Modern Atomic Theory and Periodic Trends Electromagnetic Waves












- Slides: 42

Modern Atomic Theory and Periodic Trends

Electromagnetic Waves Originate from the movement of electrical charges Do not require a medium to move

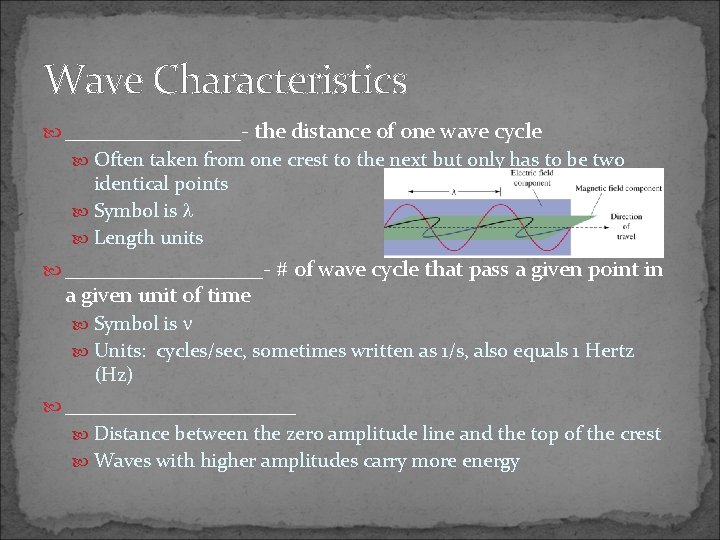
Wave Characteristics ________- the distance of one wave cycle Often taken from one crest to the next but only has to be two identical points Symbol is Length units _________- # of wave cycle that pass a given point in a given unit of time Symbol is Units: cycles/sec, sometimes written as 1/s, also equals 1 Hertz (Hz) ___________ Distance between the zero amplitude line and the top of the crest Waves with higher amplitudes carry more energy

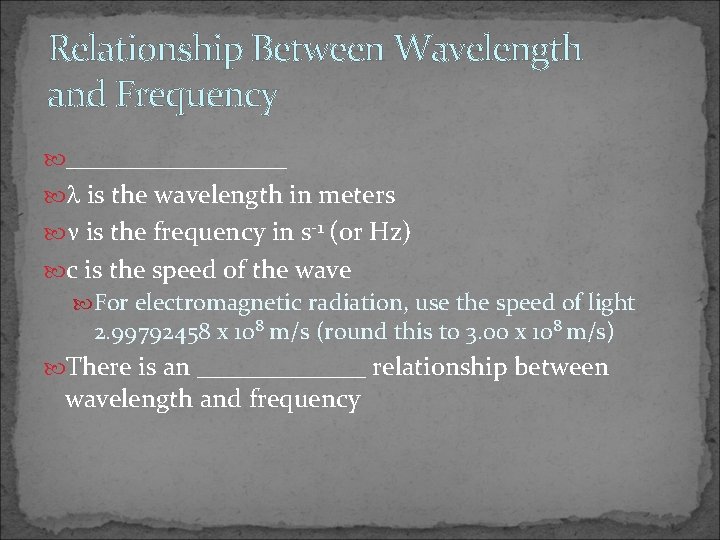
Relationship Between Wavelength and Frequency _________ is the wavelength in meters is the frequency in s-1 (or Hz) c is the speed of the wave For electromagnetic radiation, use the speed of light 2. 99792458 x 108 m/s (round this to 3. 00 x 108 m/s) There is an _______ relationship between wavelength and frequency

