Electron Configurations Chemistry 1 d Students know how



















- Slides: 47

Electron Configurations Chemistry 1 d - Students know how to use the periodic table to determine the number of electrons available for bonding.

How to identify an element: - Look at the protons How to find protons: - Look at the atomic number How to find neutrons: - Subtract atomic mass from atomic number (the # of protons) How to find electrons in a neutral (no charge) atom : - Always equals the number of protons

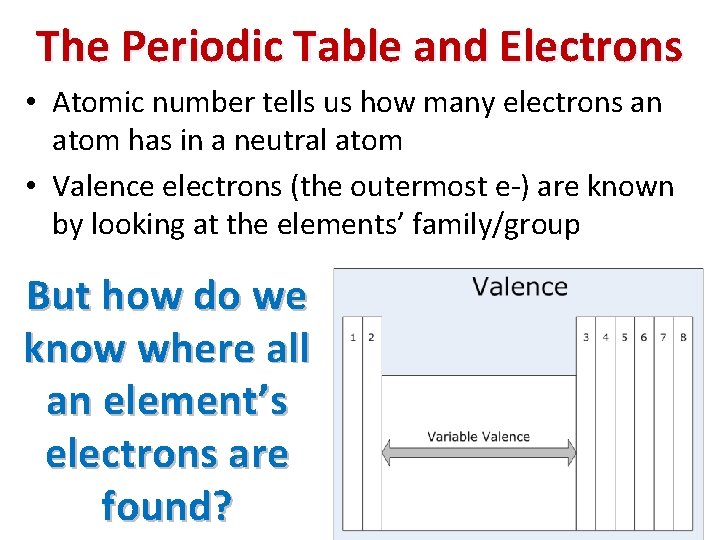
The Periodic Table and Electrons • Atomic number tells us how many electrons an atom has in a neutral atom • Valence electrons (the outermost e-) are known by looking at the elements’ family/group But how do we know where all an element’s electrons are found?

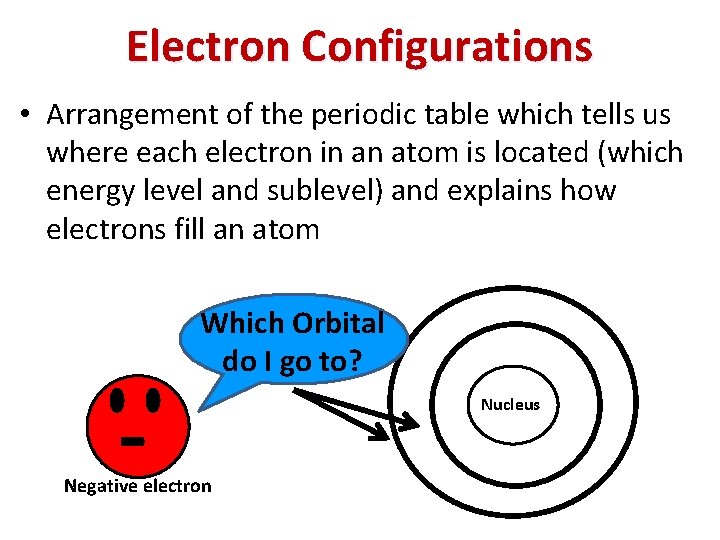
Electron Configurations • Arrangement of the periodic table which tells us where each electron in an atom is located (which energy level and sublevel) and explains how electrons fill an atom - Which Orbital do I go to? Negative electron Nucleus

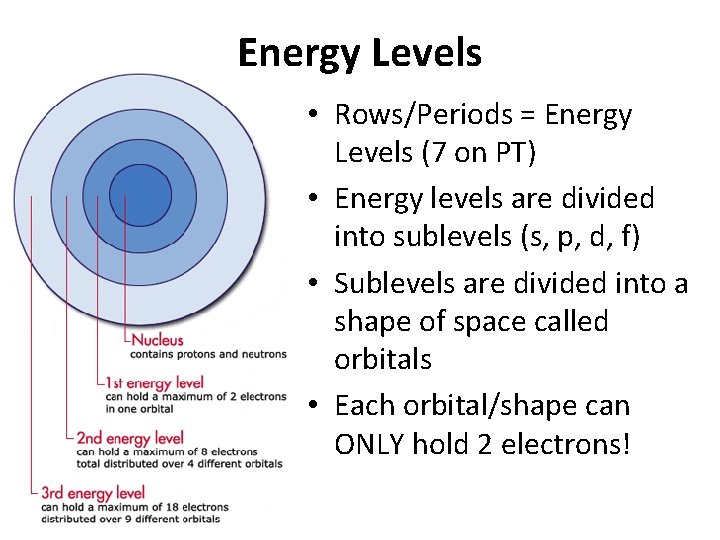
Energy Levels • Rows/Periods = Energy Levels (7 on PT) • Energy levels are divided into sublevels (s, p, d, f) • Sublevels are divided into a shape of space called orbitals • Each orbital/shape can ONLY hold 2 electrons!

