan, G. , Li, H. , Zweier, J. L. , Kuppusamy, P. J. Phys. Chem. B 104, 4047 (2000); 104, 9404 (2000); 105, 5323 (2001) Oxygen EPR Line-width (G) N 0% 0 10 20 30 40 Time (sec) 50 60

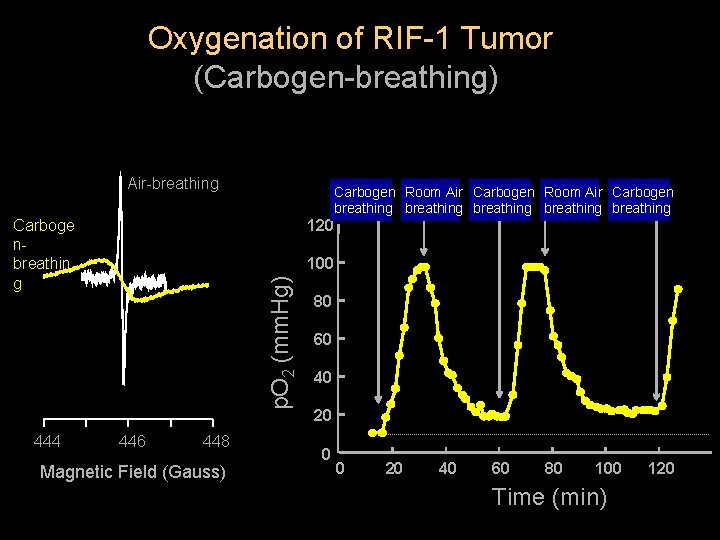
Oxygenation of RIF-1 Tumor (Carbogen-breathing) Air-breathing Carboge nbreathin g 444 Carbogen Room Air Carbogen breathing breathing 120 p. O 2 (mm. Hg) 100 446 448 Magnetic Field (Gauss) 80 60 40 20 0 0 20 40 60 80 100 Time (min) 120

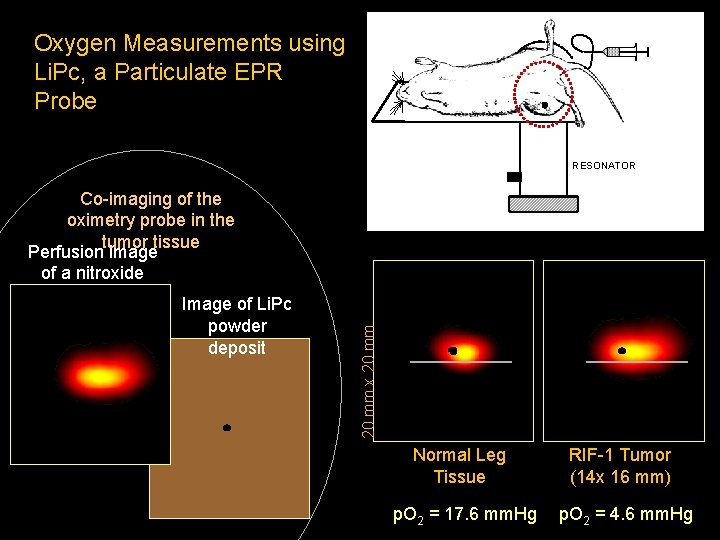
Oxygen Measurements using Li. Pc, a Particulate EPR Probe RESONATOR Image of Li. Pc powder deposit 20 mm x 20 mm Co-imaging of the oximetry probe in the tumor tissue Perfusion image of a nitroxide Normal Leg Tissue p. O 2 = 17. 6 mm. Hg RIF-1 Tumor (14 x 16 mm) p. O 2 = 4. 6 mm. Hg

