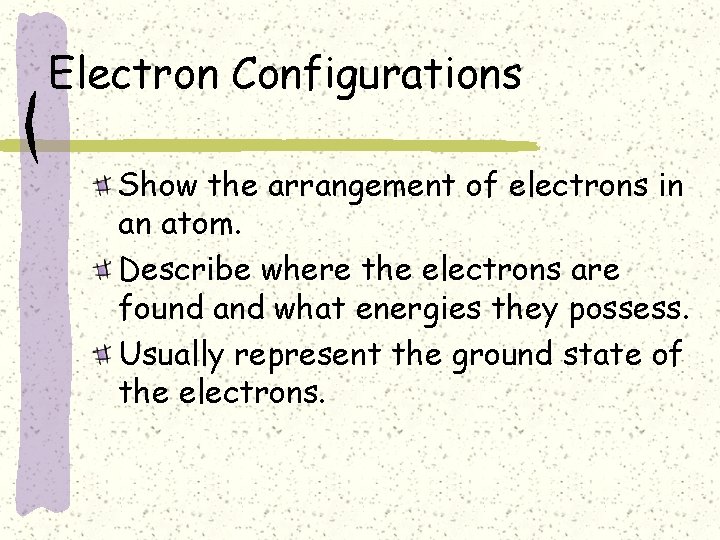
Electron Configurations Show the arrangement of electrons in




- Slides: 15

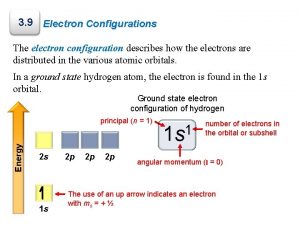
Electron Configurations Show the arrangement of electrons in an atom. Describe where the electrons are found and what energies they possess. Usually represent the ground state of the electrons.

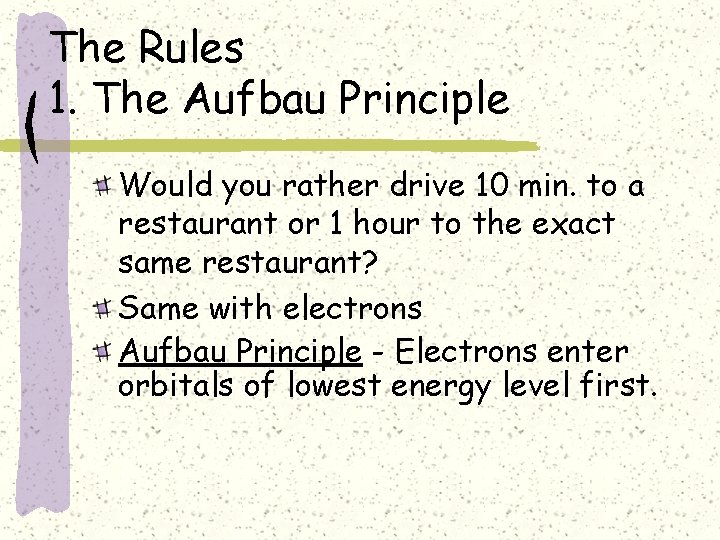
The Rules 1. The Aufbau Principle Would you rather drive 10 min. to a restaurant or 1 hour to the exact same restaurant? Same with electrons Aufbau Principle - Electrons enter orbitals of lowest energy level first.