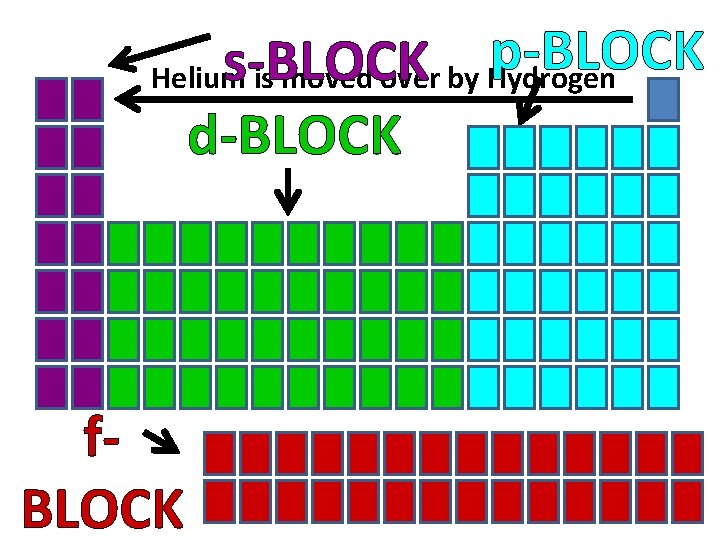
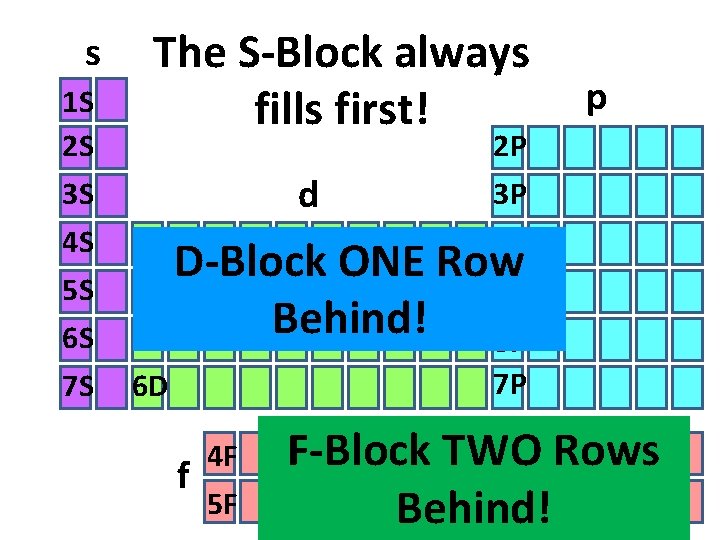
s, p, d, f Blocks • The periodic table can be divided into sections called blocks (energy sublevels) • 4 blocks/sublevels = s, p, d, f • s, p, d, f sublevels are divided into orbitals • s = 1 shape • p = 3 shapes • d = 5 shapes • f = 7 shapes


Remember…S, P, D, & F blocks refer to the shape of the orbital in which electrons are found!

p-BLOCK s-BLOCK Helium is moved over by Hydrogen d-BLOCK f. BLOCK

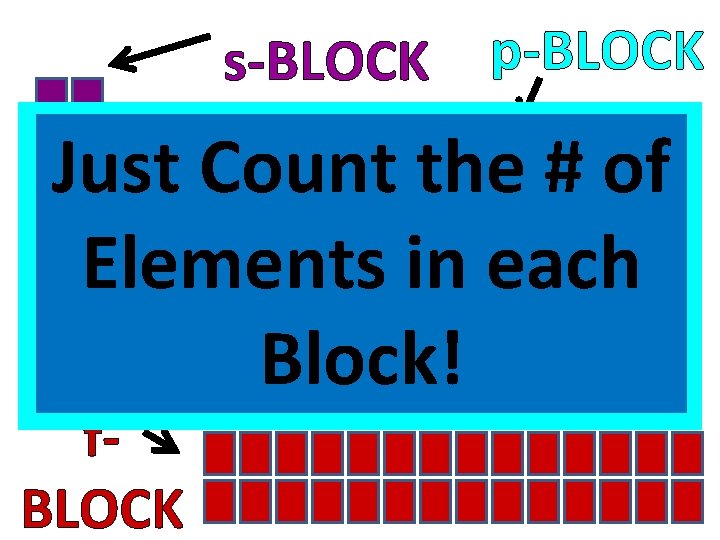
s-BLOCK d-BLOCK p-BLOCK many Just. How Count the # of Elements in each electrons can fit Block! in each Orbital? f. BLOCK

s-BLOCK 2 ELECTRONS d-BLOCK 10 Electrons f. BLOCK 14 Electrons p-BLOCK 6 Electrons