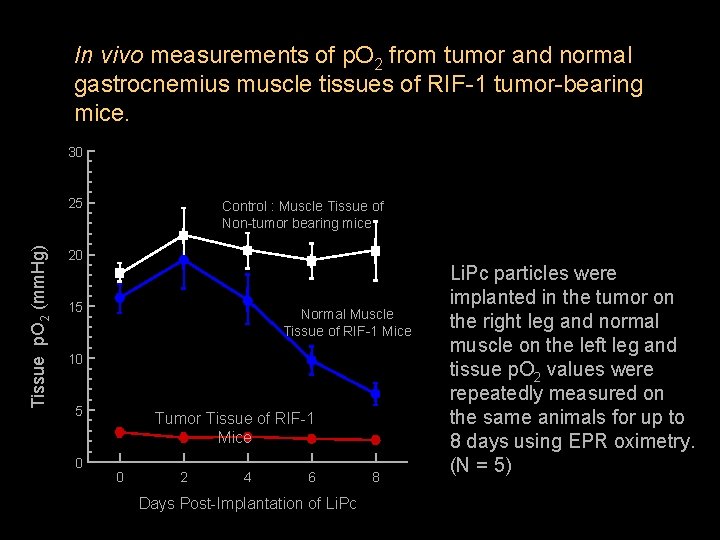
In vivo measurements of p. O 2 from tumor and normal gastrocnemius muscle tissues of RIF-1 tumor-bearing mice. 30 Tissue p. O 2 (mm. Hg) 25 Control : Muscle Tissue of Non-tumor bearing mice 20 15 Normal Muscle Tissue of RIF-1 Mice 10 5 0 Tumor Tissue of RIF-1 Mice 0 2 4 6 Days Post-Implantation of Li. Pc 8 Li. Pc particles were implanted in the tumor on the right leg and normal muscle on the left leg and tissue p. O 2 values were repeatedly measured on the same animals for up to 8 days using EPR oximetry. (N = 5)

Redox Status Redox State describe the ratio of the interconvertible oxidized and reduced form of a specific redox couple Redox Status applies to a set of redox couples. It is the summation of the products of the reduction potential and reducing capacity of all the redox couples present GSSG/2 GSH GSSG + 2 H+ + 2 e- 2 GSH Ehc = E 0– (RT/n. F) log([GSH]2/[GSSG]) Redox State = Reduction potential x concentration GSSG/2 GSH NADP+/NADPH Trx. SS/Trx(SH)2 Redox Status = [Reduction potential x concentration]i Schaefer, F. Q. & Buettner, G. R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30, 1191 -1212 (2001)

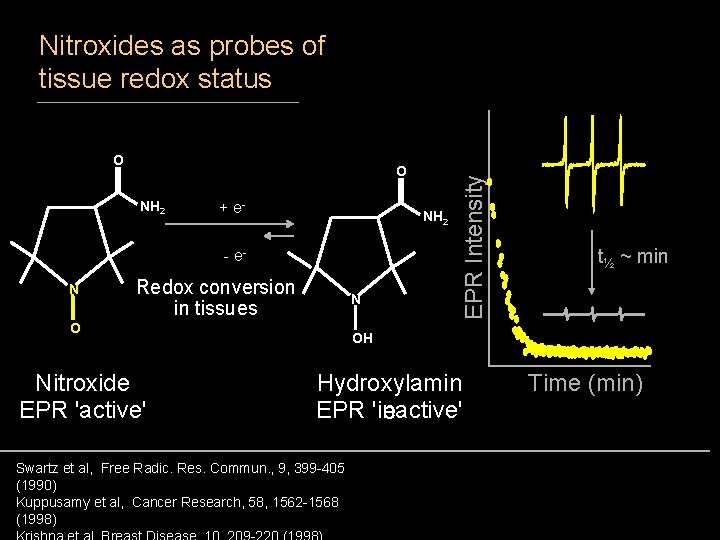
O O NH 2 + e- NH 2 - e. N Redox conversion in tissues N O Nitroxide EPR 'active' EPR Intensity Nitroxides as probes of tissue redox status t½ ~ min OH Hydroxylamin EPR 'inactive' e Swartz et al, Free Radic. Res. Commun. , 9, 399 -405 (1990) Kuppusamy et al, Cancer Research, 58, 1562 -1568 (1998) Time (min)

Reduction of 3 -CP in the Normal & Tumor Tissue NORMAL TISSUE (LEFT LEG) RIF-1 TUMOR (RIGHT LEG) 3. 0 4. 5 6. 0 7. 5 9. 0 10. 5 12. 0 13. 5 15. 0 16. 5 Time (min) Intensity --> C 3 H mice with RIF-1 tumor; ~30 g bw; dose: 100 mg/kg, iv; Measured in vivo using surface resonator at L-band (1. 25 GHz); Images: 10 x 10 mm 2 Kuppusamy, P. , et al. Cancer Research, 58, 1562 -1568 (1998).