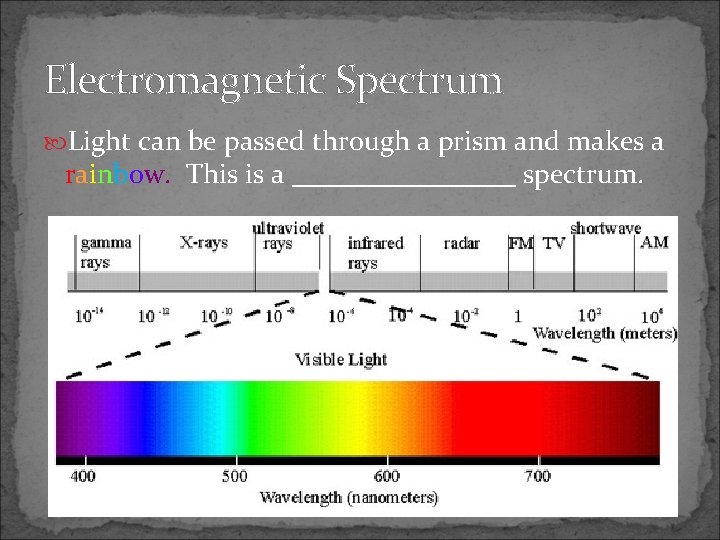
Electromagnetic Spectrum Light can be passed through a prism and makes a rainbow. This is a ________ spectrum.

Planck Proposed that electromagnetic energy comes in discreet amounts (or ___________) The energy of a quantum is expressed by ______where h= 6. 626 x 10 -34 J s Energy can only increase by multiples: 1 h , 2 h , 3 h , and so on

Einstein Applied the idea of quanta to explain how light can cause electricity: Tiny packets of light (photons) hit an atom. If they have enough energy, they can kick an electron out of an atom (photoelectric effect) So light is a wave and a “packet”

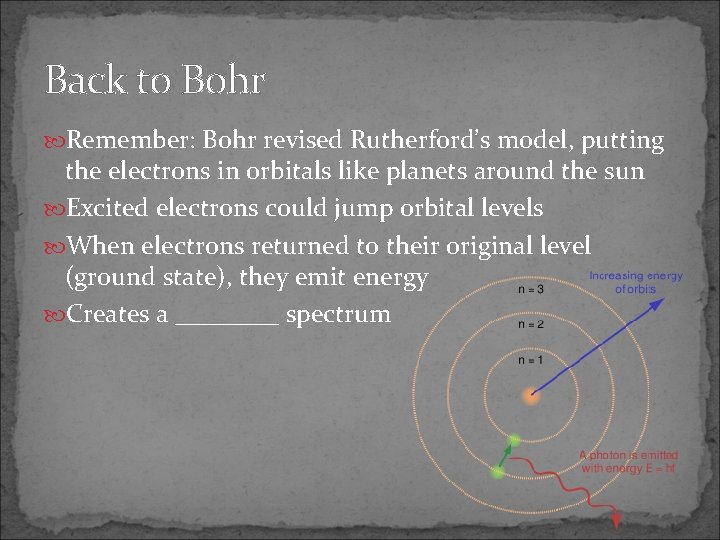
Back to Bohr Remember: Bohr revised Rutherford’s model, putting the electrons in orbitals like planets around the sun Excited electrons could jump orbital levels When electrons returned to their original level (ground state), they emit energy Creates a ____ spectrum

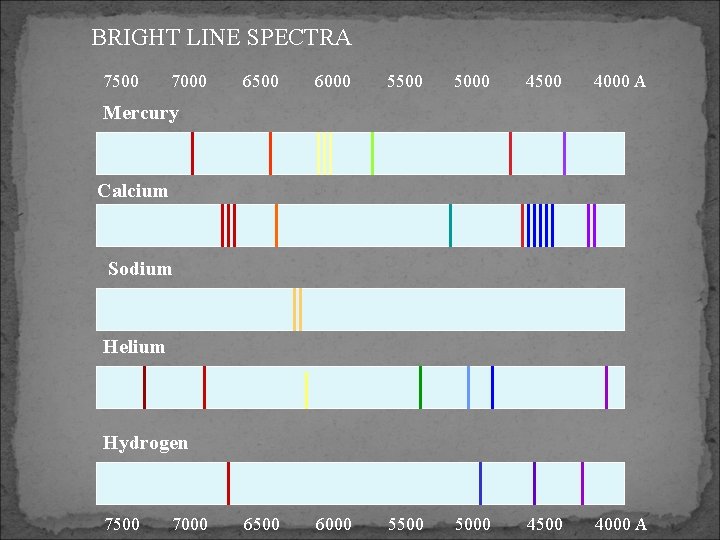
Line Spectra Created when only a few wavelengths of the electromagnetic spectrum are seen Gases of each element will make them when electricity passes through them or they’re heated up. The lines can be seen when the light is passed through a prism. Lines are different for each element (_______________)