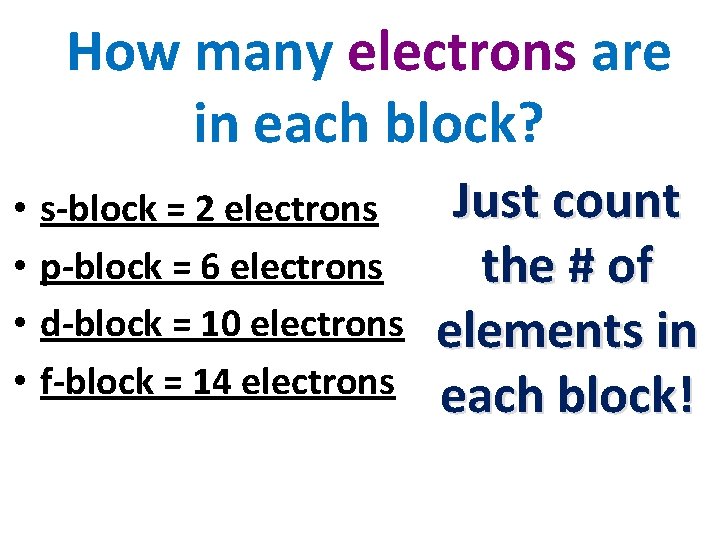
How many electrons are in each block? • • s-block = 2 electrons p-block = 6 electrons d-block = 10 electrons f-block = 14 electrons Just count the # of elements in each block!

How many electrons per energy level (row)? 1. N = 1 (1 st row/energy level): • Smallest energy level • Can only hold 2 electrons (2 = s-block) 2. N = 2 (2 nd row/energy level): • A little larger than the first • Can only hold 8 electrons (2 = s-block; 6 = pblock)

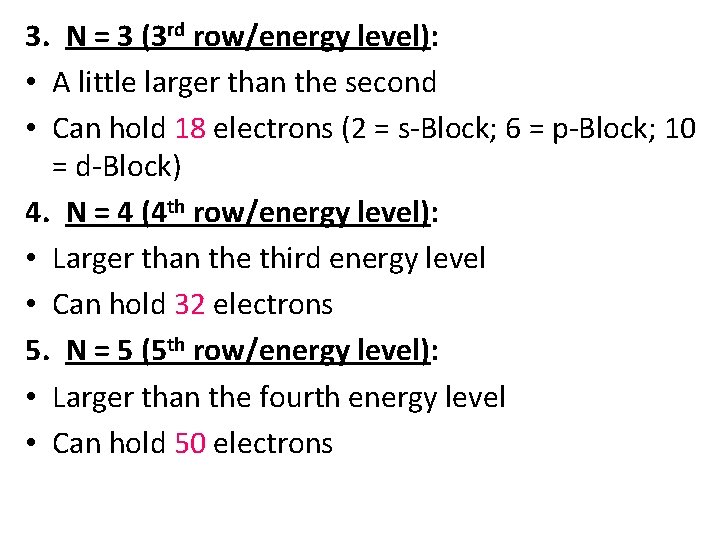
3. N = 3 (3 rd row/energy level): • A little larger than the second • Can hold 18 electrons (2 = s-Block; 6 = p-Block; 10 = d-Block) 4. N = 4 (4 th row/energy level): • Larger than the third energy level • Can hold 32 electrons 5. N = 5 (5 th row/energy level): • Larger than the fourth energy level • Can hold 50 electrons

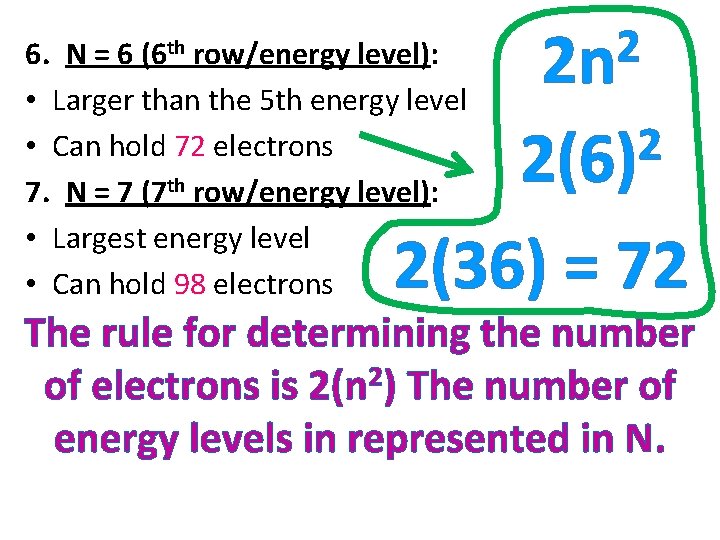
6. N = 6 (6 th row/energy level): • Larger than the 5 th energy level • Can hold 72 electrons 7. N = 7 (7 th row/energy level): • Largest energy level • Can hold 98 electrons 2 2 n 2 2(6) 2(36) = 72 The rule for determining the number of electrons is 2(n 2) The number of energy levels in represented in N.

s 1 S 2 S 3 S 4 S 5 S 6 S 7 S The S-Block always fills first! d 3 D D-Block ONE 4 D Behind! 5 D 6 D f 4 F 5 F p 2 P 3 P 4 P Row 5 P 6 P 7 P F-Block TWO Rows Behind!

