Electron Configurations Chemical Periodicity Ch 8 Electron spin

![Ionization Energy (IE) Mg (g) atom Mg (g) + Mg 3+ [Ne]2 s 735 Ionization Energy (IE) Mg (g) atom Mg (g) + Mg 3+ [Ne]2 s 735](https://slidetodoc.com/presentation_image_h/2c301ba396a2e7a6d5ddbe0542a9b935/image-41.jpg)
- Slides: 47

Electron Configurations Chemical Periodicity (Ch 8) • Electron spin & Pauli exclusion principle • configurations • spectroscopic, orbital box notation • Hund’s rule - electron filling rules • configurations of ATOMS: Na • the basis for chemical valence • configurations and properties of IONS • periodic trends in : • size • ionization energies • electron affinities + Cl Na. Cl Mg + O 2 Mg. O 6 Oct 1997 Chemical Periodicity 1

Arrangement of Electrons in Atoms Electrons in atoms are arranged as SHELLS (n) SUBSHELLS ( ) ORBITALS (m ) Each orbital can be assigned up to 2 electrons! WHY ? . . . Because there is a 4 th quantum number, the electron spin quantum number, ms. 6 Oct 1997 Chemical Periodicity 2

Electron Spin Quantum Number, ms • It can be proved experimentally that the electron has a spin. This is QUANTIZED. • The two allowed spin directions are defined by the magnetic spin quantum number, ms ms = +1/2 and -1/2 ONLY. 6 Oct 1997 Chemical Periodicity 3

Electron Spin Quantum Number MAGNETISM is a macroscopic result of quantized electron spin 5_magnet. mov Diamagnetic: NOT attracted to a magnetic field All electrons are paired N 2 Paramagnetic: attracted to a magnetic field. Substance has unpaired electrons 6 Oct 1997 Chemical Periodicity O 2 4

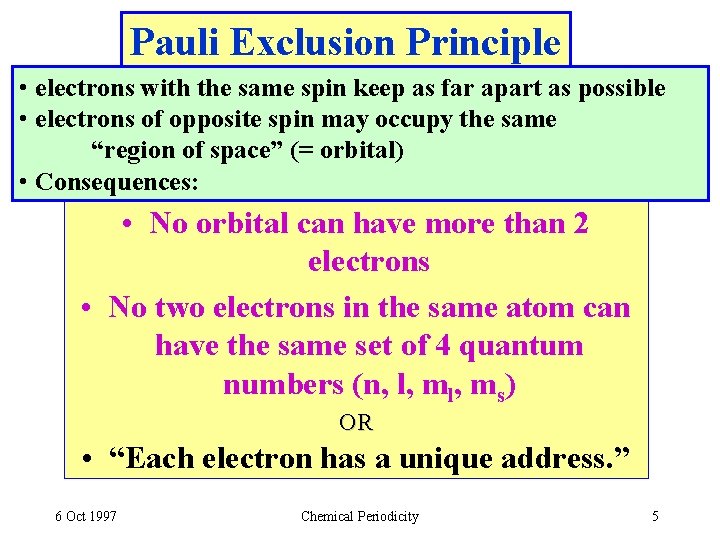
Pauli Exclusion Principle • electrons with the same spin keep as far apart as possible • electrons of opposite spin may occupy the same “region of space” (= orbital) • Consequences: • No orbital can have more than 2 electrons • No two electrons in the same atom can have the same set of 4 quantum numbers (n, l, ms) OR • “Each electron has a unique address. ” 6 Oct 1997 Chemical Periodicity 5

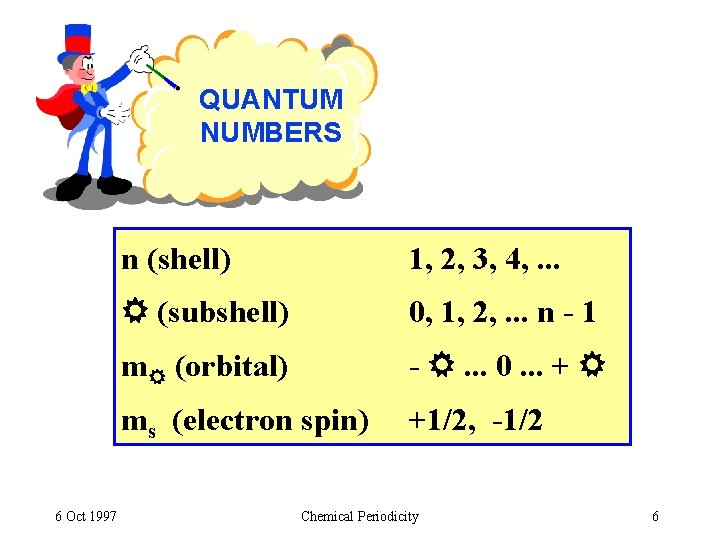
QUANTUM NUMBERS 6 Oct 1997 n (shell) 1, 2, 3, 4, . . . (subshell) 0, 1, 2, . . . n - 1 m (orbital) - . . . 0. . . + ms (electron spin) +1/2, -1/2 Chemical Periodicity 6

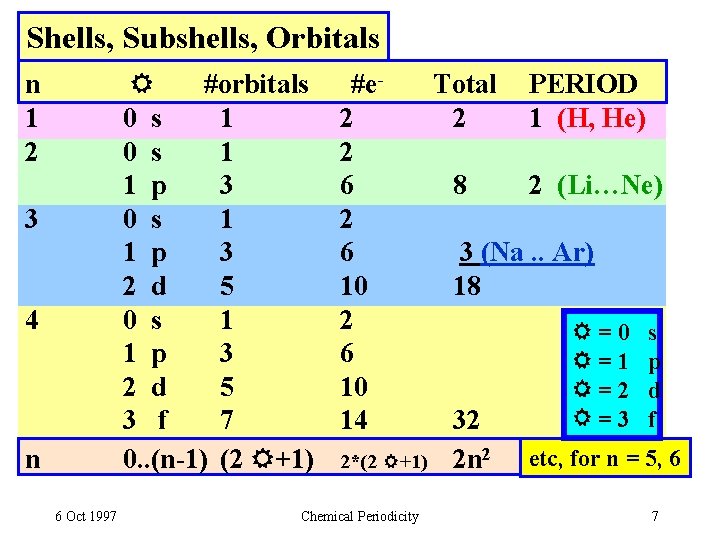
Shells, Subshells, Orbitals #orbitals 0 s 1 1 p 3 2 d 5 3 f 7 0. . (n-1) (2 +1) n 1 2 3 4 n 6 Oct 1997 #e 2 2 6 10 14 2*(2 +1) Chemical Periodicity Total 2 8 PERIOD 1 (H, He) 2 (Li…Ne) 3 (Na. . Ar) 18 32 2 n 2 =0 =1 =2 =3 s p d f etc, for n = 5, 6 7

Element Mnemonic Competition Hey! Here Lies Ben Brown. Could Not Order Fire. Near Nancy Margaret Alice Sits Peggy Sucking Clorets. Are Kids Capable ? 6 Oct 1997 Chemical Periodicity 8

