Electron Configuration Electron configurations show the arrangement of









![Abbreviated Configuration 3 LI [He] 2 s 1 26 Fe [Ar] 3 d 6 Abbreviated Configuration 3 LI [He] 2 s 1 26 Fe [Ar] 3 d 6](https://slidetodoc.com/presentation_image_h2/32f54ff2b24850be059c8b48ac83fc18/image-19.jpg)



- Slides: 30

Electron Configuration

• Electron configurations show the arrangement of electrons within atoms. • Remember: Atom = nucleus + electron cloud

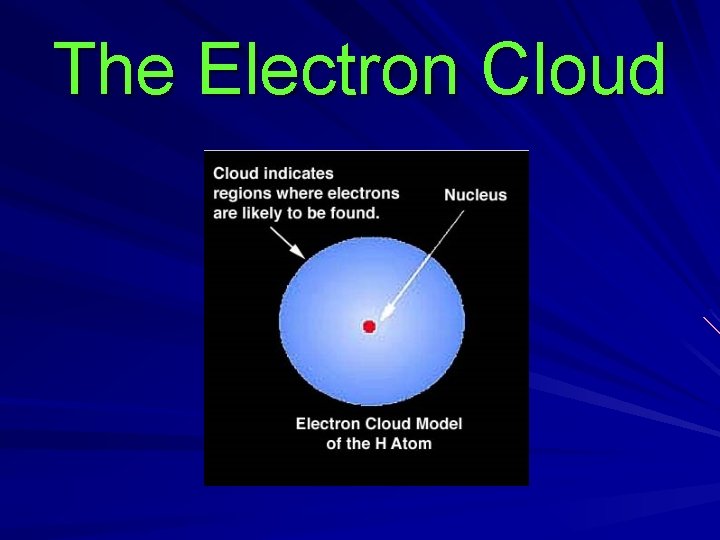
The Electron Cloud

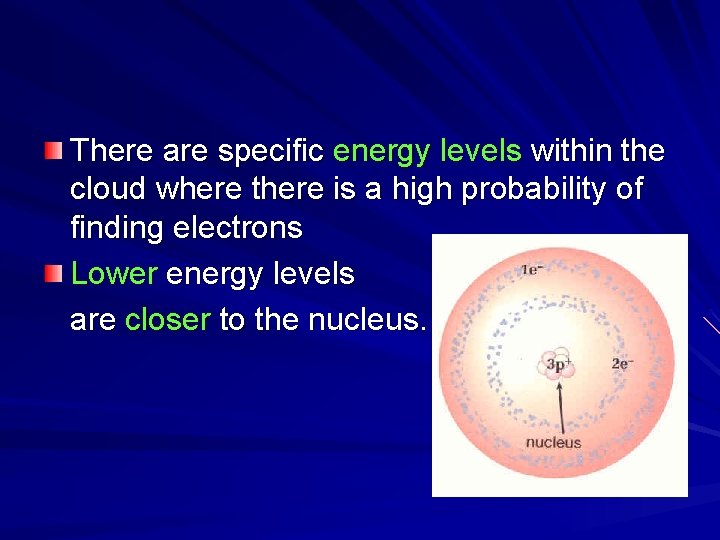
There are specific energy levels within the cloud where there is a high probability of finding electrons Lower energy levels are closer to the nucleus.

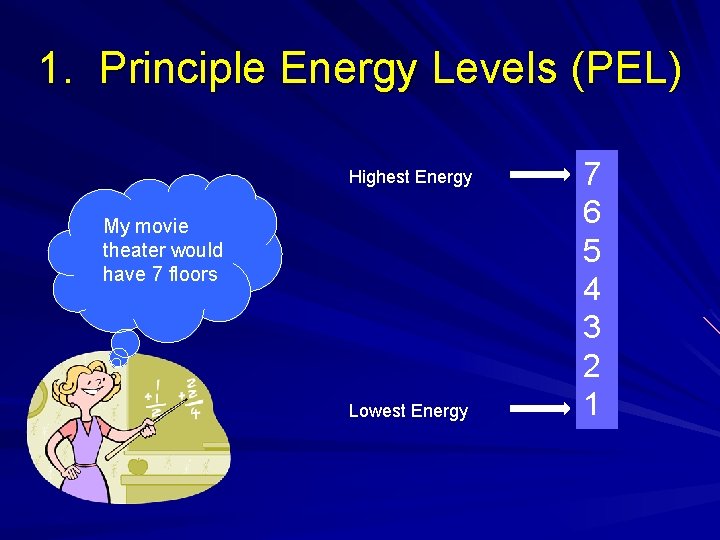
1. Principle Energy Levels (PEL) Highest Energy My movie theater would have 7 floors Lowest Energy 7 6 5 4 3 2 1