BRIGHT LINE SPECTRA 7500 7000 6500 6000 5500 5000 4500 4000 A Mercury Calcium Sodium Helium Hydrogen 7500 7000

Modern Atomic Model Electron behave like ______ (matter) and ______ (electromagnetic radiation) If this is true, electrons can’t be tied to a fixed orbit We can’t even be certain of both an electron’s momentum and position at any one point in time (Heisenberg uncertainty principle) Can only create shaded areas around the nucleus where there is a high _________ of finding electrons

Quantum Numbers System of numbers that gives each electron a unique address in any atom

Principle Quantum Number Also called ______ Represents the primary energy levels around the nucleus (kind of like Bohr’s rings) There are 7 energy levels so _______ Electrons with a higher n are in higher energy orbital and spend more time away from the nucleus Electrons with the same n are in the same principle shell

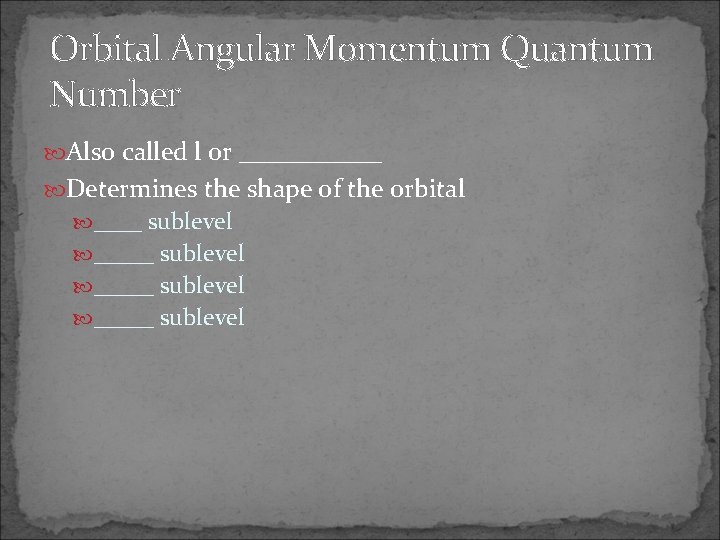
Orbital Angular Momentum Quantum Number Also called l or ______ Determines the shape of the orbital ____ sublevel _____ sublevel