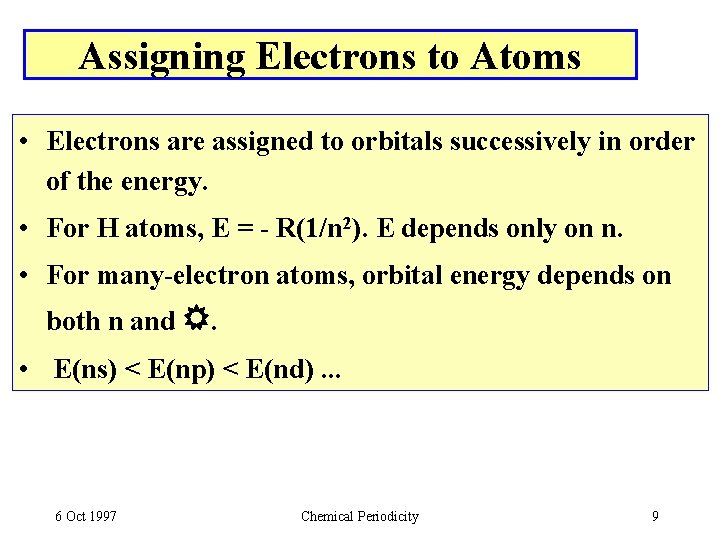
Assigning Electrons to Atoms • Electrons are assigned to orbitals successively in order of the energy. • For H atoms, E = - R(1/n 2). E depends only on n. • For many-electron atoms, orbital energy depends on both n and . • E(ns) < E(np) < E(nd). . . 6 Oct 1997 Chemical Periodicity 9

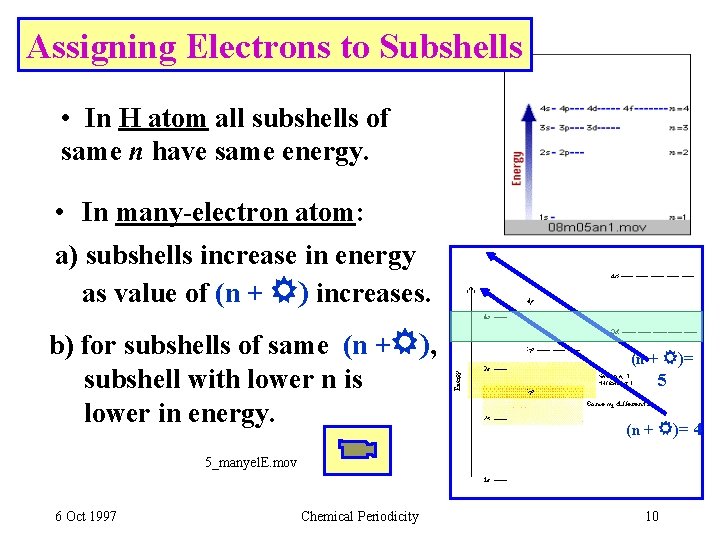
Assigning Electrons to Subshells • In H atom all subshells of same n have same energy. • In many-electron atom: a) subshells increase in energy as value of (n + ) increases. b) for subshells of same (n + ), subshell with lower n is lower in energy. (n + )= 5 (n + )= 4 5_manyel. E. mov 6 Oct 1997 Chemical Periodicity 10

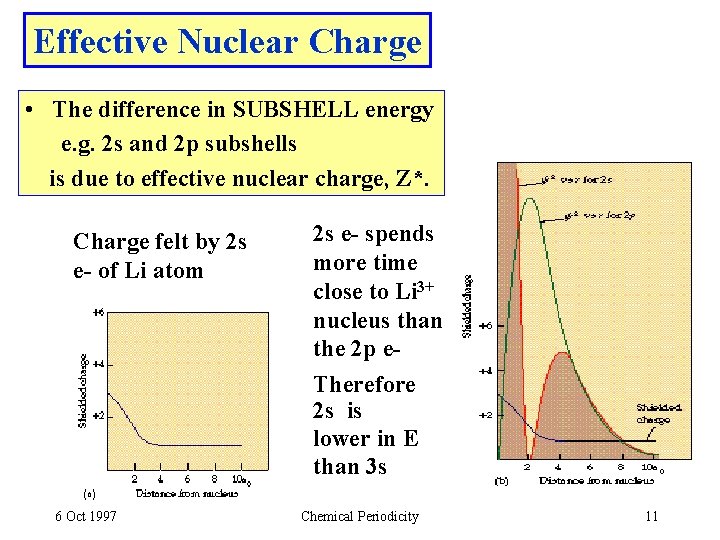
Effective Nuclear Charge • The difference in SUBSHELL energy e. g. 2 s and 2 p subshells is due to effective nuclear charge, Z*. Charge felt by 2 s e- of Li atom 6 Oct 1997 2 s e- spends more time close to Li 3+ nucleus than the 2 p e. Therefore 2 s is lower in E than 3 s Chemical Periodicity 11

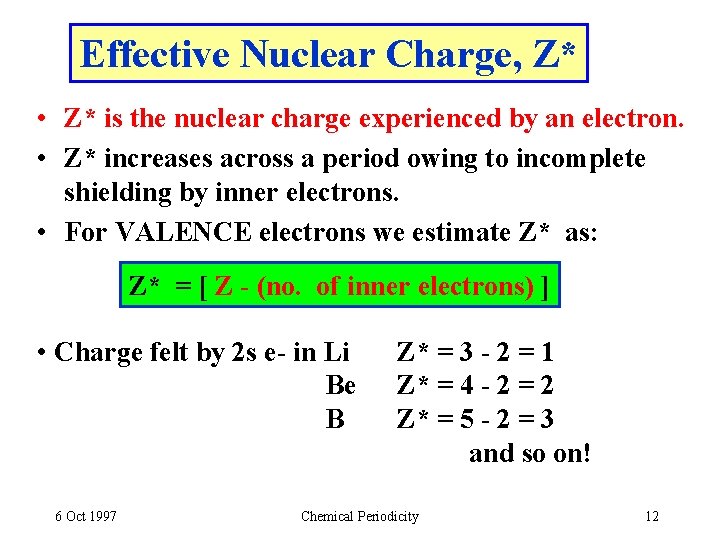
Effective Nuclear Charge, Z* • Z* is the nuclear charge experienced by an electron. • Z* increases across a period owing to incomplete shielding by inner electrons. • For VALENCE electrons we estimate Z* as: Z* = [ Z - (no. of inner electrons) ] • Charge felt by 2 s e- in Li Be B 6 Oct 1997 Z* = 3 - 2 = 1 Z* = 4 - 2 = 2 Z* = 5 - 2 = 3 and so on! Chemical Periodicity 12

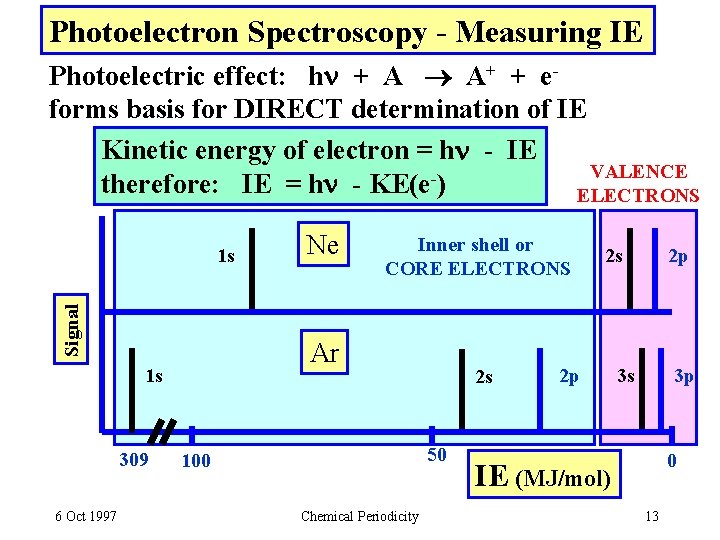
Photoelectron Spectroscopy - Measuring IE Photoelectric effect: h + A A+ + eforms basis for DIRECT determination of IE Kinetic energy of electron = h - IE VALENCE therefore: IE = h - KE(e ) ELECTRONS Signal 1 s 6 Oct 1997 Inner shell or CORE ELECTRONS Ar 1 s 309 Ne 2 s 50 100 Chemical Periodicity 2 s 2 p 2 p 3 s 3 p 0 IE (MJ/mol) 13