Intensity Reconstruction of Tissue ‘Redox Status’ Image ‘kx, y’ x, y Time x, y Intensity Map Reduction constant, kx, y Redox Map [64 x 64] 8 -12 points, first order [64 x 64] Kuppusamy, P. , & Krishna, MC. , Curr. Topics in Biophys. (2002)

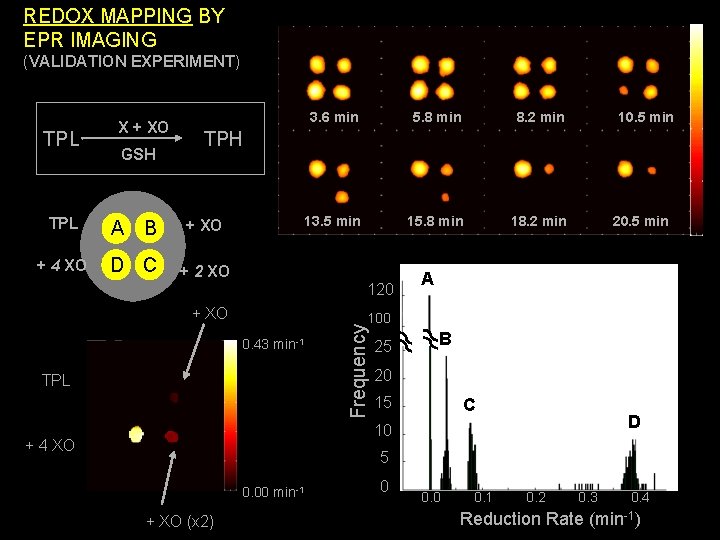
REDOX MAPPING BY EPR IMAGING (VALIDATION EXPERIMENT) TPL X + XO GSH 3. 6 min 5. 8 min 8. 2 min 13. 5 min 15. 8 min 18. 2 min 10. 5 min TPH TPL A B + XO + 4 XO D C + 2 XO 120 + XO 20. 5 min A 0. 43 min-1 TPL Frequency 100 25 B 20 15 C D 10 + 4 XO 5 0. 00 min-1 + XO (x 2) 0 0. 1 0. 2 0. 3 0. 4 Reduction Rate (min-1)

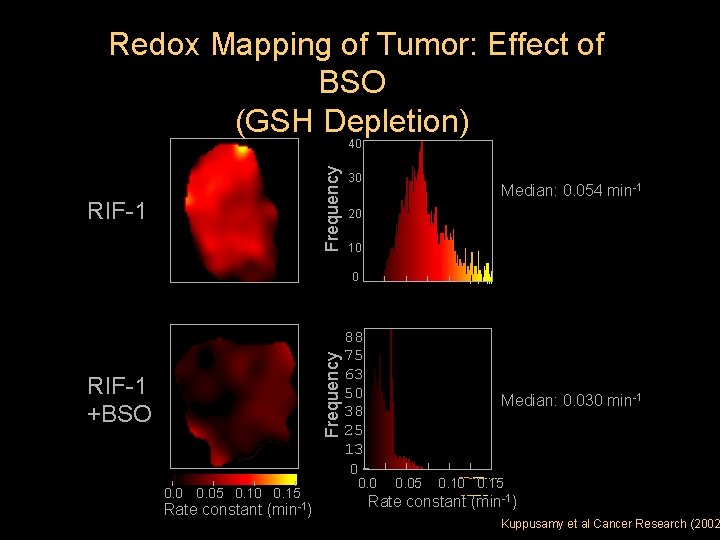
Redox Mapping of Tumor: Effect of BSO (GSH Depletion) Frequency 40 RIF-1 30 Median: 0. 054 min-1 20 10 Frequency 0 RIF-1 +BSO 0. 05 0. 10 0. 15 Rate constant (min-1) 88 75 63 50 38 25 13 0 Median: 0. 030 min-1 0. 05 0. 10 0. 15 Rate constant (min-1) Kuppusamy et al Cancer Research (2002