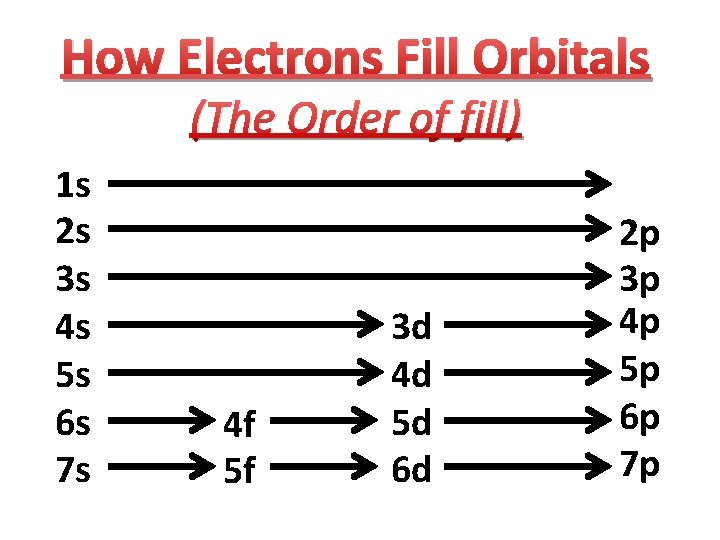
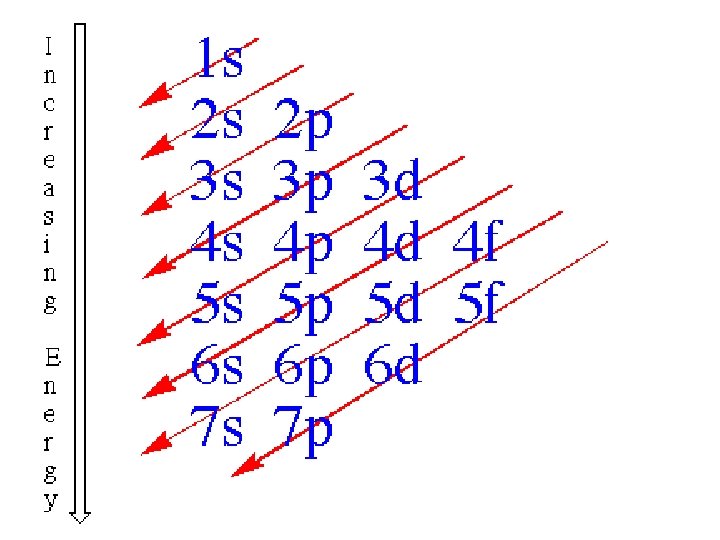
How Electrons Fill Orbitals (The Order of fill) 1 s 2 s 3 s 4 s 5 s 6 s 7 s 4 f 5 f 3 d 4 d 5 d 6 d 2 p 3 p 4 p 5 p 6 p 7 p


s p 1 2 d 3 4 5 6 7 f

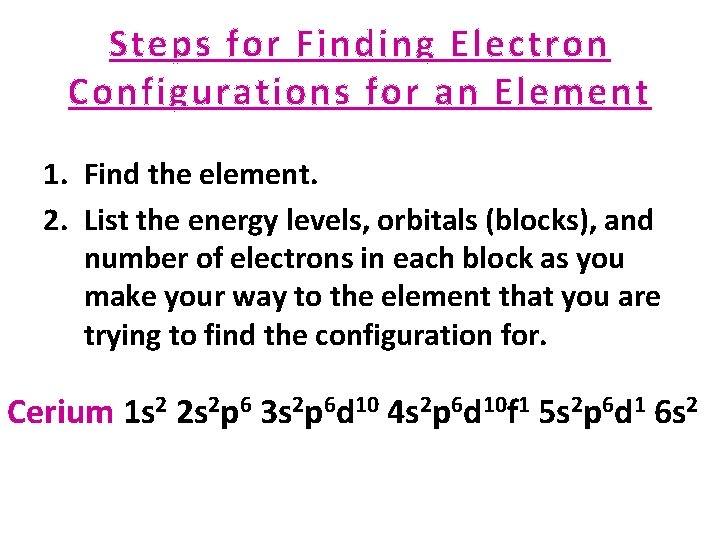
Steps for Finding Electron Configurations for an Element 1. Find the element. 2. List the energy levels, orbitals (blocks), and number of electrons in each block as you make your way to the element that you are trying to find the configuration for. Cerium 1 s 2 2 s 2 p 6 3 s 2 p 6 d 10 4 s 2 p 6 d 10 f 1 5 s 2 p 6 d 1 6 s 2

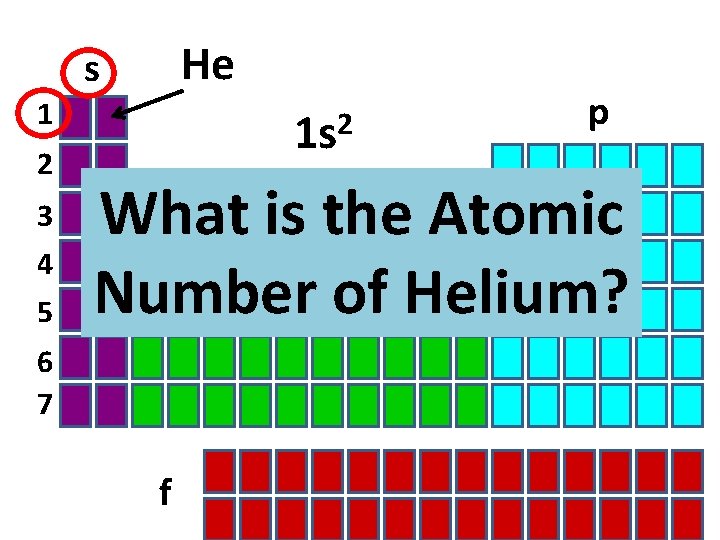
He s 1 2 3 4 5 2 1 s p What is the Atomic Number of Helium? d 6 7 f