2. Orbital Sublevels s p d f The first floor would have one row, the “s” row!

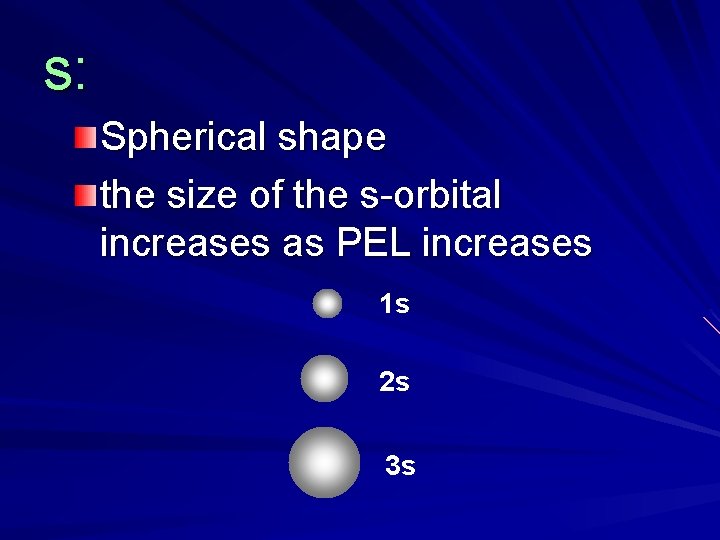
s: Spherical shape the size of the s-orbital increases as PEL increases 1 s 2 s 3 s

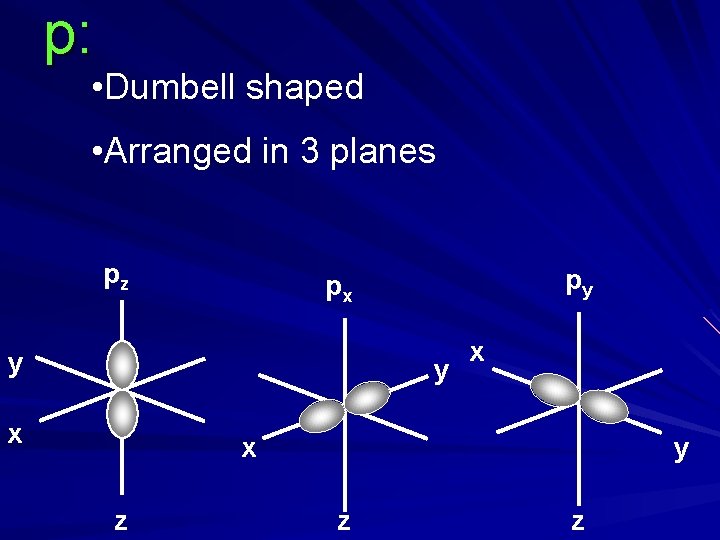
p: • Dumbell shaped • Arranged in 3 planes pz py px y y x x x z y z z

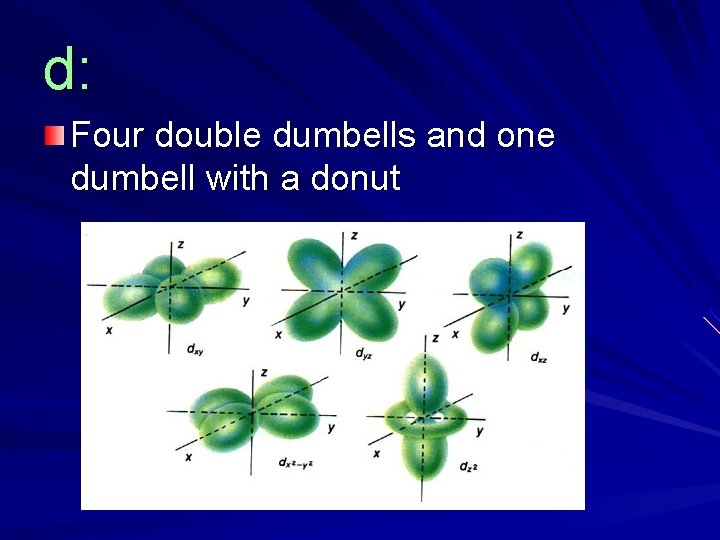
d: Four double dumbells and one dumbell with a donut