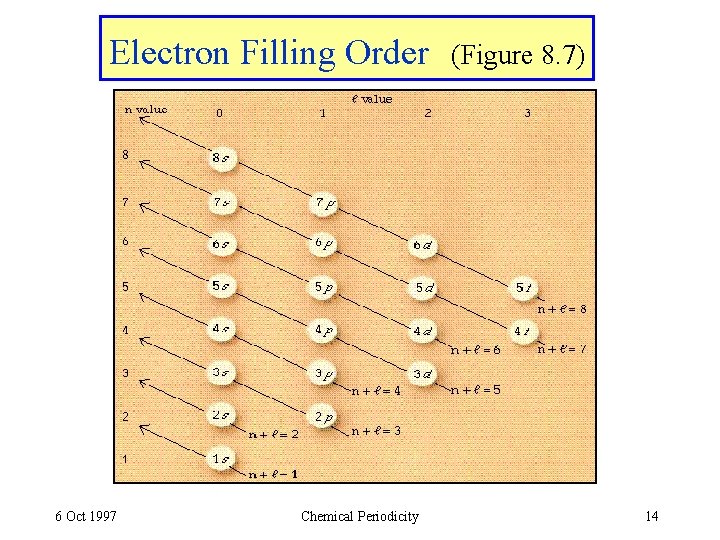
Electron Filling Order 6 Oct 1997 Chemical Periodicity (Figure 8. 7) 14

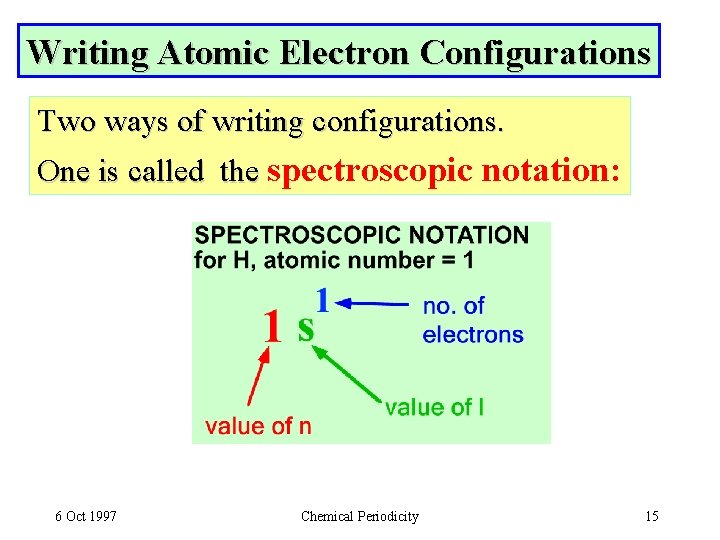
Writing Atomic Electron Configurations Two ways of writing configurations. One is called the spectroscopic notation: 6 Oct 1997 Chemical Periodicity 15

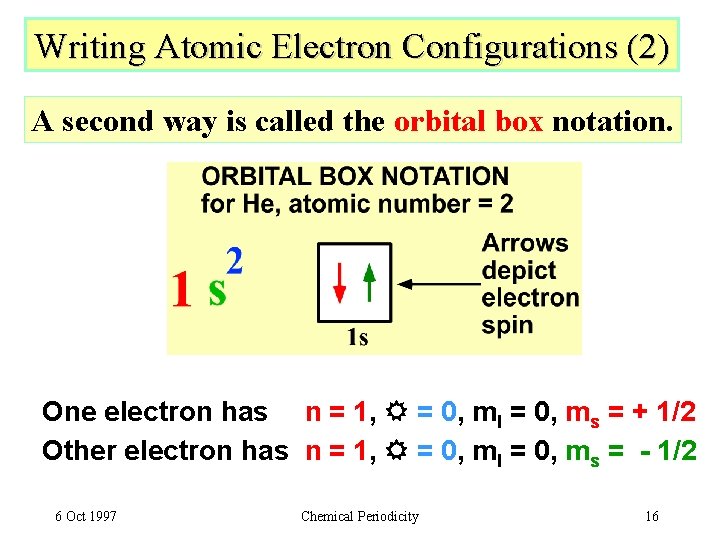
Writing Atomic Electron Configurations (2) A second way is called the orbital box notation. One electron has n = 1, = 0, ml = 0, ms = + 1/2 Other electron has n = 1, = 0, ml = 0, ms = - 1/2 6 Oct 1997 Chemical Periodicity 16

Electron Configuration tool - see “toolbox”. 6 Oct 1997 Chemical Periodicity 17

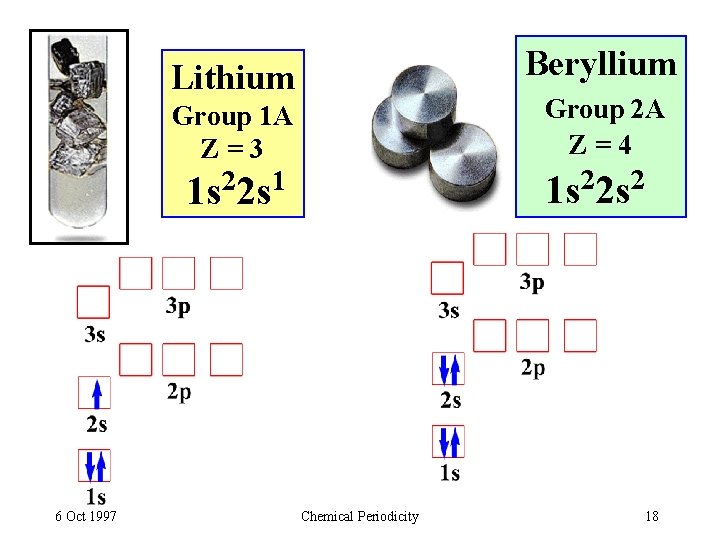
Beryllium Lithium Group 2 A Z=4 1 s 22 s 2 Group 1 A Z=3 1 s 22 s 1 6 Oct 1997 Chemical Periodicity 18

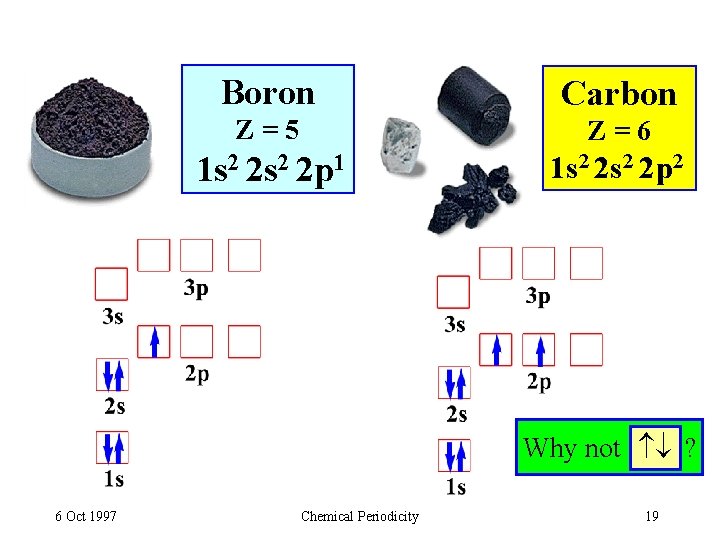
Boron Carbon Z=5 Z=6 1 s 2 2 p 1 1 s 2 2 p 2 Why not ? 6 Oct 1997 Chemical Periodicity 19