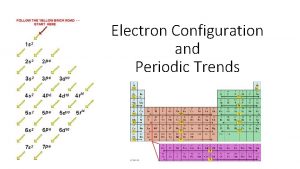
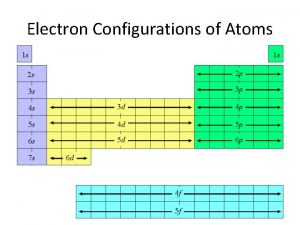
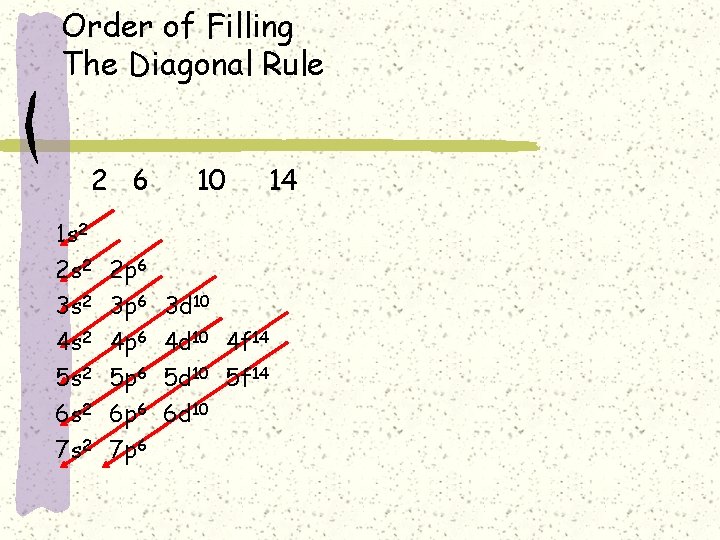
Order of Filling The Diagonal Rule 2 6 10 14 1 s 2 2 p 6 3 s 2 4 s 2 5 s 2 6 s 2 7 s 2 3 p 6 4 p 6 5 p 6 6 p 6 7 p 6 3 d 10 4 f 14 5 d 10 5 f 14 6 d 10

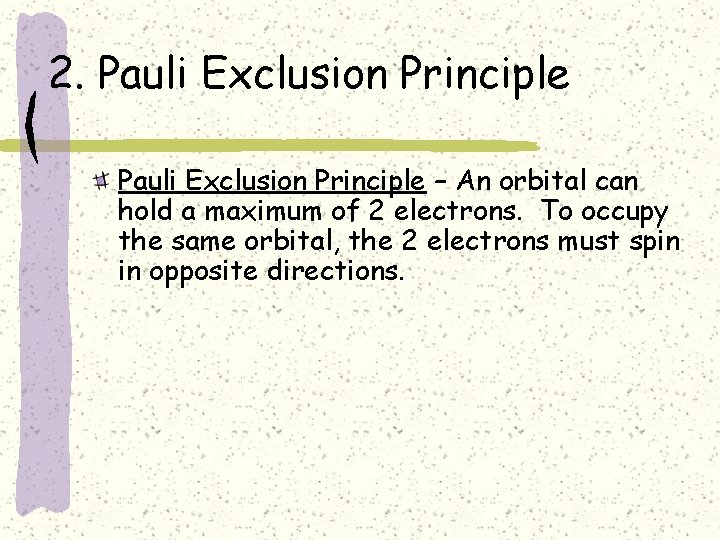
2. Pauli Exclusion Principle – An orbital can hold a maximum of 2 electrons. To occupy the same orbital, the 2 electrons must spin in opposite directions.

3. Hund’s Rule - one electron enters each orbital until each orbital contain one electron with parallel spins before a second electron is added. Electrons like to stay unpaired for as long as possible Orbitals of equal energy are each occupied by one electron first before any orbital is occupied by another electron Unpaired electrons in the same orbital have the SAME spin

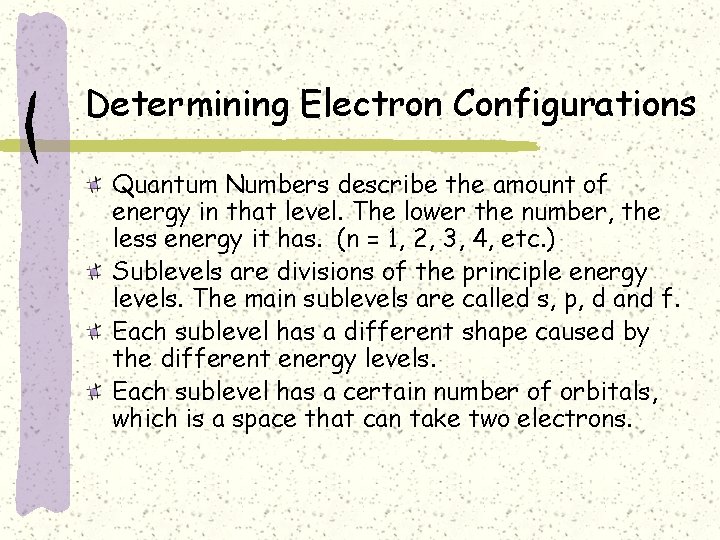
Determining Electron Configurations Quantum Numbers describe the amount of energy in that level. The lower the number, the less energy it has. (n = 1, 2, 3, 4, etc. ) Sublevels are divisions of the principle energy levels. The main sublevels are called s, p, d and f. Each sublevel has a different shape caused by the different energy levels. Each sublevel has a certain number of orbitals, which is a space that can take two electrons.