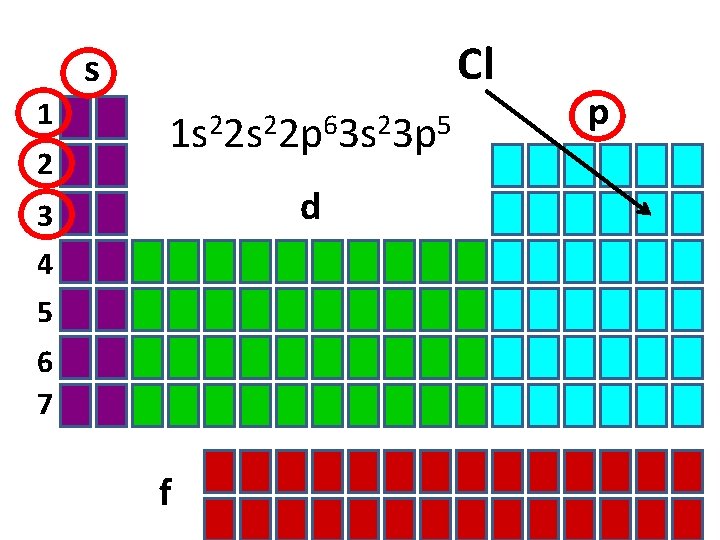
Cl s 1 2 1 s 22 p 63 s 23 p 5 d 3 4 5 6 7 f p

2 2 6 2 5 1 s 2 s 2 p 3 s 3 p Energy Levels

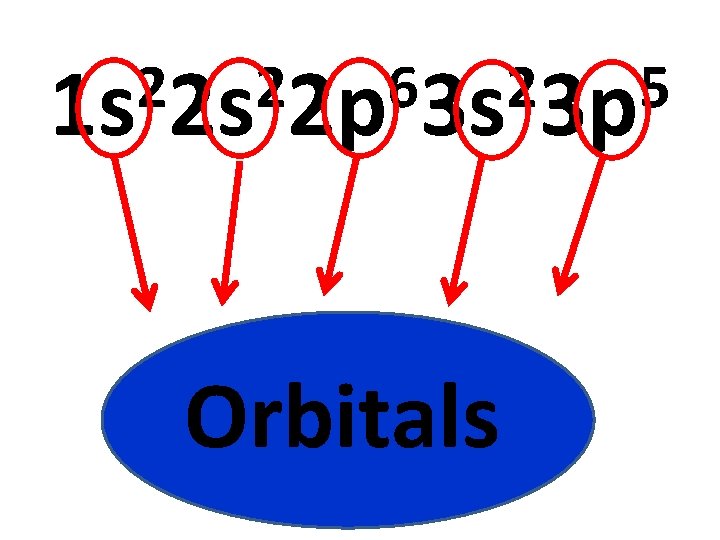
2 2 6 2 5 1 s 2 s 2 p 3 s 3 p Orbitals

2 2 6 2 5 1 s 2 s 2 p 3 s 3 p Check work by counting number of electrons! Electrons

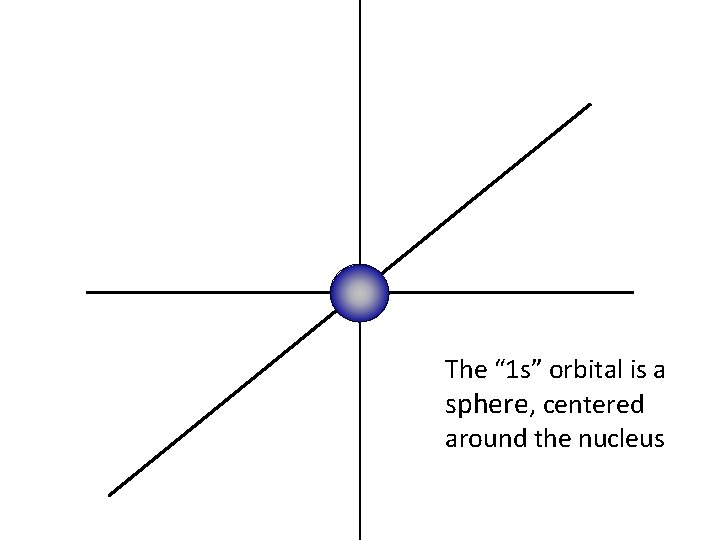
z y For this presentation, the nucleus of the atom is at the center of the three axes. x