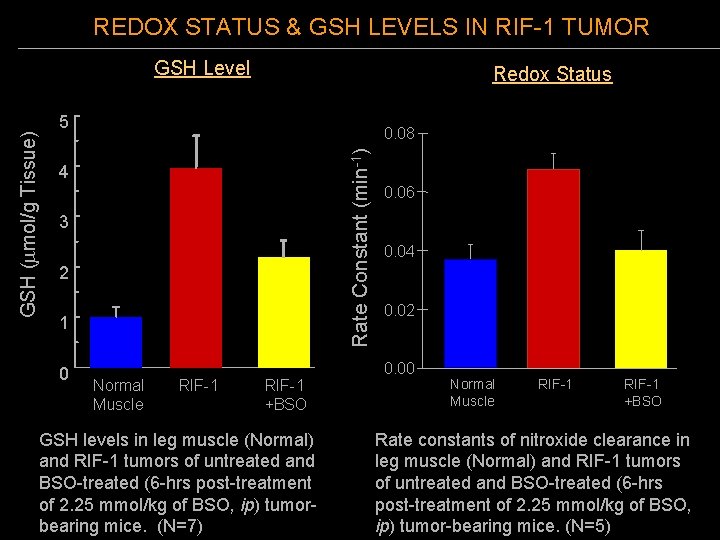
REDOX STATUS & GSH LEVELS IN RIF-1 TUMOR Redox Status 5 0. 08 Rate Constant (min-1) GSH (mmol/g Tissue) GSH Level 4 3 2 1 0 Normal Muscle RIF-1 +BSO GSH levels in leg muscle (Normal) and RIF-1 tumors of untreated and BSO-treated (6 -hrs post-treatment of 2. 25 mmol/kg of BSO, ip) tumorbearing mice. (N=7) 0. 06 0. 04 0. 02 0. 00 Normal Muscle RIF-1 +BSO Rate constants of nitroxide clearance in leg muscle (Normal) and RIF-1 tumors of untreated and BSO-treated (6 -hrs post-treatment of 2. 25 mmol/kg of BSO, ip) tumor-bearing mice. (N=5)

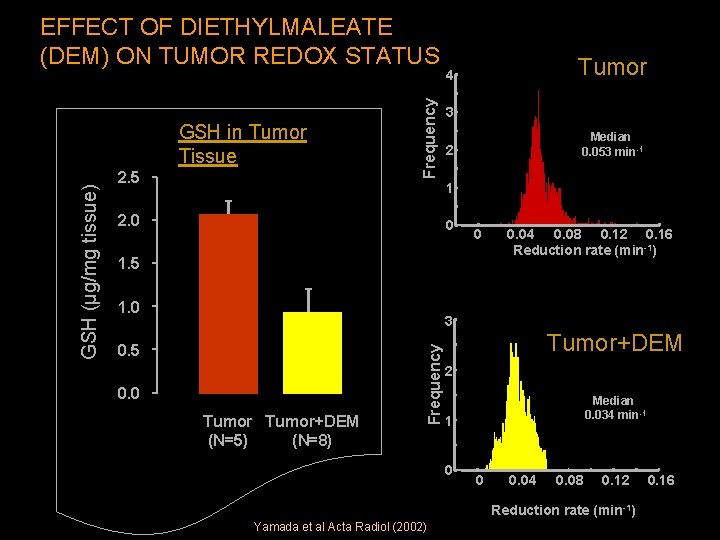
2. 5 Tumor 4 3 Median 0. 053 min-1 2 1 2. 0 0 0 1. 5 1. 0 0. 04 0. 08 0. 12 0. 16 Reduction rate (min-1) 3 0. 5 0. 0 Tumor+DEM (N=5) (N=8) Frequency GSH (µg/mg tissue) GSH in Tumor Tissue Frequency EFFECT OF DIETHYLMALEATE (DEM) ON TUMOR REDOX STATUS Tumor+DEM 2 Median 0. 034 min-1 1 0 0 0. 04 0. 08 0. 12 Reduction rate (min-1) Yamada et al Acta Radiol (2002) 0. 16