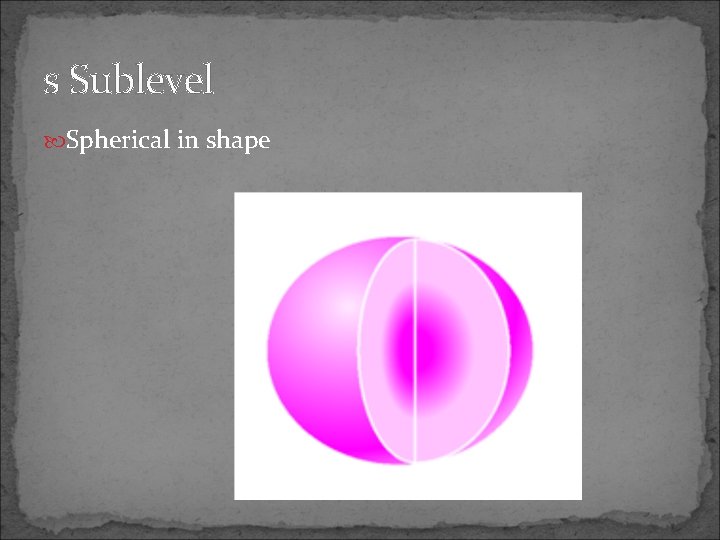
s Sublevel Spherical in shape

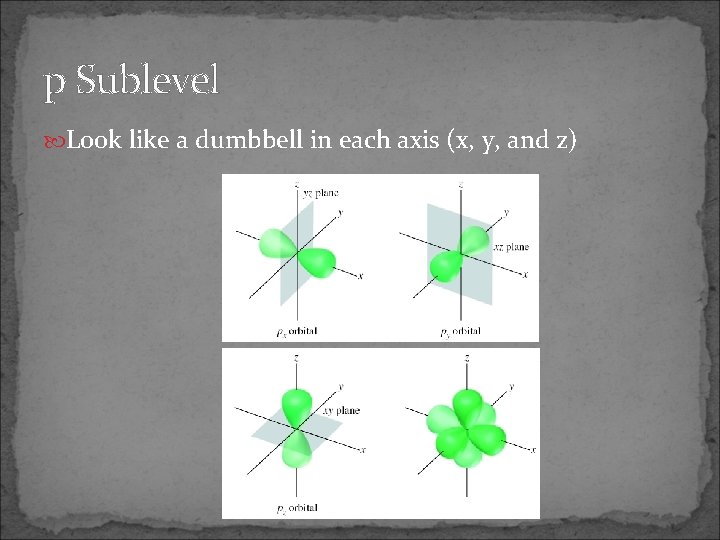
p Sublevel Look like a dumbbell in each axis (x, y, and z)

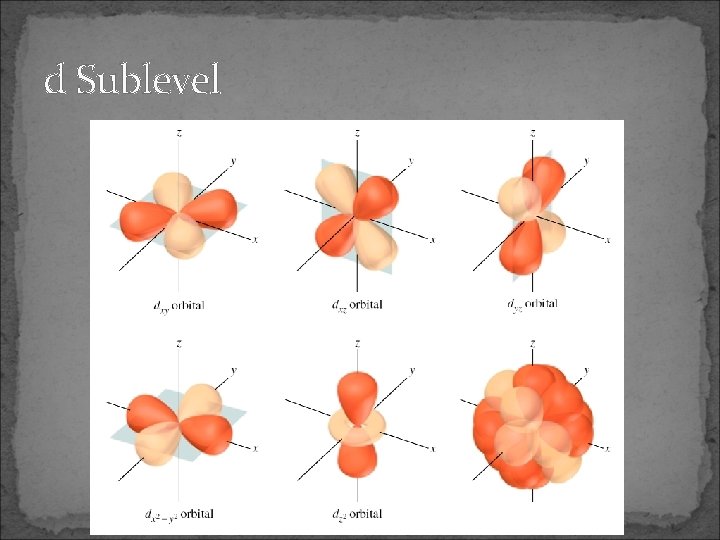
d Sublevel

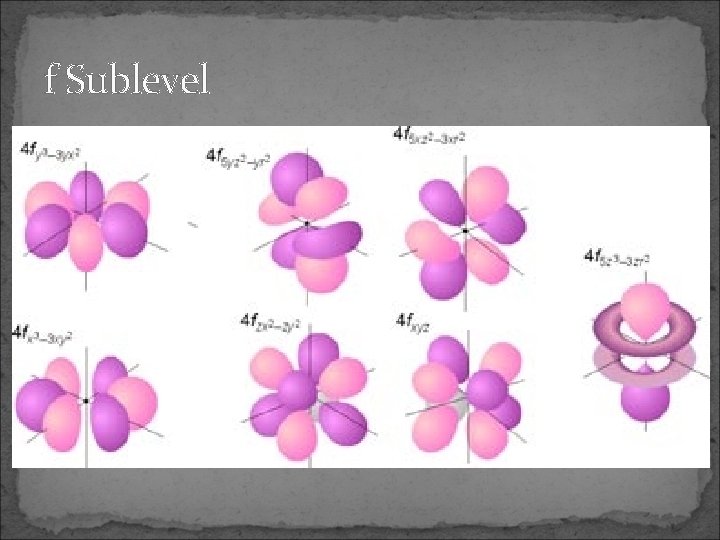
f Sublevel

Magnetic Quantum Number Also called ml or the orbital If s subshell……_____ orbital p subshell……_____ orbitals d subshell……_____ orbitals f subshell…. . ______ orbitals