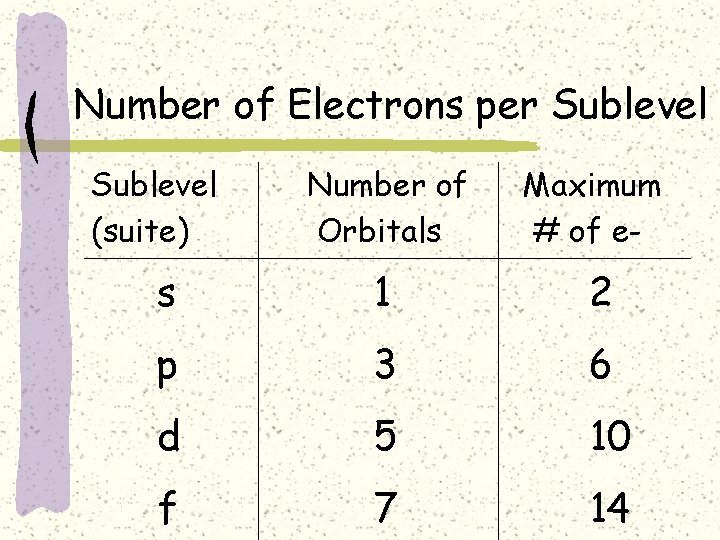
Number of Electrons per Sublevel (suite) Number of Orbitals Maximum # of e- s 1 2 p 3 6 d 5 10 f 7 14

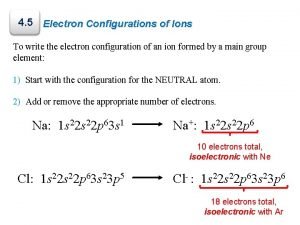
Let’s Try an Example An atom of carbon has 6 electrons arranged in the following order. 1 s 22 p 2 Carbon’s electrons require two energy levels. (Second quantum number. ) It has 4 electrons in its highest level. Superscripts must add up to the atomic number of the element. (#p+ = #e-) Carbon has 6 p+ and 6 e-. (2+2+2)

How about another one? ? ? An atom of sodium has 11 electrons arranged in the following order. 1 s 22 p 63 s 1 Sodium’s electrons require three energy levels. (Third quantum number. ) It has 1 electron in its highest level. Superscripts must add up to the atomic number of the element. (#p+ = #e-) Sodium has 11 p+ and 11 e-. (2+2+6+1)