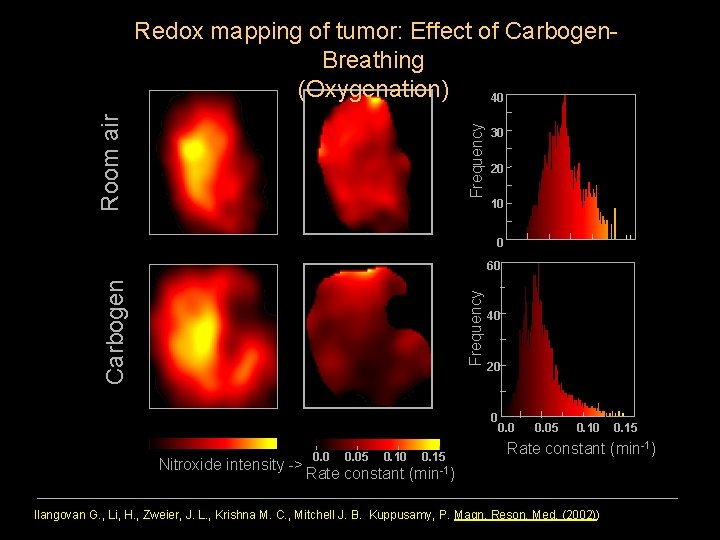
Frequency Room air Redox mapping of tumor: Effect of Carbogen. Breathing (Oxygenation) 40 30 20 10 0 Frequency Carbogen 60 40 20 0 Nitroxide intensity -> 0. 05 0. 10 0. 15 Rate constant (min-1) Ilangovan G. , Li, H. , Zweier, J. L. , Krishna M. C. , Mitchell J. B. Kuppusamy, P. Magn. Reson. Med. (2002))

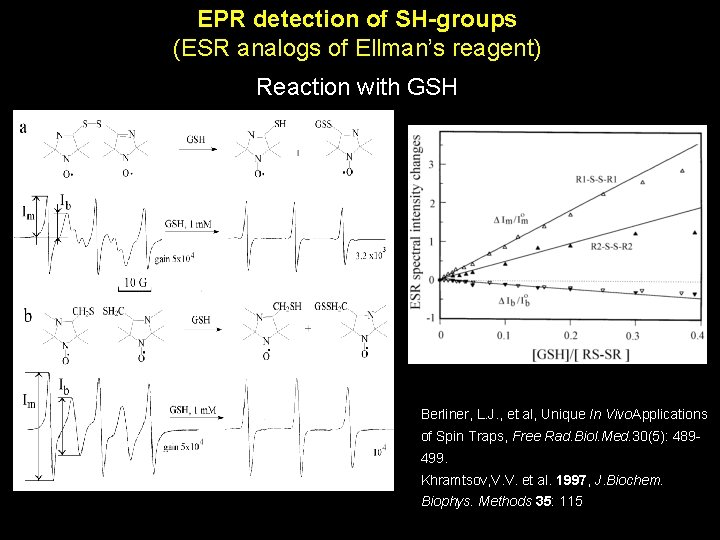
EPR detection of SH-groups (ESR analogs of Ellman’s reagent) Reaction with GSH Berliner, L. J. , et al, Unique In Vivo. Applications of Spin Traps, Free Rad. Biol. Med. 30(5): 489499. Khramtsov, V. V. et al. 1997, J. Biochem. Biophys. Methods 35: 115

Summary 1. EPR spectroscopy is a direct & definitive technique for detection and quantitation of free radicals and paramagnetic species. 2. Low-frequency EPR spectroscopy enables measurement of free radicals (endogenous/exogenous) in biological systems including intact tissues, isolated organs and small animals. 3. In vivo EPR spectroscopy and imaging methods enable noninvasive measurement and mapping of tissue p. O 2, redox status and p. H.