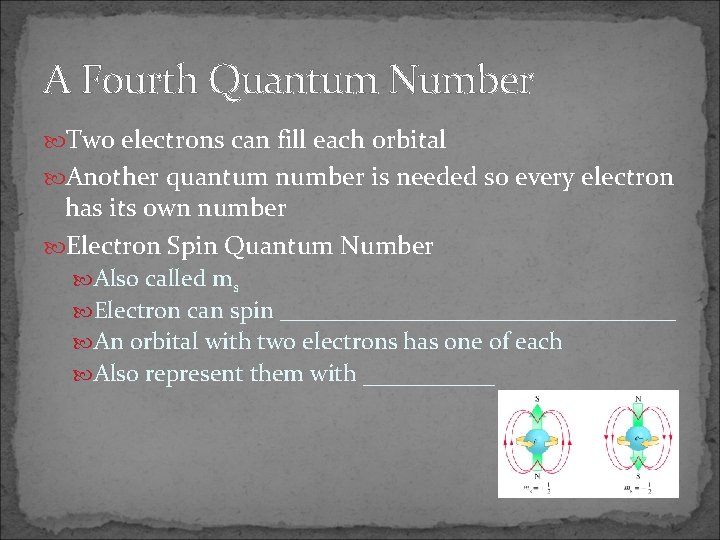
A Fourth Quantum Number Two electrons can fill each orbital Another quantum number is needed so every electron has its own number Electron Spin Quantum Number Also called ms Electron can spin _________________ An orbital with two electrons has one of each Also represent them with ______

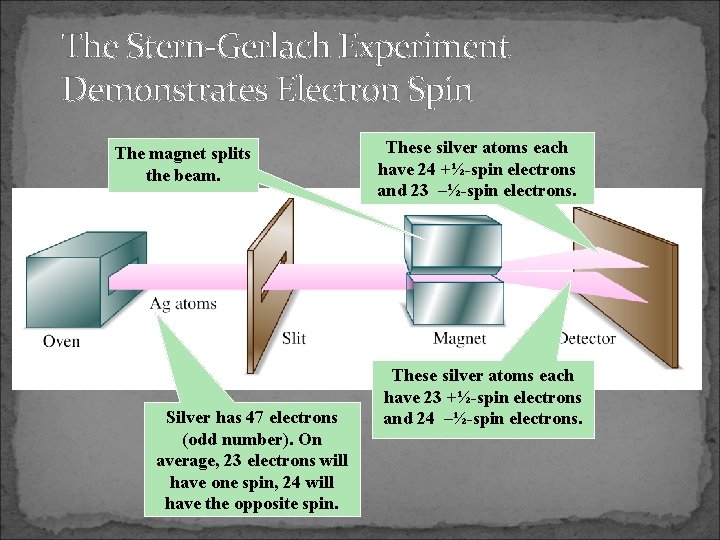
The Stern-Gerlach Experiment Demonstrates Electron Spin The magnet splits the beam. Silver has 47 electrons (odd number). On average, 23 electrons will have one spin, 24 will have the opposite spin. These silver atoms each have 24 +½-spin electrons and 23 –½-spin electrons. These silver atoms each have 23 +½-spin electrons and 24 –½-spin electrons.