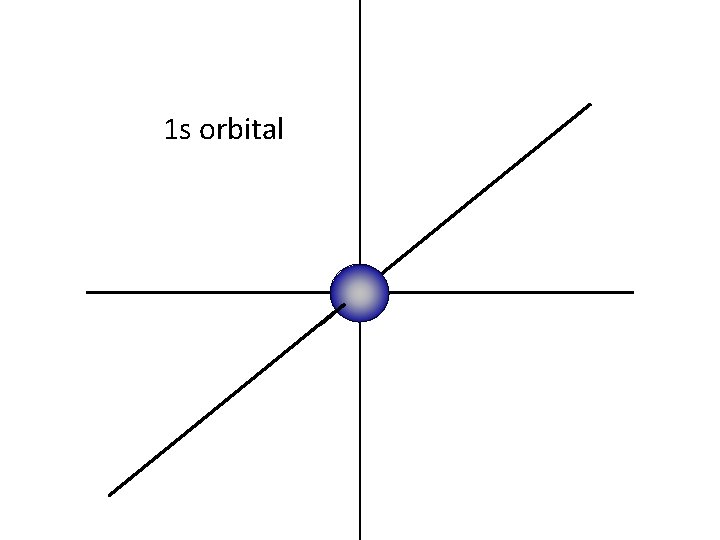
The “ 1 s” orbital is a sphere, centered around the nucleus



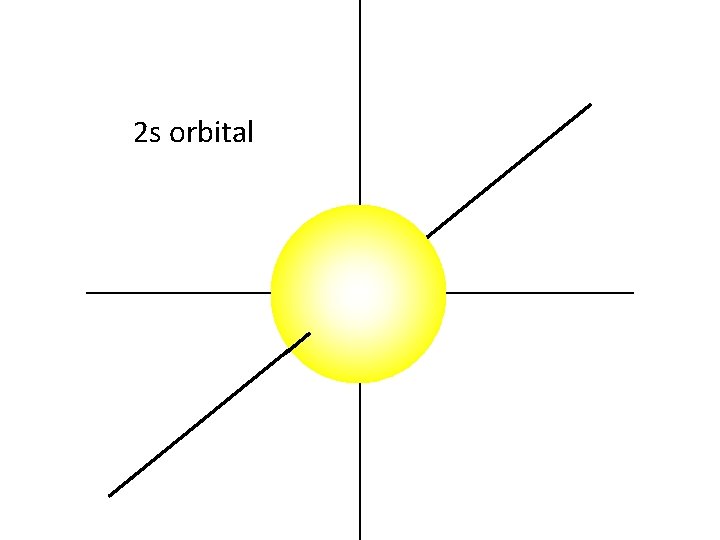
The 2 s orbital is also a sphere.

The 2 s electrons have a higher energy than the 1 s electrons. Therefore, the 2 s electrons are generally more distant from the nucleus, making the 2 s orbital larger than the 1 s orbital.