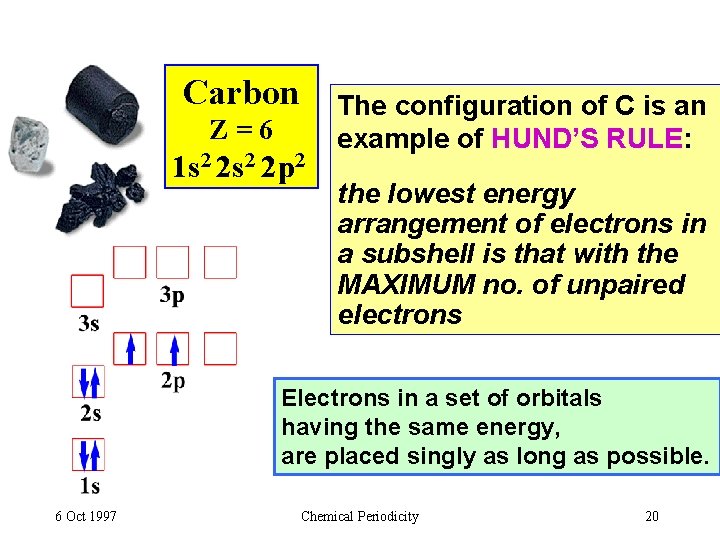
Carbon Z=6 1 s 2 2 p 2 The configuration of C is an example of HUND’S RULE: the lowest energy arrangement of electrons in a subshell is that with the MAXIMUM no. of unpaired electrons Electrons in a set of orbitals having the same energy, are placed singly as long as possible. 6 Oct 1997 Chemical Periodicity 20

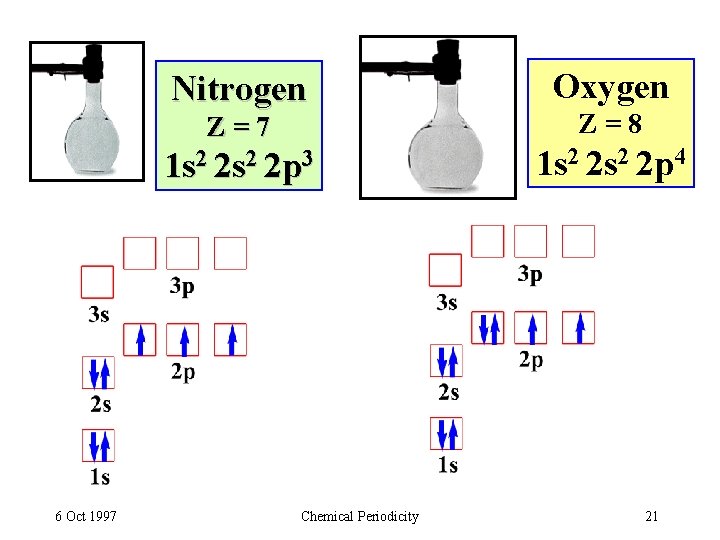
Nitrogen Z=7 1 s 2 2 p 3 6 Oct 1997 Chemical Periodicity Oxygen Z=8 1 s 2 2 p 4 21

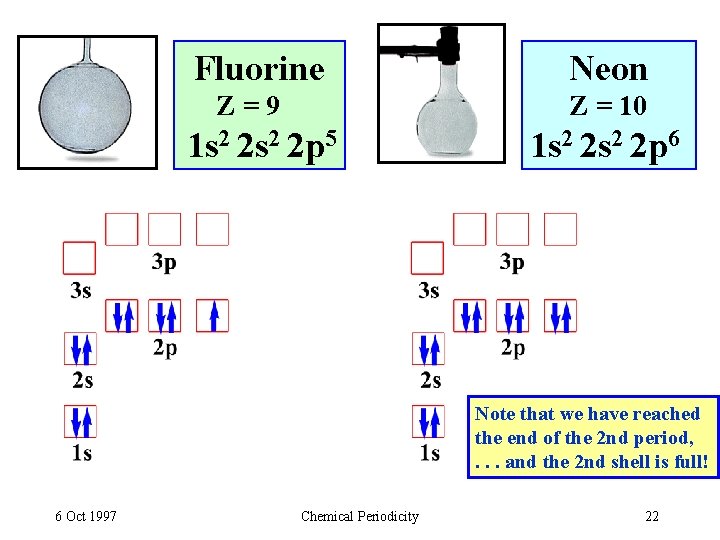
Fluorine Z=9 Neon Z = 10 1 s 2 2 p 5 1 s 2 2 p 6 Note that we have reached the end of the 2 nd period, . . . and the 2 nd shell is full! 6 Oct 1997 Chemical Periodicity 22

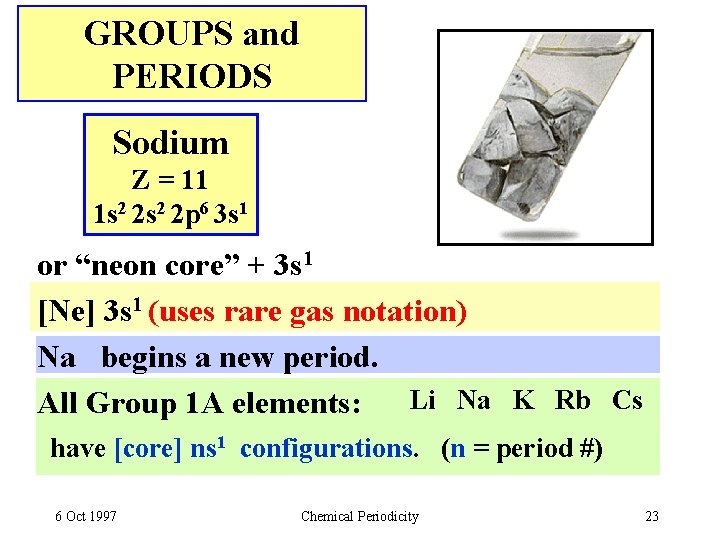
GROUPS and PERIODS Sodium Z = 11 1 s 2 2 p 6 3 s 1 or “neon core” + 3 s 1 [Ne] 3 s 1 (uses rare gas notation) Na begins a new period. All Group 1 A elements: Li Na K Rb Cs have [core] ns 1 configurations. (n = period #) 6 Oct 1997 Chemical Periodicity 23