f: Too complicated to draw!

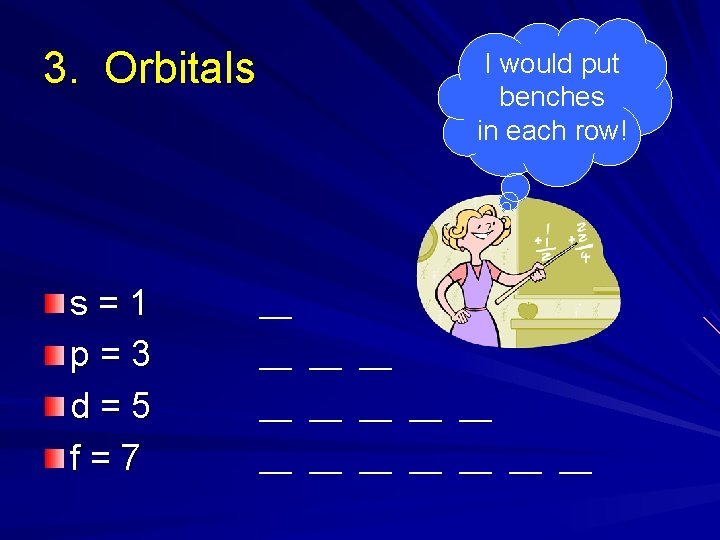
3. Orbitals s=1 p=3 d=5 f=7 I would put benches in each row! ___ ___ ___ ___

4. Electrons Two people could sit on each bench!

5 Ways to Write Electron Configurations 1. Complete 2. Abbreviated 3. Orbital Notation 4. Electron Dot Diagram 5. Quantum Numbers

Let’s stop and practice some different ways to write electron configurations!

Complete Configuration · Remember, electrons are located in orbitals, regions around the nucleus that correspond to specific energy levels. · A complete configuration shows the PEL, sublevel, and orbital for each electron in an atom.

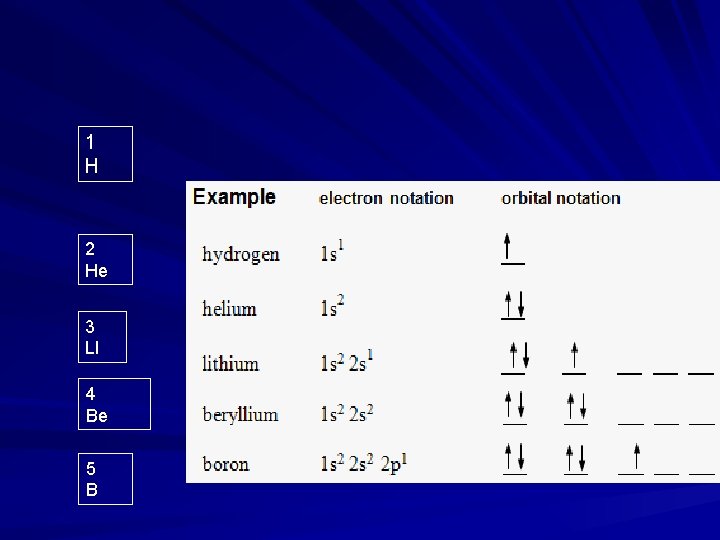
1 H 2 He 3 LI 4 Be 5 B

· d’s are “cheaters”, they dive back in one level · Why? Because energy-wise, it is easier to fill the 4 s level with two electrons than the 3 d with 10 electrons · Just follow the Aufbau diagram! =1 s = 22 s 22 p 63 s 23 p 64 s 23 d 6 26 Fe