1 s orbital

2 s orbital

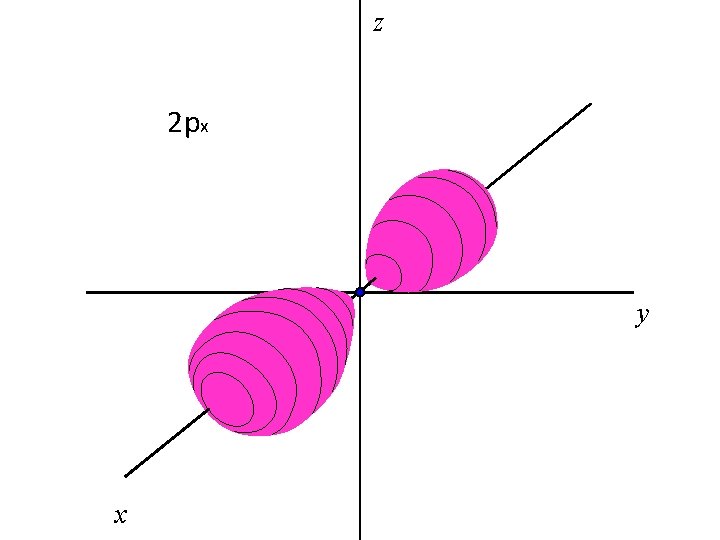
z 2 px y x

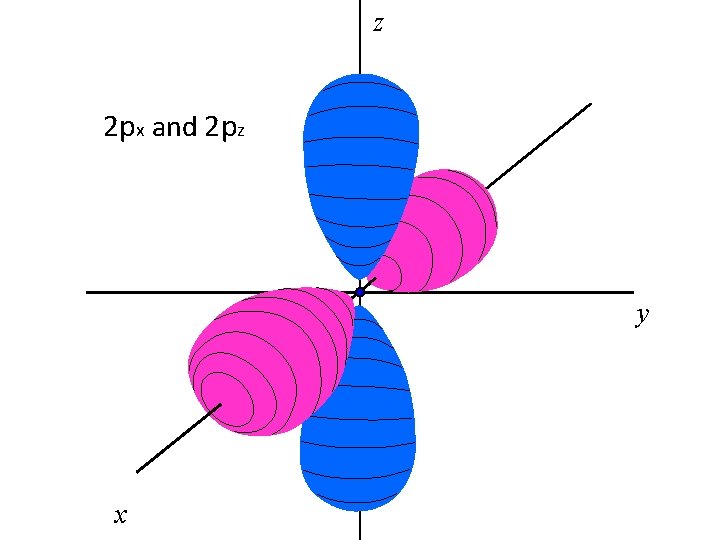
z 2 px and 2 pz y x

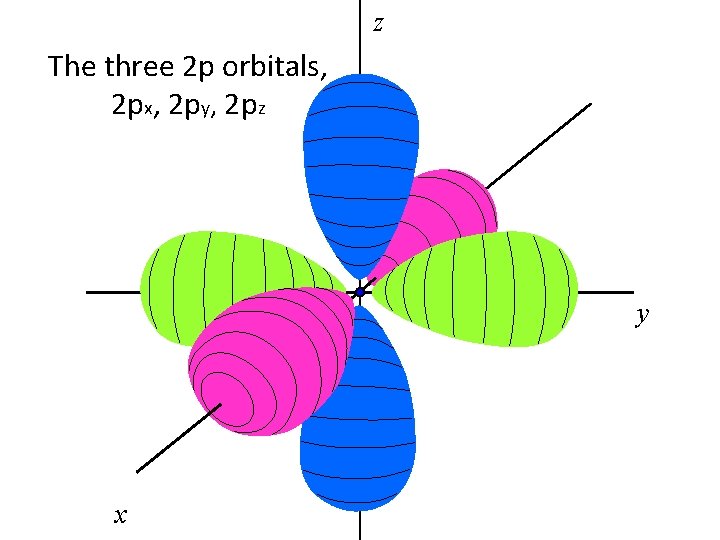
z The three 2 p orbitals, 2 px, 2 py, 2 pz y x

once the 1 s orbital is filled,

the 2 s orbital begins to fill

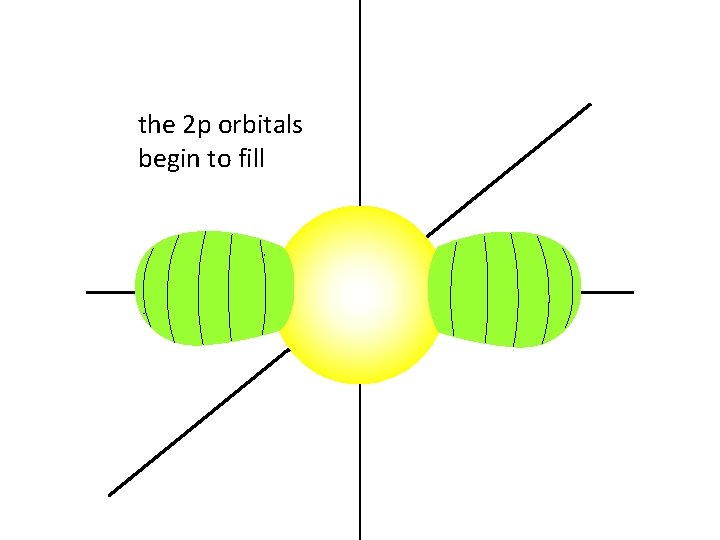
once the 2 s orbital is filled,

the 2 p orbitals begin to fill

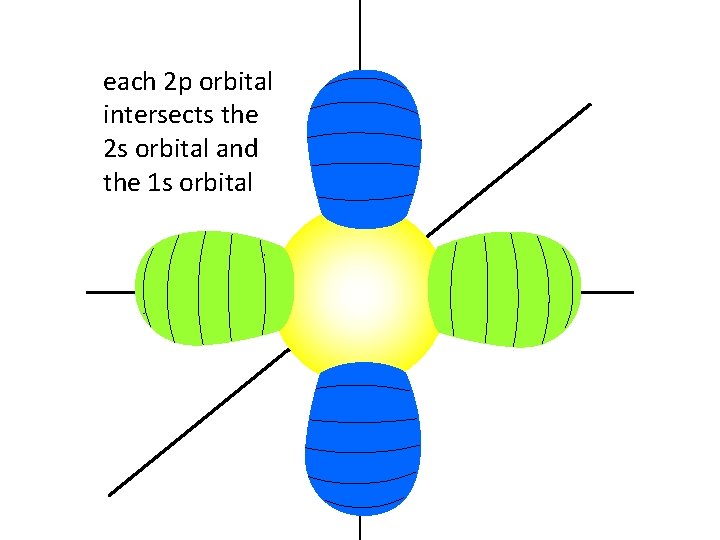
each 2 p orbital intersects the 2 s orbital and the 1 s orbital