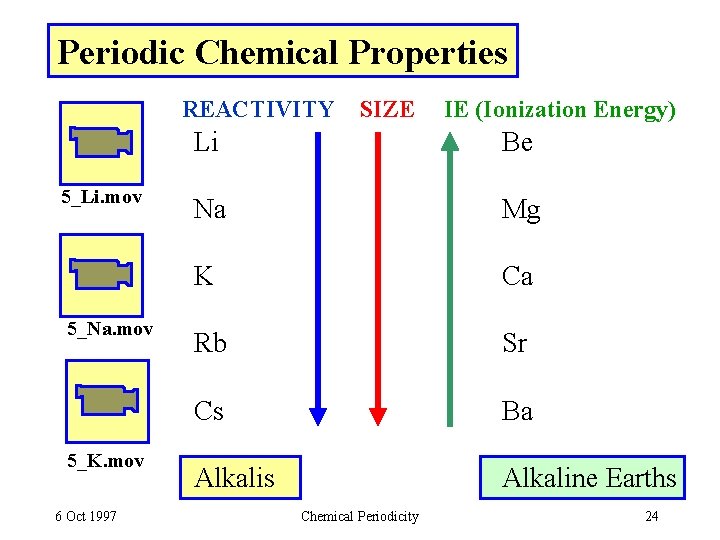
Periodic Chemical Properties REACTIVITY 5_Li. mov 5_Na. mov 5_K. mov 6 Oct 1997 SIZE IE (Ionization Energy) Li Be Na Mg K Ca Rb Sr Cs Ba Alkalis Alkaline Earths Chemical Periodicity 24

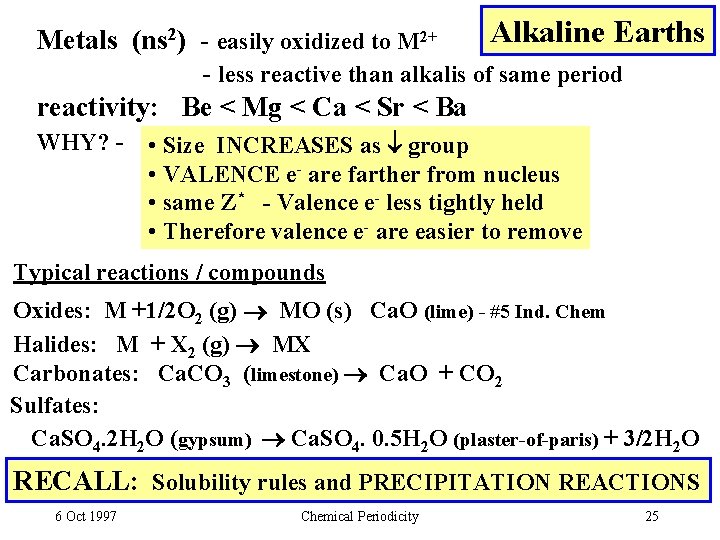
Alkaline Earths Metals (ns 2) - easily oxidized to M 2+ - less reactive than alkalis of same period reactivity: Be < Mg < Ca < Sr < Ba WHY? - • Size INCREASES as group • VALENCE e- are farther from nucleus • same Z* - Valence e- less tightly held • Therefore valence e- are easier to remove Typical reactions / compounds Oxides: M +1/2 O 2 (g) MO (s) Ca. O (lime) - #5 Ind. Chem Halides: M + X 2 (g) MX Carbonates: Ca. CO 3 (limestone) Ca. O + CO 2 Sulfates: Ca. SO 4. 2 H 2 O (gypsum) Ca. SO 4. 0. 5 H 2 O (plaster-of-paris) + 3/2 H 2 O RECALL: Solubility rules and PRECIPITATION REACTIONS 6 Oct 1997 Chemical Periodicity 25

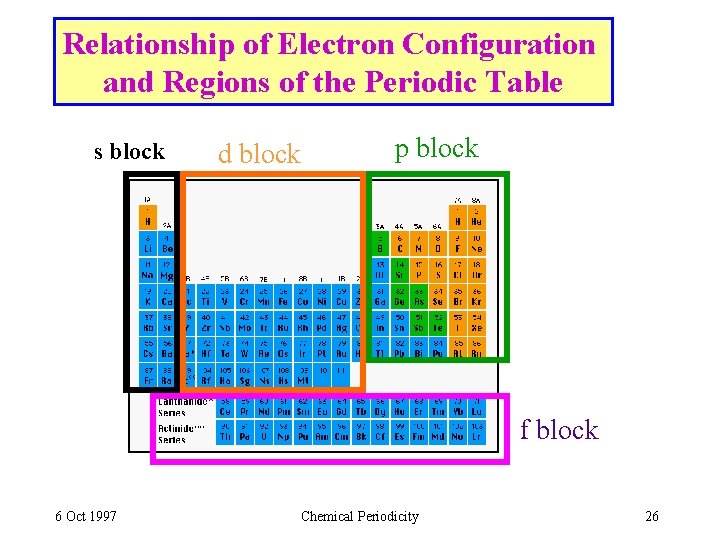
Relationship of Electron Configuration and Regions of the Periodic Table s block d block p block f block 6 Oct 1997 Chemical Periodicity 26

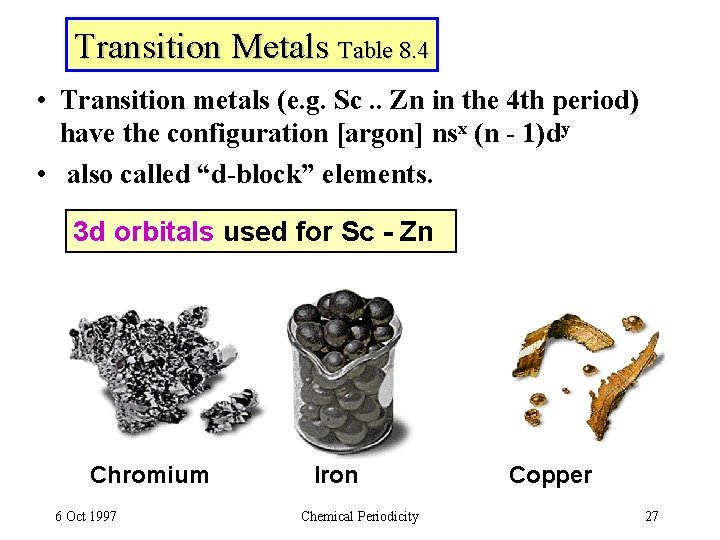
Transition Metals Table 8. 4 • Transition metals (e. g. Sc. . Zn in the 4 th period) have the configuration [argon] nsx (n - 1)dy • also called “d-block” elements. 3 d orbitals used for Sc - Zn Chromium 6 Oct 1997 Iron Chemical Periodicity Copper 27

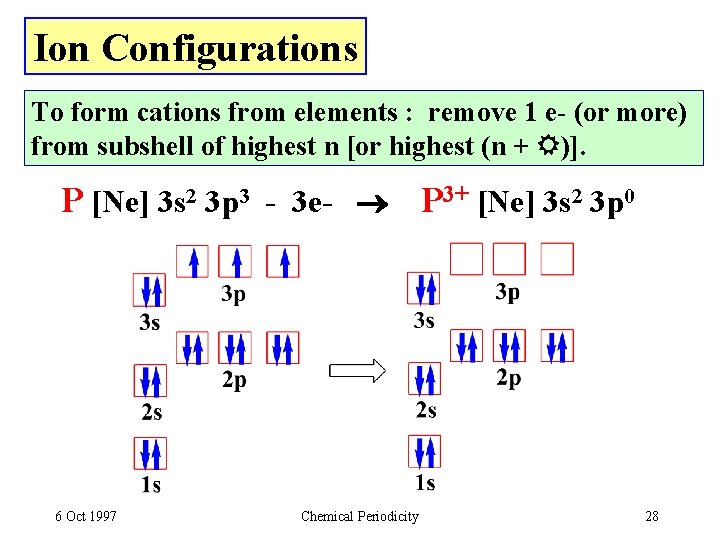
Ion Configurations To form cations from elements : remove 1 e- (or more) from subshell of highest n [or highest (n + )]. P [Ne] 3 s 2 3 p 3 - 3 e- P 3+ [Ne] 3 s 2 3 p 0 6 Oct 1997 Chemical Periodicity 28