· f’s are “really big cheaters”, they dive back in two levels · Just follow the Aufbau diagram. · Double check the number of electrons when you are finished (OK to use calculator). 22 s 22 p 63 s 23 p 64 s 23 d 10 82 =1 s = Pb 4 p 65 s 24 d 105 p 66 s 24 f 145 d 106 p 2
![Abbreviated Configuration 3 LI He 2 s 1 26 Fe Ar 3 d 6 Abbreviated Configuration 3 LI [He] 2 s 1 26 Fe [Ar] 3 d 6](https://slidetodoc.com/presentation_image_h2/32f54ff2b24850be059c8b48ac83fc18/image-19.jpg)
Abbreviated Configuration 3 LI [He] 2 s 1 26 Fe [Ar] 3 d 6 4 s 2 11 Na [Ne] 3 s 1 17 Cl [Ne] 3 s 2 3 p 5 82 Pb [Xe] 4 f 14 5 d 10 6 s 2 6 p 2

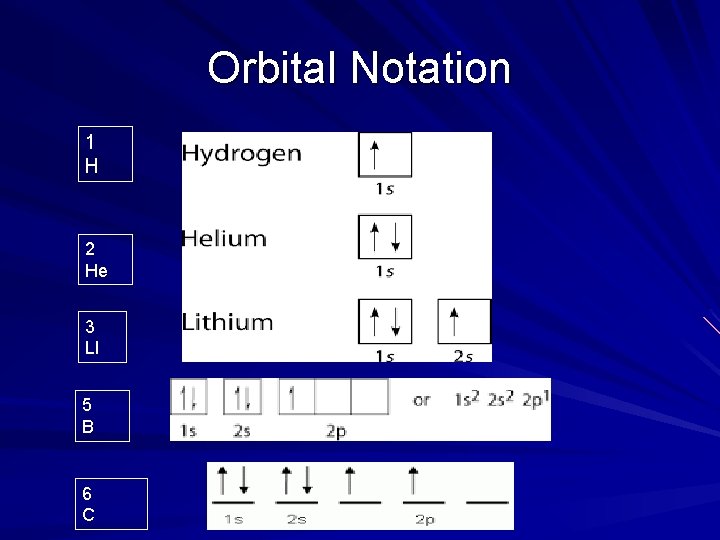
Orbital Notation 1 H 2 He 3 LI 5 B 6 C

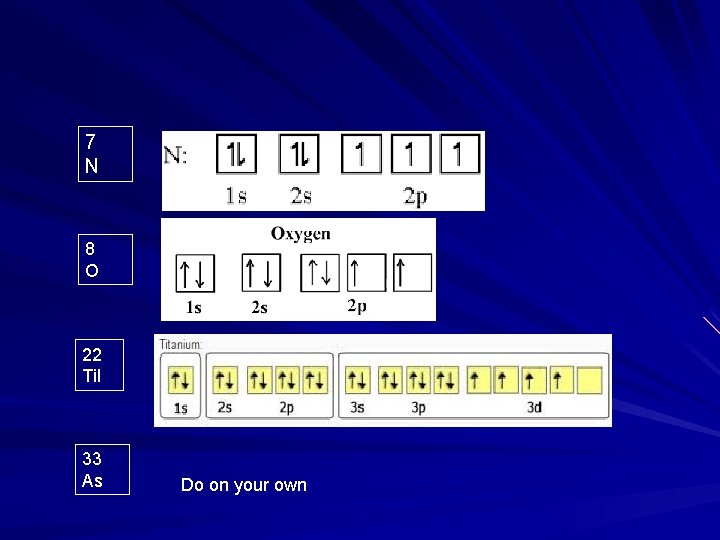
7 N 8 O 22 Ti. I 33 As Do on your own

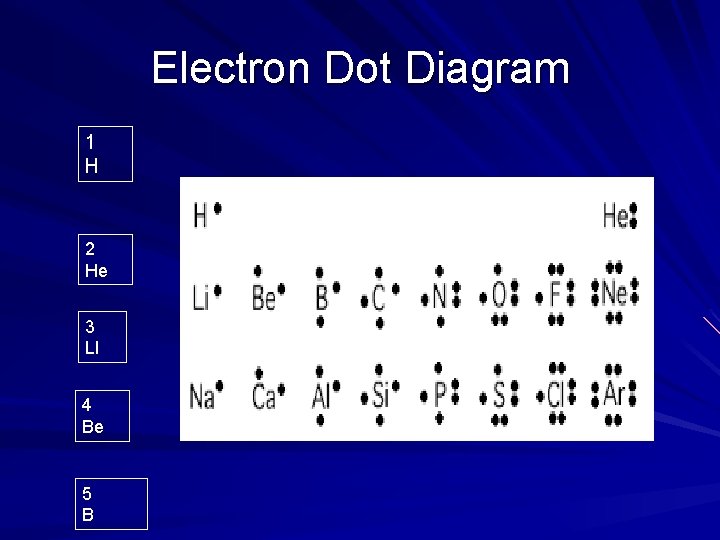
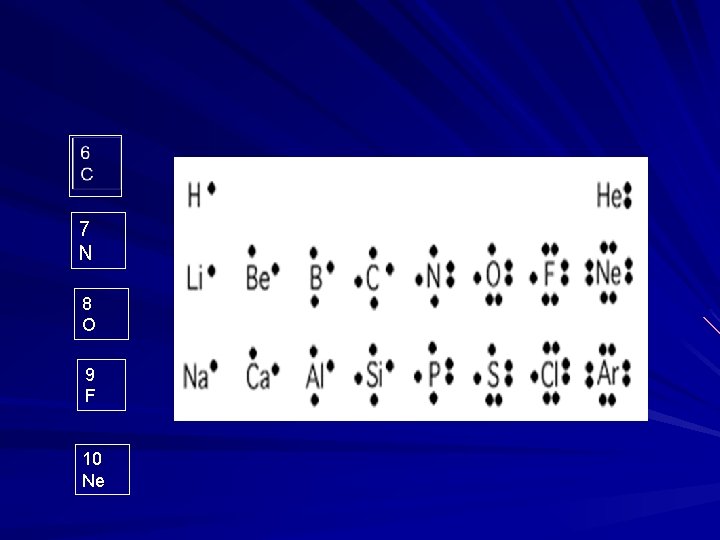
Electron Dot Diagram 1 H 2 He 3 LI 4 Be 5 B

7 N 8 O 9 F 10 Ne

Electrons and Light

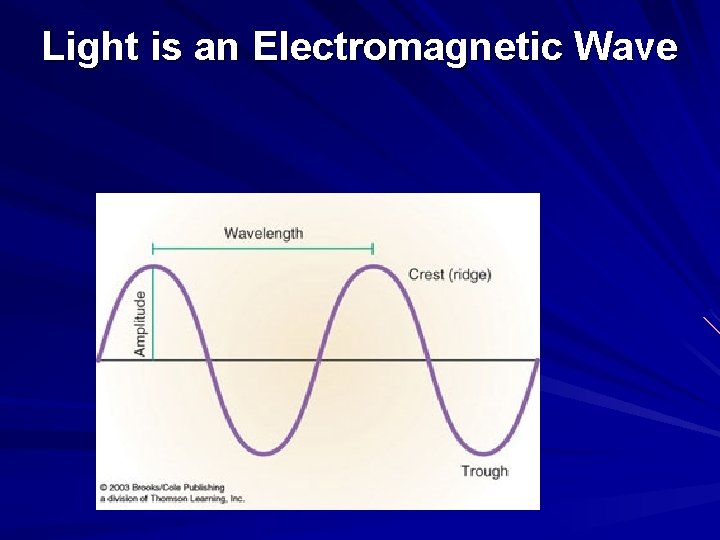
Electrons Act Like Both Particles and Waves When electrons move about the nucleus within their orbitals, they are behaving as particles with mass. However, electrons also behave as waves of energy.