BOOKS Rosen, G. M. , Britigan, B. E. , Halpern, H. J. , and Pou, S. Free Radicals: Biology and Detection by Spin Trapping. New York: Oxford University Press, 1999 Eaton, G. R. , Eaton, S. S. , and Ohno, K. EPR imaging and in vivo EPR: CRC Press, Inc, 1991. REFERENCES Buettner, G. R. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med, 3: 259 -303, 1987. Berliner, L. J. , Khramtsov, V. , Fujii, H. , and Clanton, T. L. Unique in vivo applications of spin traps. Free Radic Biol Med, 30: 489 -499, 2001. Mc. Cay, P. B. Application of ESR spectroscopy in toxicology. Arch Toxicol, 60: 133 -137, 1987. Kuppusamy, P. , Chzhan, M. , Vij, K. , Shteynbuk, M. , Lefer, D. J. , Giannella, E. , and Zweier, J. L. Threedimensional spectral-spatial EPR imaging of free radicals in the heart: a technique for imaging tissue metabolism and oxygenation. Proc Natl Acad Sci U S A, 91: 3388 -3392, 1994. Kuppusamy, P. , Ohnishi, S. T. , Numagami, Y. , Ohnishi, T. , and Zweier, J. L. Three-dimensional imaging of nitric oxide production in the rat brain subjected to ischemia-hypoxia. J Cereb Blood Flow Metab, 15: 899 -903, 1995. Kuppusamy, P. , Wang, P. , and Zweier, J. L. Three-dimensional spatial EPR imaging of the rat heart. Magn Reson Med, 34: 99 -105, 1995. Kuppusamy, P. , Chzhan, M. , Wang, P. , and Zweier, J. L. Three-dimensional gated EPR imaging of the beating heart: time-resolved measurements of free radical distribution during the cardiac contractile cycle. Magn Reson Med, 35: 323 -328, 1996. Kuppusamy, P. , Wang, P. , Samouilov, A. , and Zweier, J. L. Spatial mapping of nitric oxide generation in the ischemic heart using electron paramagnetic resonance imaging. Magn Reson Med, 36: 212 -218, 1996. Kuppusamy, P. , Wang, P. , Zweier, J. L. , Krishna, M. C. , Mitchell, J. B. , Ma, L. , Trimble, C. E. , and Hsia, C. J. Electron paramagnetic resonance imaging of rat heart with nitroxide and polynitroxyl-albumin. Biochemistry, 35: 7051 -7057, 1996. Khramtsov, V. V. , Yelinova, V. I. , Glazachev Yu, I. , Reznikov, V. A. , and Zimmer, G. Quantitative determination and reversible modification of thiols using imidazolidine biradical disulfide label. J Biochem Biophys Methods, 35: 115 -128, 1997. Kuppusamy, P. , Afeworki, M. , Shankar, R. A. , Coffin, D. , Krishna, M. C. , Hahn, S. M. , Mitchell, J. B. , and Zweier, J. L. In vivo electron paramagnetic resonance imaging of tumor heterogeneity and oxygenation in a murine model. Cancer Res, 58: 1562 -1568, 1998. Kuppusamy, P. , Shankar, R. A. , and Zweier, J. L. In vivo measurement of arterial and venous oxygenation in the rat using 3 D spectral-spatial electron paramagnetic resonance imaging. Phys Med Biol, 43:

determination and reversible modification of thiols using imidazolidine biradical disulfide label. J Biochem Biophys Methods, 35: 115 -128, 1997. Kuppusamy, P. , Afeworki, M. , Shankar, R. A. , Coffin, D. , Krishna, M. C. , Hahn, S. M. , Mitchell, J. B. , and Zweier, J. L. In vivo electron paramagnetic resonance imaging of tumor heterogeneity and oxygenation in a murine model. Cancer Res, 58: 1562 -1568, 1998. Kuppusamy, P. , Shankar, R. A. , and Zweier, J. L. In vivo measurement of arterial and venous oxygenation in the rat using 3 D spectral-spatial electron paramagnetic resonance imaging. Phys Med Biol, 43: 1837 -1844, 1998. Velan, S. S. , Spencer, R. G. , Zweier, J. L. , and Kuppusamy, P. Electron paramagnetic resonance oxygen mapping (EPROM): direct visualization of oxygen concentration in tissue. Magn Reson Med, 43: 804 -809, 2000. Kuppusamy, P. , Shankar, R. A. , Roubaud, V. M. , and Zweier, J. L. Whole body detection and imaging of nitric oxide generation in mice following cardiopulmonary arrest: detection of intrinsic nitrosoheme complexes. Magn Reson Med, 45: 700 -707, 2001. Ilangovan, G. , Li, H. , Zweier, J. L. , Krishna, M. C. , Mitchell, J. B. , and Kuppusamy, P. In vivo measurement of regional oxygenation and imaging of redox status in RIF-1 murine tumor: Effect of carbogen-breathing. Magn Reson Med, 48: 723 -730, 2002. Kuppusamy, P. , Li, H. , Ilangovan, G. , Cardounel, A. J. , Zweier, J. L. , Yamada, K. , Krishna, M. C. , and Mitchell, J. B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res, 62: 307 -312, 2002. Yamada, K. I. , Kuppusamy, P. , English, S. , Yoo, J. , Irie, A. , Subramanian, S. , Mitchell, J. B. , and Krishna, M. C. Feasibility and assessment of non-invasive in vivo redox status using electron paramagnetic resonance imaging. Acta Radiol, 43: 433 -440, 2002. WEB SITES The Illinois EPR Research Center: http: //ierc. scs. uiuc. edu/ The National Biomedical EPR Center at the Medical College of Wisconsin : http: //www. biophysics. mcw. edu/bri-epr. html NIEHS - Spin Trap Database: http: //epr. niehs. nih. gov/stdb. html
 Unpaired electrons in electron configuration
Unpaired electrons in electron configuration Selenium unpaired electrons
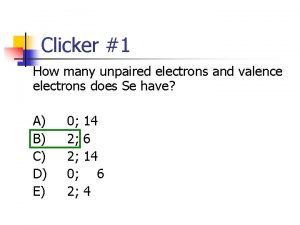
Selenium unpaired electrons How many unpaired electrons does selenium have
How many unpaired electrons does selenium have Atoms with unpaired electrons are called diamagnetic.
Atoms with unpaired electrons are called diamagnetic. Vanadium number of valence electrons
Vanadium number of valence electrons Manageengine desktop central spying
Manageengine desktop central spying Hewlett-packard spying scandal case study
Hewlett-packard spying scandal case study Unpaired vs paired t test
Unpaired vs paired t test Paired vs unpaired t test
Paired vs unpaired t test Difference between a paired and unpaired t test
Difference between a paired and unpaired t test Unpaired tags in html examples
Unpaired tags in html examples Paired t-test formula
Paired t-test formula Unpaired consonants in english
Unpaired consonants in english Epr
Epr Sylvie richard edf
Sylvie richard edf Epr sue jennings
Epr sue jennings Epr paradox
Epr paradox Epr wwarszawa
Epr wwarszawa Epr cms
Epr cms Epr.ulaanbaatar
Epr.ulaanbaatar Epr example
Epr example Targeted delivery
Targeted delivery Epr einstein podolsky rosen
Epr einstein podolsky rosen Epr paradox
Epr paradox Whole airman concept epr bullets
Whole airman concept epr bullets Ir
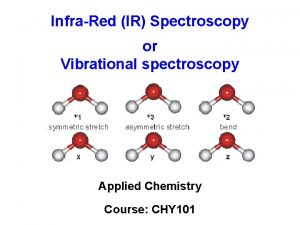
Ir Ir spectroscopy provides information about
Ir spectroscopy provides information about Mossbauer spectroscopy
Mossbauer spectroscopy Ftir spectroscopy theory
Ftir spectroscopy theory Beer lambert law
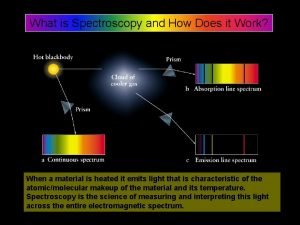
Beer lambert law What is spectroscopy
What is spectroscopy Total consumption burner
Total consumption burner Deep learning spectroscopy
Deep learning spectroscopy Principles of atomic emission spectroscopy
Principles of atomic emission spectroscopy Atomic emission spectroscopy lecture notes
Atomic emission spectroscopy lecture notes Isms spectroscopy
Isms spectroscopy Periklis papadopoulos
Periklis papadopoulos Flame aas
Flame aas Spectroscopy problem set
Spectroscopy problem set Site:slidetodoc.com
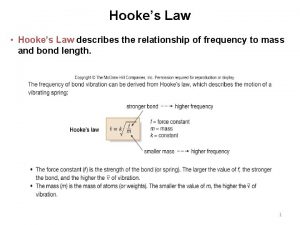
Site:slidetodoc.com Hooke's law in ir spectroscopy
Hooke's law in ir spectroscopy Mass spec principle
Mass spec principle Woodward fieser rule in uv spectroscopy
Woodward fieser rule in uv spectroscopy Uv vis spectroscopy in pharmaceutical analysis
Uv vis spectroscopy in pharmaceutical analysis Mossbauer spectroscopy
Mossbauer spectroscopy Why are raman and ir complementary
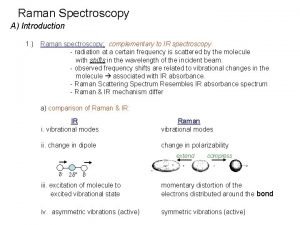
Why are raman and ir complementary