Electron Configurations Electron configurations show the arrangement of





- Slides: 11


Electron Configurations • Electron configurations show the arrangement of electrons in an atom. • A distinct electron configuration exists for atoms of each element. • Electrons like to assume arrangements that have the lowest possible energies.

Rules Governing Electron Configurations • To build up electron configurations for the ground state of any particular atom, first the energy levels of the orbitals are determined. • Then electrons are added to the orbitals one by one according to three basic rules.

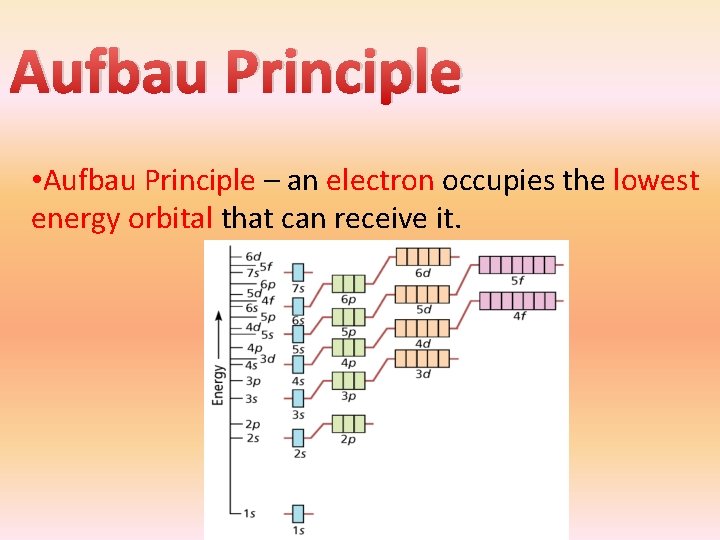
Aufbau Principle • Aufbau Principle – an electron occupies the lowest energy orbital that can receive it.

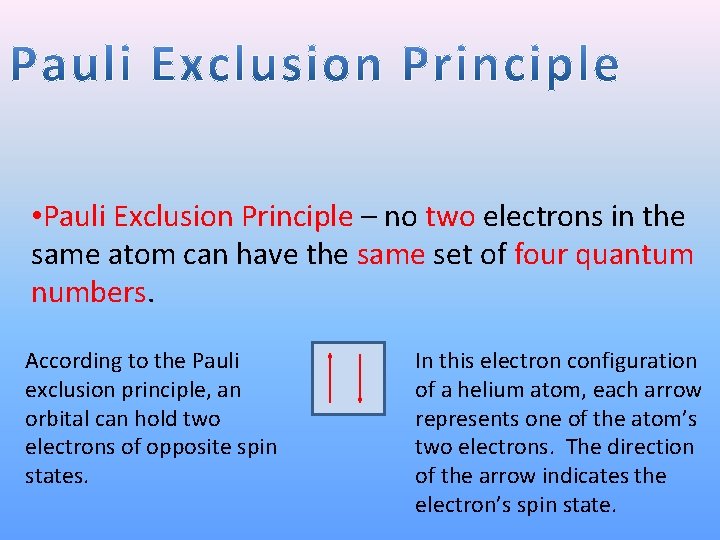
• Pauli Exclusion Principle – no two electrons in the same atom can have the same set of four quantum numbers. According to the Pauli exclusion principle, an orbital can hold two electrons of opposite spin states. In this electron configuration of a helium atom, each arrow represents one of the atom’s two electrons. The direction of the arrow indicates the electron’s spin state.

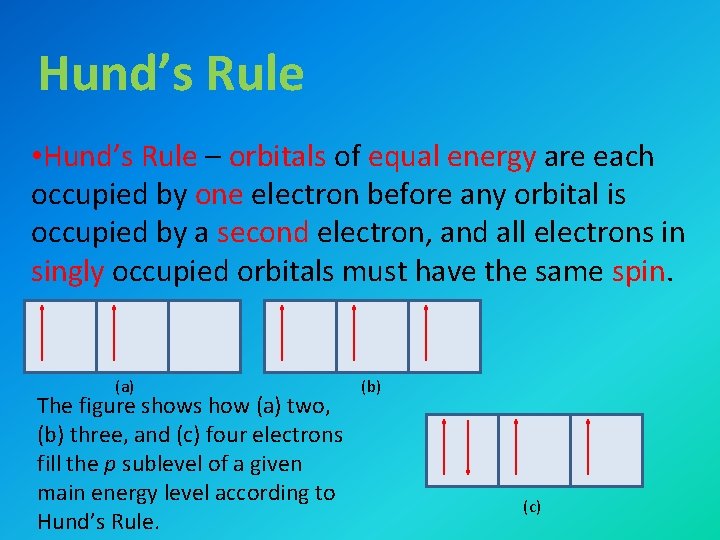
Hund’s Rule • Hund’s Rule – orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin. (a) The figure shows how (a) two, (b) three, and (c) four electrons fill the p sublevel of a given main energy level according to Hund’s Rule. (b) (c)

Representing Electron Configurations • Orbital Notation – an unoccupied orbital is represented by a line _____, with the orbital’s name underneath. Arrows represent electrons. • Beryllium’s orbital notation would be 1 s 2 s Beryllium has four electrons which are represented by four arrows in the orbital notation above.