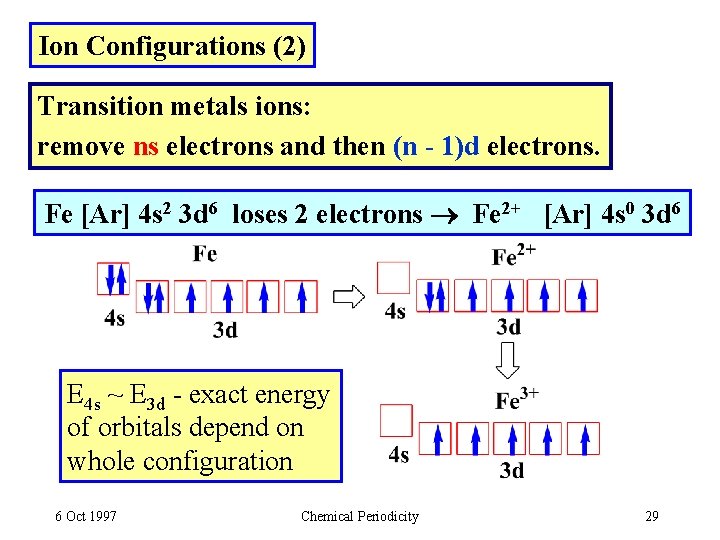
Ion Configurations (2) Transition metals ions: remove ns electrons and then (n - 1)d electrons. Fe [Ar] 4 s 2 3 d 6 loses 2 electrons Fe 2+ [Ar] 4 s 0 3 d 6 E 4 s ~ E 3 d - exact energy of orbitals depend on whole configuration 6 Oct 1997 Chemical Periodicity 29

Ion Configurations (3) How do we know the configurations of ions? From the magnetic properties of ions. Ions (or atoms) with UNPAIRED ELECTRONS are: PARAMAGNETIC. Ions (or atoms) without unpaired electrons are: DIAMAGNETIC. 6 Oct 1997 Chemical Periodicity 30

General Periodic Trends • Atomic and ionic radii : SIZE • Ionization energy : E(A+) - E(A) • Electron affinity : E(A-) - E(A) 6 Oct 1997 Chemical Periodicity 31

Atomic Size INCREASES down a Group • Size goes UP on going down a GROUP • Because electrons are added further from the nucleus, there is less attraction. 6 Oct 1997 Chemical Periodicity 32

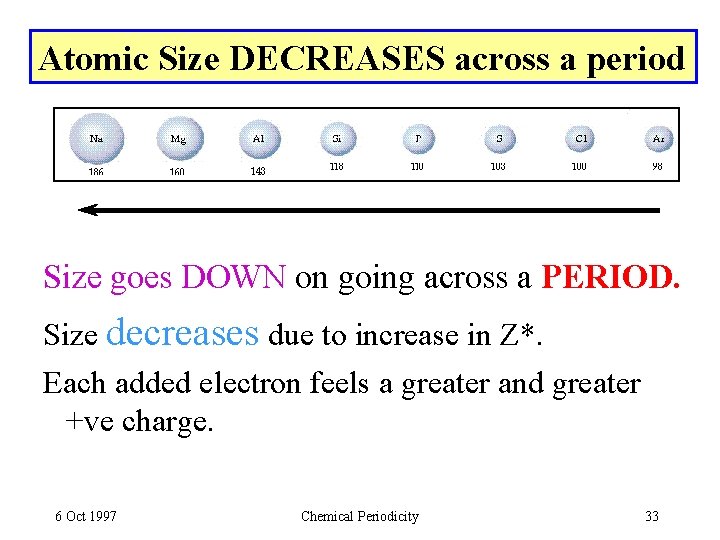
Atomic Size DECREASES across a period Size goes DOWN on going across a PERIOD. Size decreases due to increase in Z*. Each added electron feels a greater and greater +ve charge. 6 Oct 1997 Chemical Periodicity 33

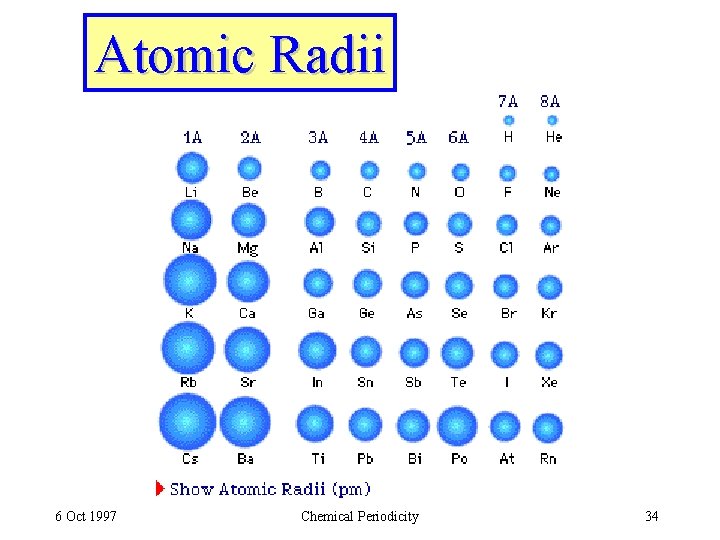
Atomic Radii 6 Oct 1997 Chemical Periodicity 34

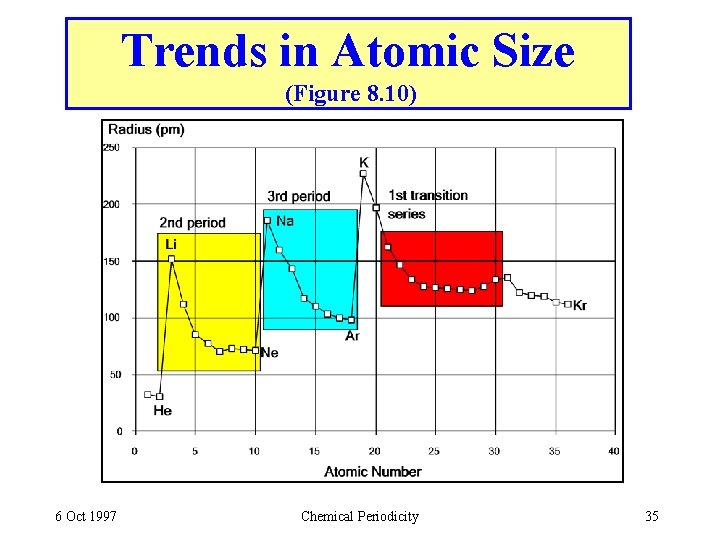
Trends in Atomic Size (Figure 8. 10) 6 Oct 1997 Chemical Periodicity 35