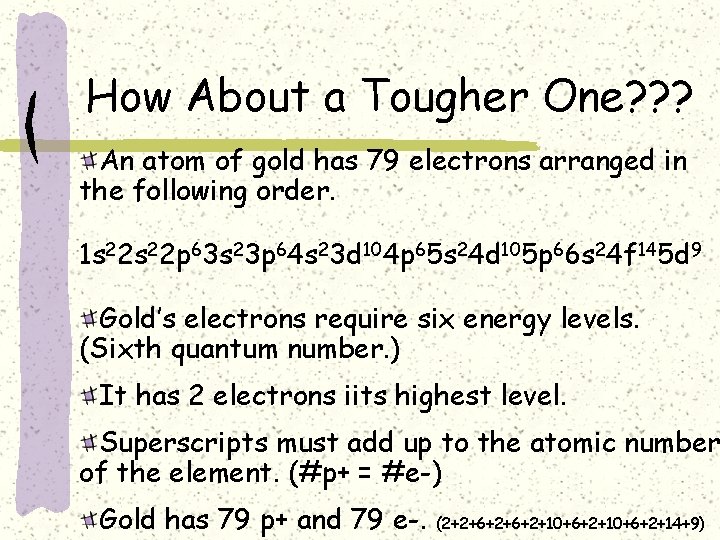
How About a Tougher One? ? ? An atom of gold has 79 electrons arranged in the following order. 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 145 d 9 Gold’s electrons require six energy levels. (Sixth quantum number. ) It has 2 electrons iits highest level. Superscripts must add up to the atomic number of the element. (#p+ = #e-) Gold has 79 p+ and 79 e-. (2+2+6+2+10+6+2+14+9)

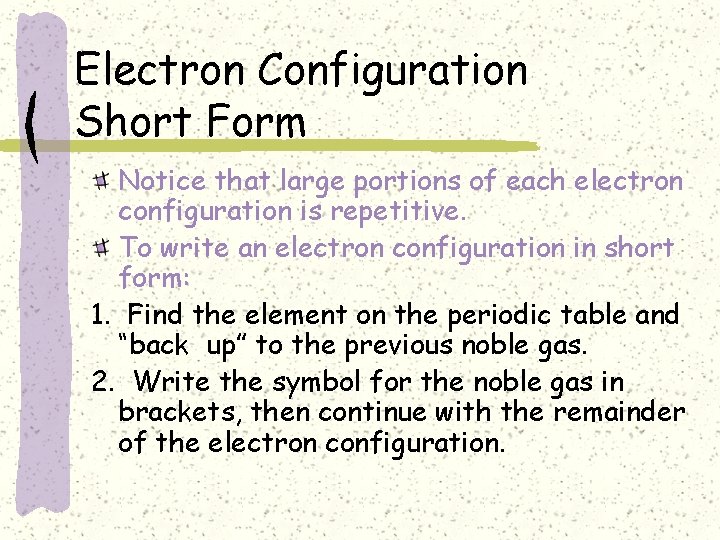
Electron Configuration Short Form Notice that large portions of each electron configuration is repetitive. To write an electron configuration in short form: 1. Find the element on the periodic table and “back up” to the previous noble gas. 2. Write the symbol for the noble gas in brackets, then continue with the remainder of the electron configuration.

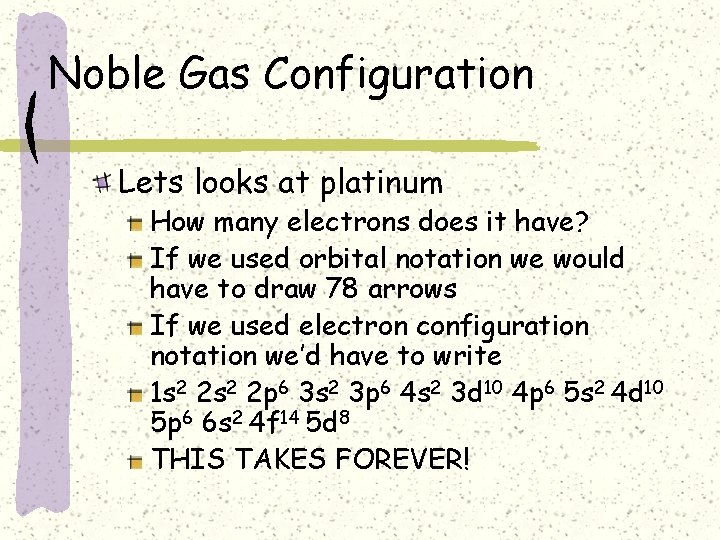
Noble Gas Configuration Lets looks at platinum How many electrons does it have? If we used orbital notation we would have to draw 78 arrows If we used electron configuration notation we’d have to write 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 14 5 d 8 THIS TAKES FOREVER!