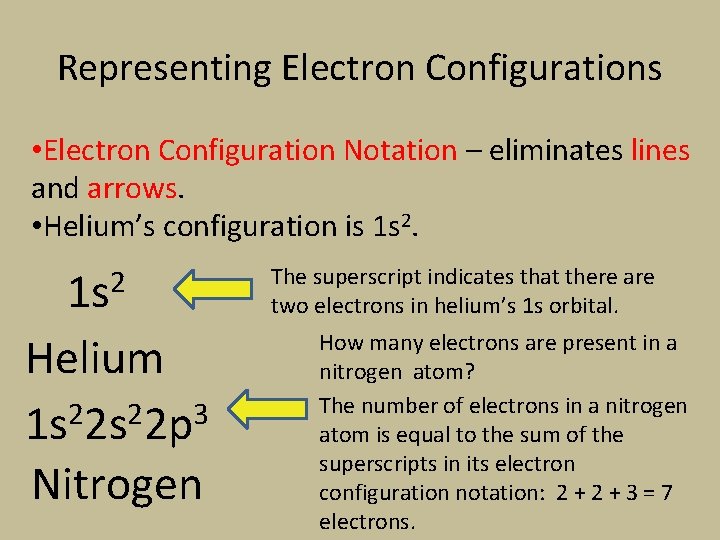
Representing Electron Configurations • Electron Configuration Notation – eliminates lines and arrows. • Helium’s configuration is 1 s 2 Helium 1 s 22 p 3 Nitrogen The superscript indicates that there are two electrons in helium’s 1 s orbital. How many electrons are present in a nitrogen atom? The number of electrons in a nitrogen atom is equal to the sum of the superscripts in its electron configuration notation: 2 + 3 = 7 electrons.

Noble Gas Notation Compare the configuration of a sodium atom with that of an atom of neon. Sodium, Na Neon, Ne 1 s 22 p 63 s 1 [Ne]3 s 1 1 s 22 p 6 Notice that the first 10 electrons in a sodium atom have the same configuration as a neon atom. This similarity allows us to use a shorthand notation for the electron configuration of sodium.

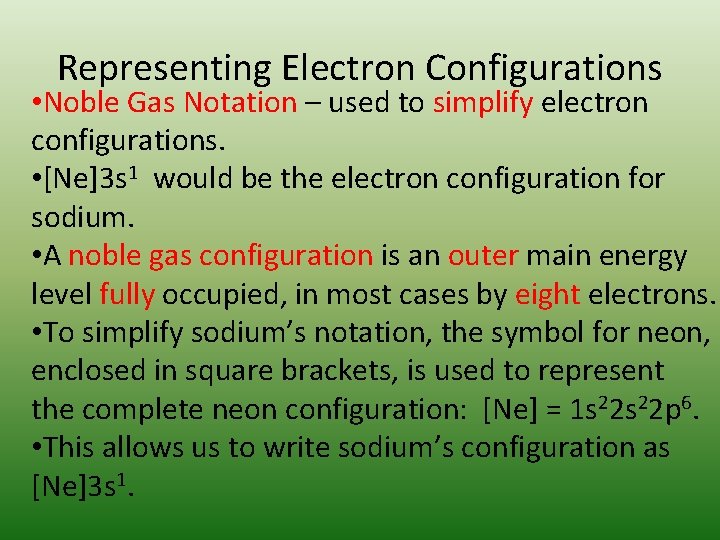
Representing Electron Configurations • Noble Gas Notation – used to simplify electron configurations. • [Ne]3 s 1 would be the electron configuration for sodium. • A noble gas configuration is an outer main energy level fully occupied, in most cases by eight electrons. • To simplify sodium’s notation, the symbol for neon, enclosed in square brackets, is used to represent the complete neon configuration: [Ne] = 1 s 22 p 6. • This allows us to write sodium’s configuration as [Ne]3 s 1.

Exceptions • Electron configurations of some atom, such as chromium and copper, deviate from the predictions of the Aufbau principle, but the ground-state configuration that results is the configuration with the minimum possible energy.