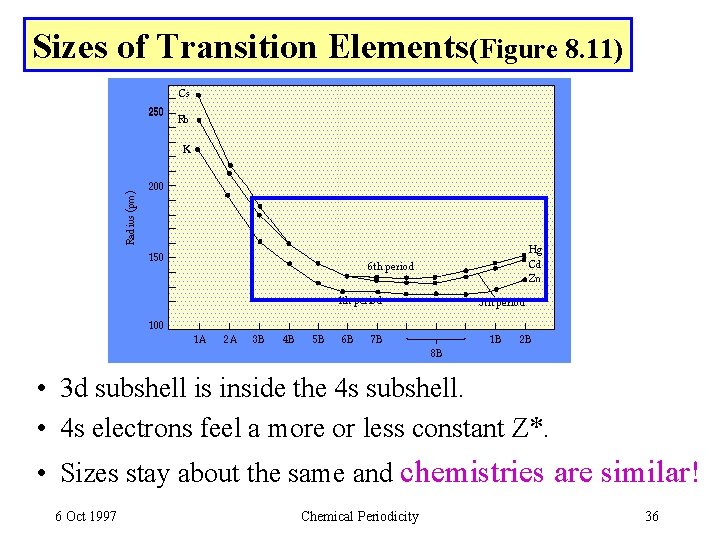
Sizes of Transition Elements(Figure 8. 11) • 3 d subshell is inside the 4 s subshell. • 4 s electrons feel a more or less constant Z*. • Sizes stay about the same and chemistries are similar! 6 Oct 1997 Chemical Periodicity 36

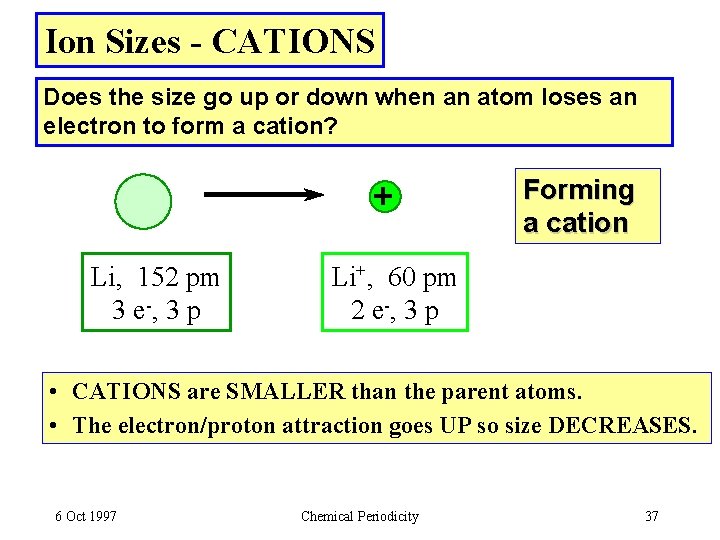
Ion Sizes - CATIONS Does the size go up or down when an atom loses an electron to form a cation? + Li, 152 pm 3 e -, 3 p Forming a cation Li+, 60 pm 2 e -, 3 p • CATIONS are SMALLER than the parent atoms. • The electron/proton attraction goes UP so size DECREASES. 6 Oct 1997 Chemical Periodicity 37

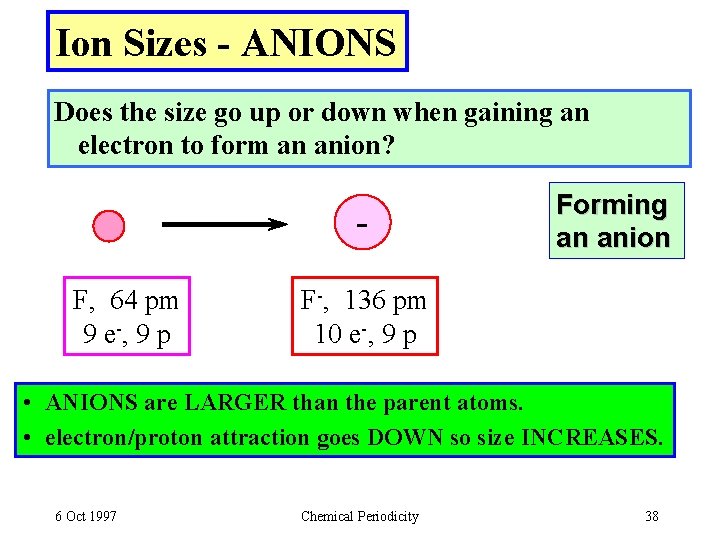
Ion Sizes - ANIONS Does the size go up or down when gaining an electron to form an anion? F, 64 pm 9 e -, 9 p Forming an anion F-, 136 pm 10 e-, 9 p • ANIONS are LARGER than the parent atoms. • electron/proton attraction goes DOWN so size INCREASES. 6 Oct 1997 Chemical Periodicity 38

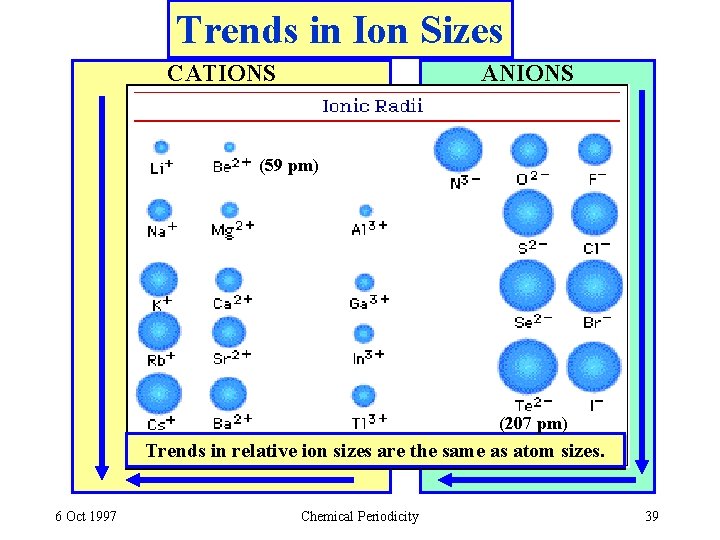
Trends in Ion Sizes CATIONS ANIONS (59 pm) (207 pm) Trends in relative ion sizes are the same as atom sizes. 6 Oct 1997 Chemical Periodicity 39

Oxidation-Reduction Reactions • Why do metals lose electrons in their reactions? • Why does Mg form Mg 2+ ions and not Mg 3+? • Why do nonmetals take on electrons? - related to IE and EA 6 Oct 1997 Chemical Periodicity 40
![Ionization Energy IE Mg g atom Mg g Mg 3 Ne2 s 735 Ionization Energy (IE) Mg (g) atom Mg (g) + Mg 3+ [Ne]2 s 735](https://slidetodoc.com/presentation_image_h/2c301ba396a2e7a6d5ddbe0542a9b935/image-41.jpg)
Ionization Energy (IE) Mg (g) atom Mg (g) + Mg 3+ [Ne]2 s 735 k. J Mg+ (g) + e- [Ne]2 s 1 Mg+ (g) + 1451 k. J Mg 2+ (g) + e- [Ne]2 s 0 Mg 2+ (g) + 7733 k. J Mg 3+ (g) + e- [He]2 s 22 p 5 Mg 2+ • Energy ‘cost’ is very high to remove an INNER SHELL e- (shell of n < n. VALENCE). • This is why oxidation. no. = Group no. 6 Oct 1997 Chemical Periodicity Mg+ Mg 41