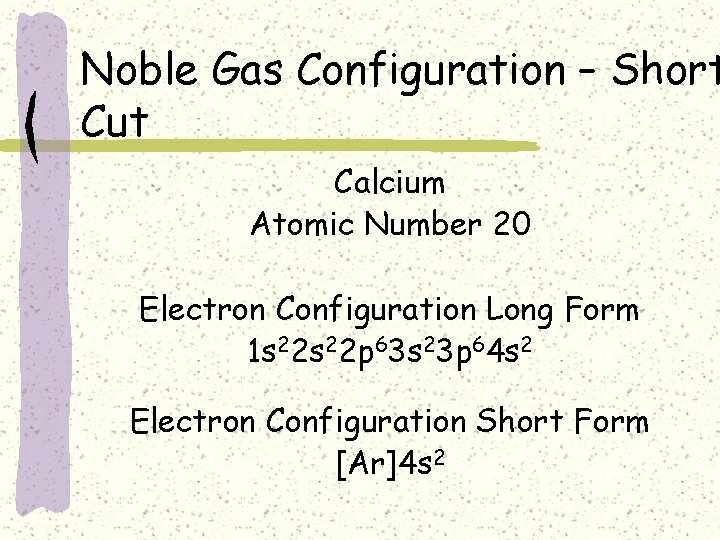
Noble Gas Configuration – Short Cut Calcium Atomic Number 20 Electron Configuration Long Form 1 s 22 p 63 s 23 p 64 s 2 Electron Configuration Short Form [Ar]4 s 2

Thank you, may I have another? ? ? Tin Atomic Number 50 Electron Configuration Long Form 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 2 Electron Configuration Short Form [Kr]5 s 24 d 105 p 2

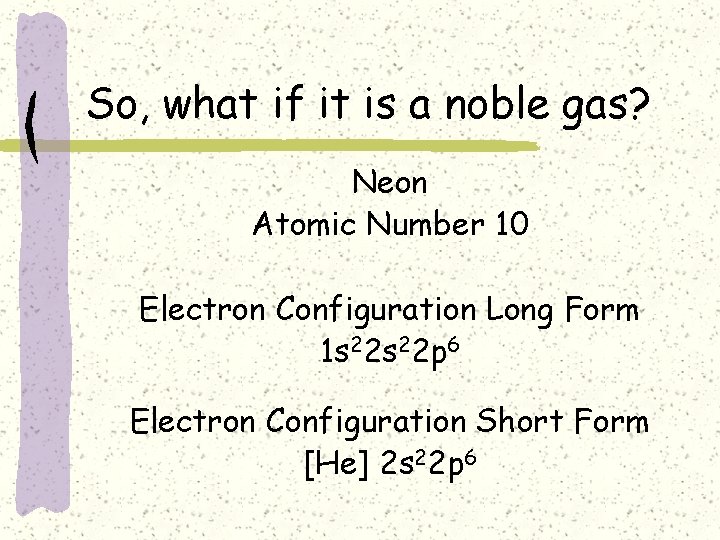
So, what if it is a noble gas? Neon Atomic Number 10 Electron Configuration Long Form 1 s 22 p 6 Electron Configuration Short Form [He] 2 s 22 p 6
 Electrons configurations
Electrons configurations Electrons in atoms section 3 electron configuration
Electrons in atoms section 3 electron configuration Electrons configurations
Electrons configurations Calcium ion formula
Calcium ion formula Stable electron configurations
Stable electron configurations Stable electron configurations are likely to contain
Stable electron configurations are likely to contain Complete ground state electron configuration
Complete ground state electron configuration An orbital is a region of space in an atom where there is
An orbital is a region of space in an atom where there is 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Electron configurations of ions
Electron configurations of ions Hund's rule vs pauli exclusion principle
Hund's rule vs pauli exclusion principle Chapter 5 review arrangement of electrons in atoms
Chapter 5 review arrangement of electrons in atoms Electronic configuration is arrangement of electrons in
Electronic configuration is arrangement of electrons in Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 4 arrangement of electrons in atoms test
Chapter 4 arrangement of electrons in atoms test