Electron Configurations Unit 3 What are electron configurations











- Slides: 17

Electron Configurations Unit 3

What are electron configurations? l According to the wave mechanical model, the electrons in an atom move around in the orbitals surrounding the nucleus. l There are 7 orbitals! ¡They are numbered 1 – 7! ¡Each orbital has sublevels!

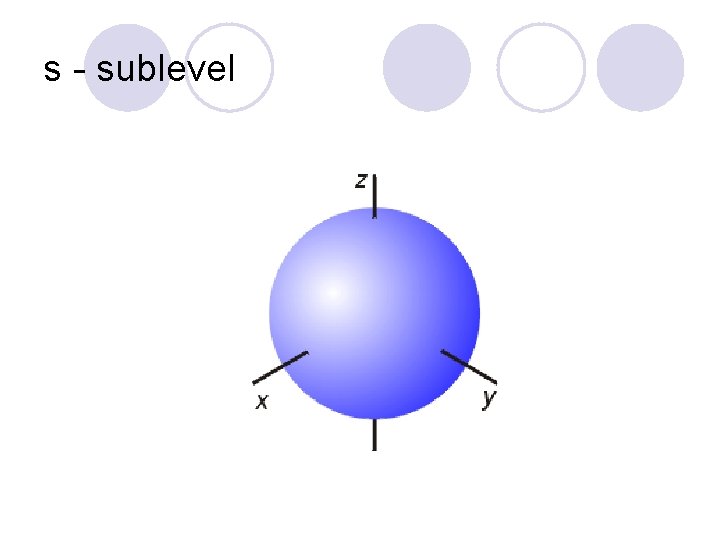
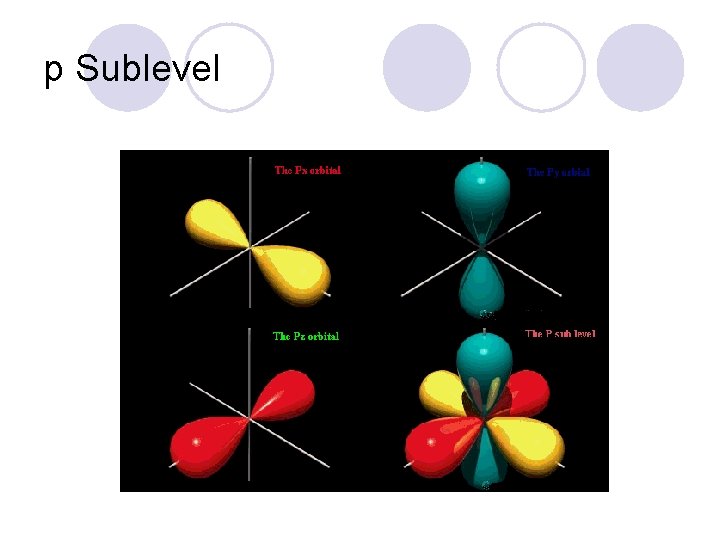
Sublevels! l They are labeled s, p, d, and f! ¡Each sublevel has a different shape to it!

s - sublevel

p Sublevel

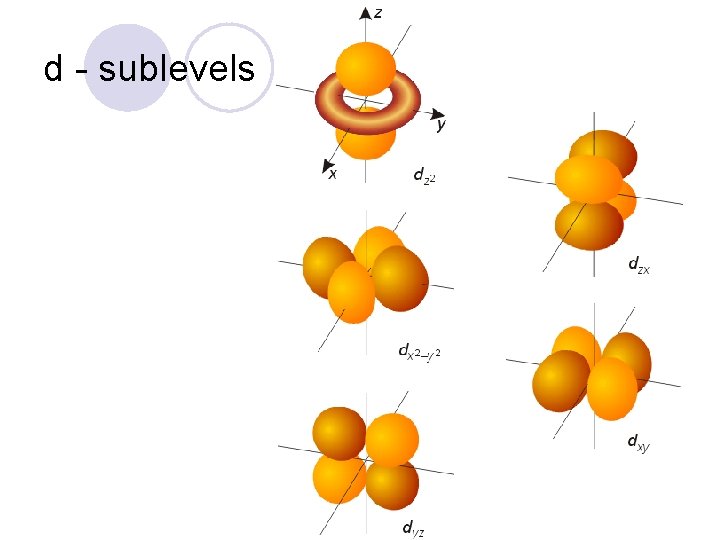
d - sublevels

f - sublevels

Each sublevel has a certain # of “rooms” that 2 electrons reside in. l s has 1 “room” l p has 3 “rooms” l d has 5 “rooms” l f has 7 “rooms” l How many electrons fit in each sublevel total?

Electron Configurations l Are a way of writing out where all the electrons are in an atom. l It specifies the orbital, sublevel, and how many electrons are in the sublevel.

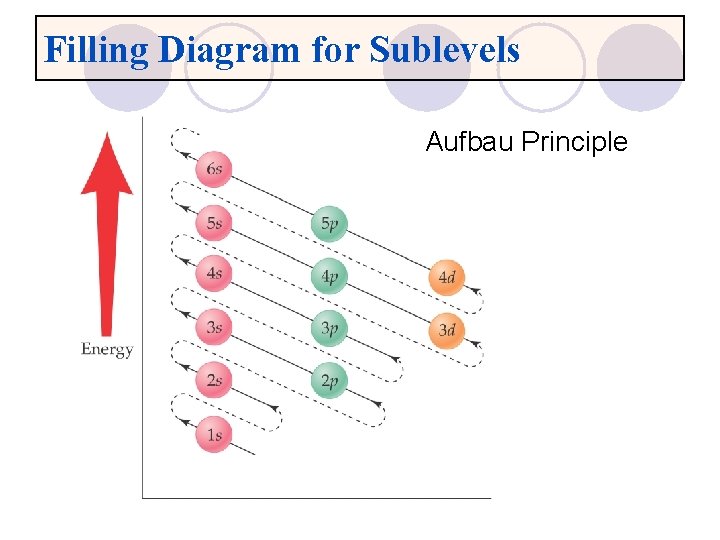
Order of filling

Filling Diagram for Sublevels Aufbau Principle

Rules for writing electron configurations l Pauli exclusion principle ¡Each “room” can hold a maximum of 2 electrons, but they must be spinning in opposite directions. l Aufbau Principle ¡Electrons enter orbital in order of increasing energy. l Hund’s Rule ¡One electron must fill each “room” in a sublevel before there can be 2.

Examples l Nitrogen ¡ 1 s 22 p 3 l Iron ¡ 1 s 22 p 63 s 23 p 64 s 23 d 6

Exceptions to the rules. l Chromium ¡ 1 s 22 p 63 s 23 p 64 s 13 d 5 l Molybdenum ¡ 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 5 s 14 d 5 l Tungsten ¡ 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 5 s 24 d 105 p 66 s 15 d 5 l Copper ¡ 1 s 22 p 63 s 23 p 64 s 13 d 10 l Silver ¡ 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 5 s 14 d 10 l Gold ¡ 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 5 s 24 d 105 p 66 s 15 d 10

The Periodic Table and Noble Gas Configurations l Noble gas configurations are a short hand way of writing electron configurations & the Periodic Table can be used to help you write them!


Let’s try some! l Nitrogen ¡Electron configuration - 1 s 22 p 3 ¡Noble Gas configuration – [He] 2 s 22 p 3 l Iron ¡Electron configuration - 1 s 22 p 63 s 23 p 64 s 23 d 6 ¡Noble Gas configuration – [Ar] 4 s 23 d 6