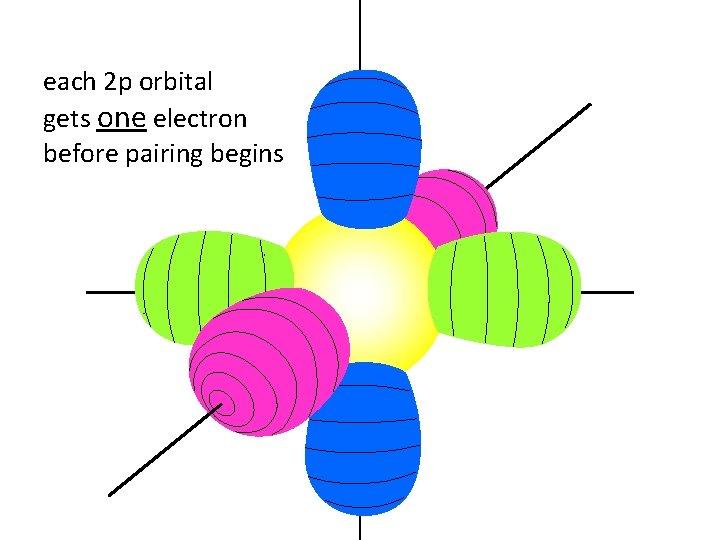
each 2 p orbital gets one electron before pairing begins

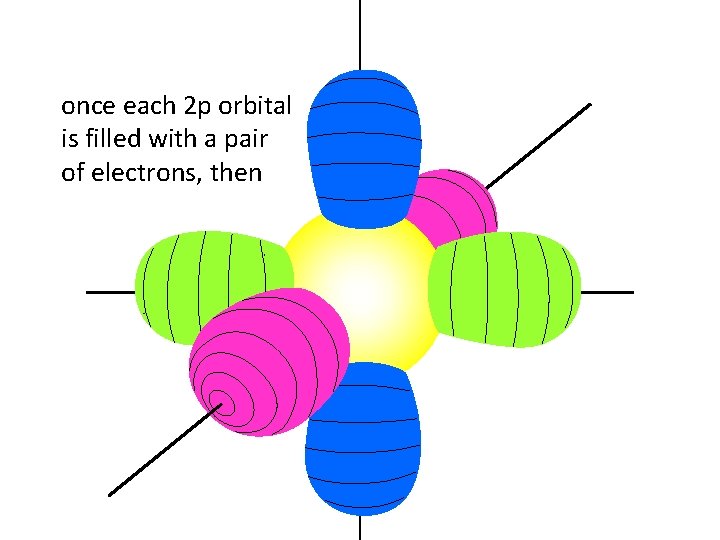
once each 2 p orbital is filled with a pair of electrons, then

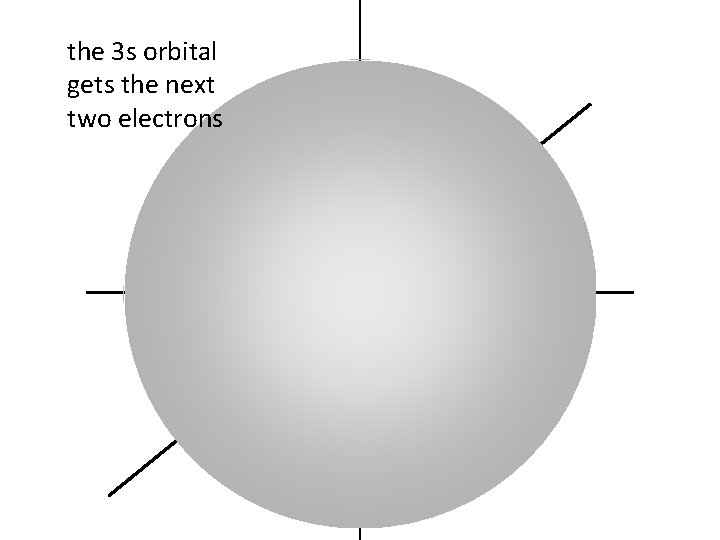
the 3 s orbital gets the next two electrons

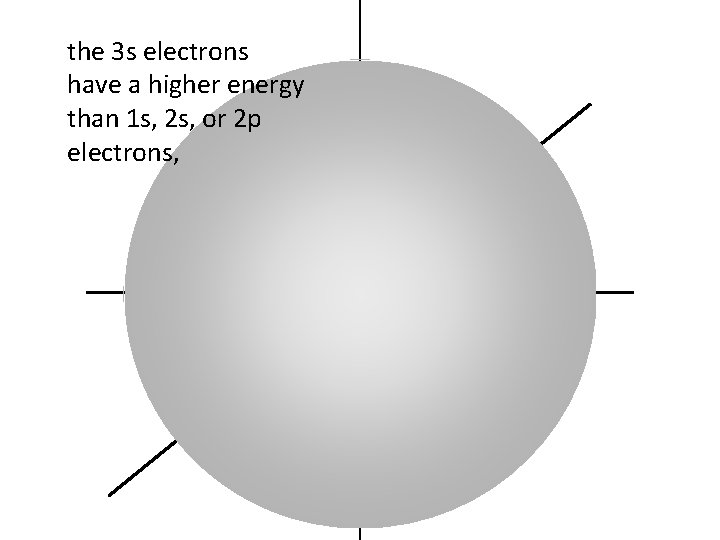
the 3 s electrons have a higher energy than 1 s, 2 s, or 2 p electrons,

so 3 s electrons are generally found further from the nucleus than 1 s, 2 s, or 2 p electrons