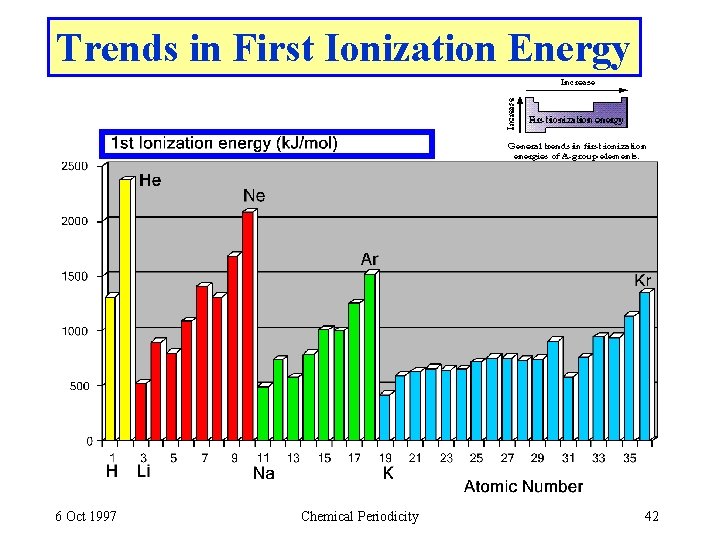
Trends in First Ionization Energy 6 Oct 1997 Chemical Periodicity 42

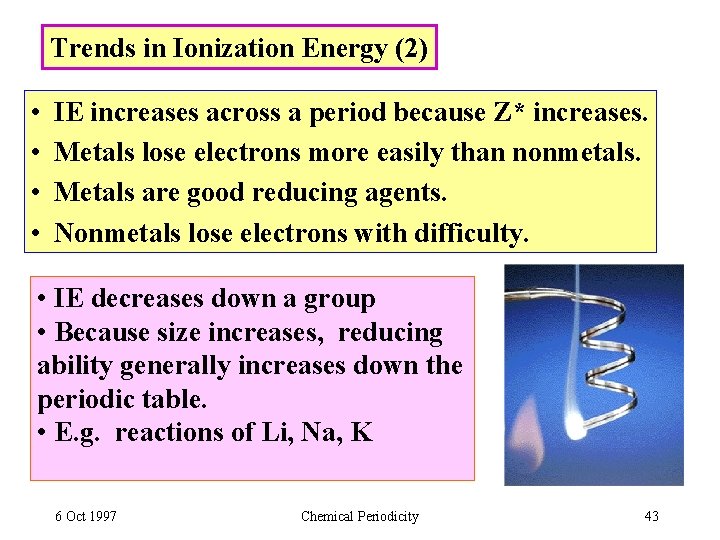
Trends in Ionization Energy (2) • • IE increases across a period because Z* increases. Metals lose electrons more easily than nonmetals. Metals are good reducing agents. Nonmetals lose electrons with difficulty. • IE decreases down a group • Because size increases, reducing ability generally increases down the periodic table. • E. g. reactions of Li, Na, K 6 Oct 1997 Chemical Periodicity 43

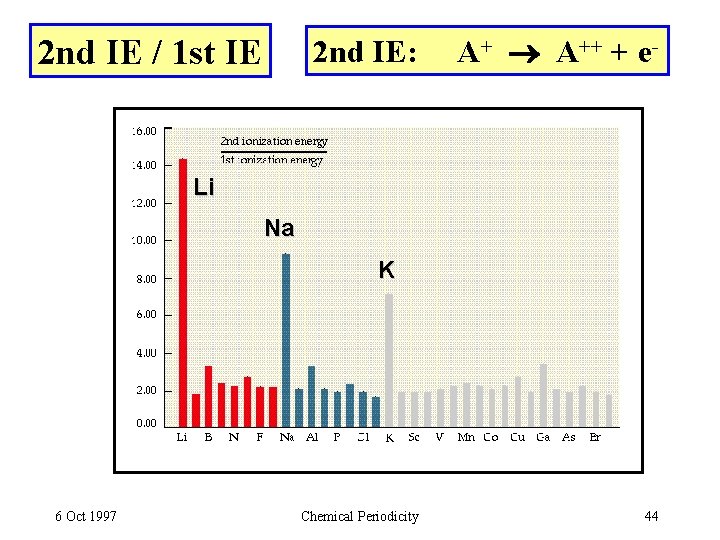
2 nd IE / 1 st IE 2 nd IE: A++ + e- Li Na K 6 Oct 1997 Chemical Periodicity 44

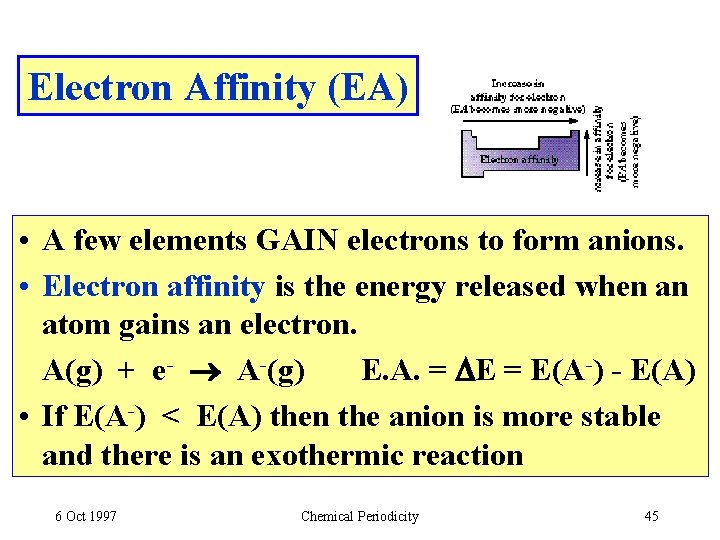
Electron Affinity (EA) • A few elements GAIN electrons to form anions. • Electron affinity is the energy released when an atom gains an electron. A(g) + e- A-(g) E. A. = DE = E(A-) - E(A) • If E(A-) < E(A) then the anion is more stable and there is an exothermic reaction 6 Oct 1997 Chemical Periodicity 45

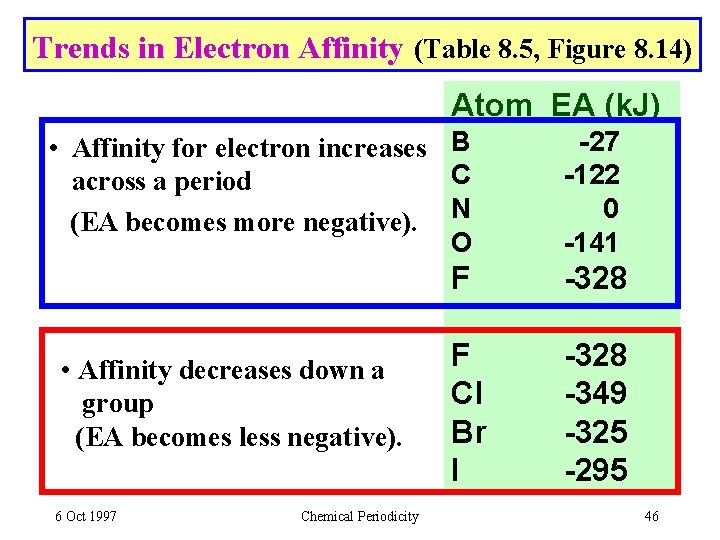
Trends in Electron Affinity (Table 8. 5, Figure 8. 14) Atom EA (k. J) • Affinity for electron increases B C across a period (EA becomes more negative). N O -27 -122 0 -141 F -328 F Cl Br I -328 -349 -325 -295 • Affinity decreases down a group (EA becomes less negative). 6 Oct 1997 Chemical Periodicity 46

SUMMARY • Electron spin: diamagnetism vs. paramagnetism • Pauli exclusion principle - allowable quantum numbers • configurations • spectroscopic notation • orbital box notation • Hund’s rule - electron filling rules • configurations of ATOMS: the basis for chemical valence • period 2 ; groups • transition metals • configurations and properties of IONS • periodic trends in : • size • ionization energies • electron affinities 6 Oct 1997 Chemical Periodicity 47