Light is an Electromagnetic Wave

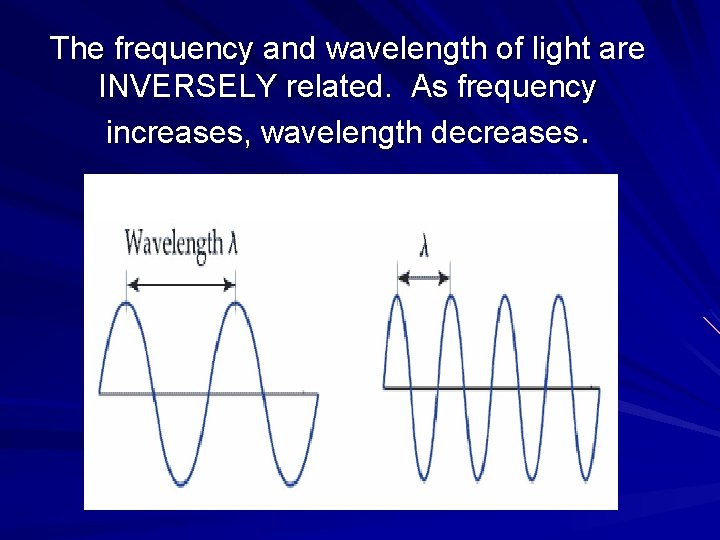
The frequency and wavelength of light are INVERSELY related. As frequency increases, wavelength decreases.

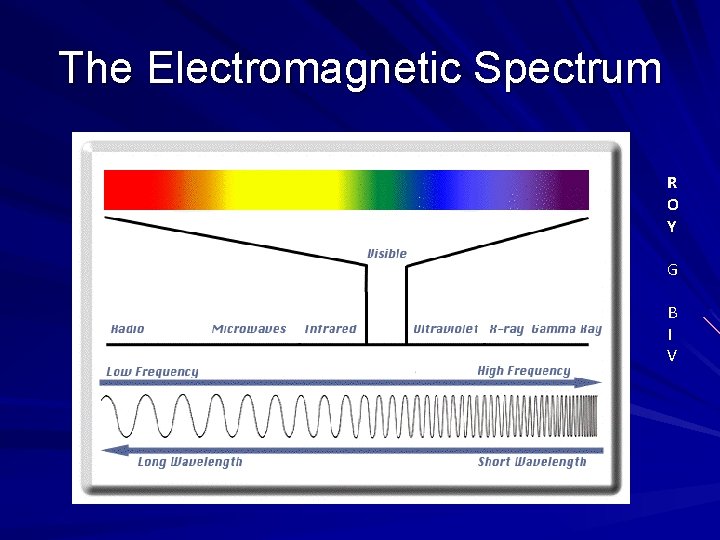
The Electromagnetic Spectrum R O Y G B I V

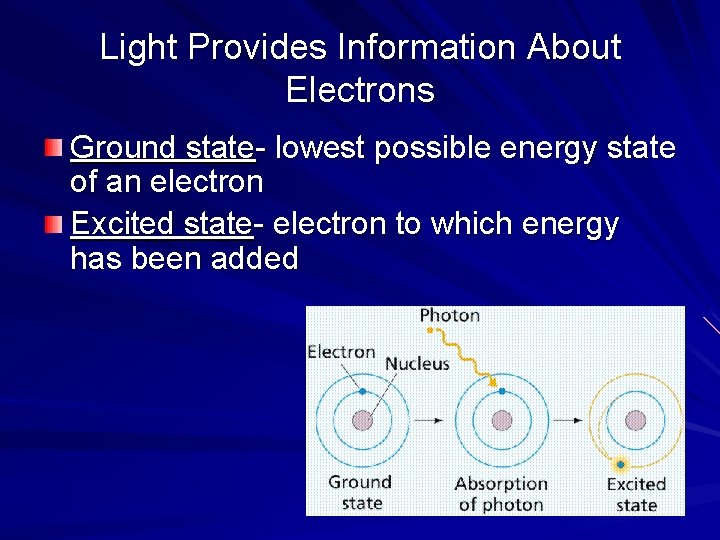
Light Provides Information About Electrons Ground state- lowest possible energy state of an electron Excited state- electron to which energy has been added

An electron in its excited state will not stay there very long. It will release a specific quantity of energy as it “falls” back to its ground state. Each element releases energy as a unique set of wavelengths. Metal ions release energy as visible light and can be identified by their emission spectra.