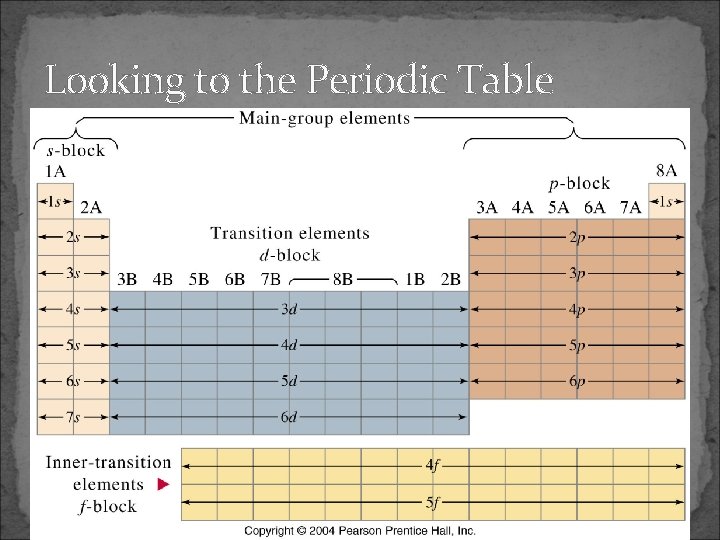
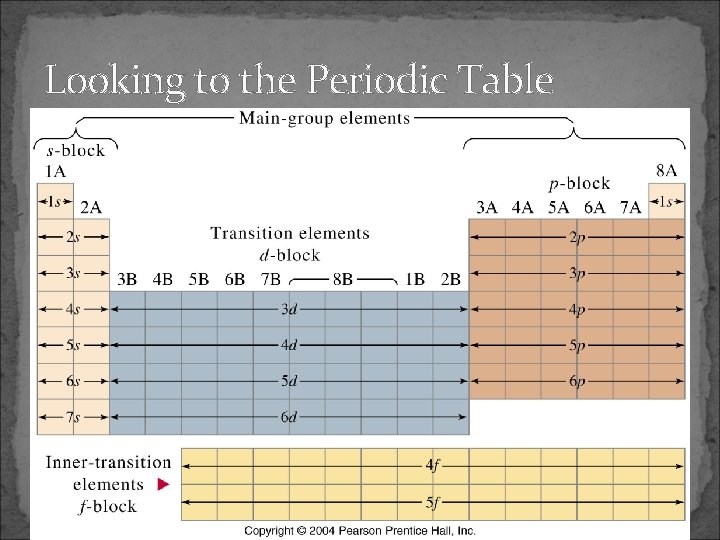
Looking to the Periodic Table

Electron Configurations Description of the placement of all the electrons in an atom of an element Rules Electrons occupy the lowest energy orbitals available (ground state). Filling order is determined by Aufbau Principle. Orbitals can only hold 2 electrons and they must have opposing spins (Pauli exclusion principle) Electrons will fill empty orbitals when possible. Half-filled orbitals all have parallel spin. (Hund’s rule)

Filling order (Aufbau Principle)

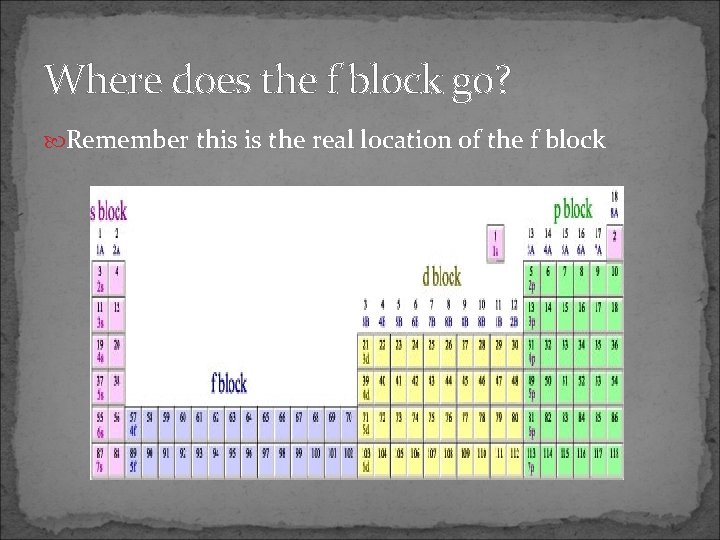
Where does the f block go? Remember this is the real location of the f block

Looking to the Periodic Table

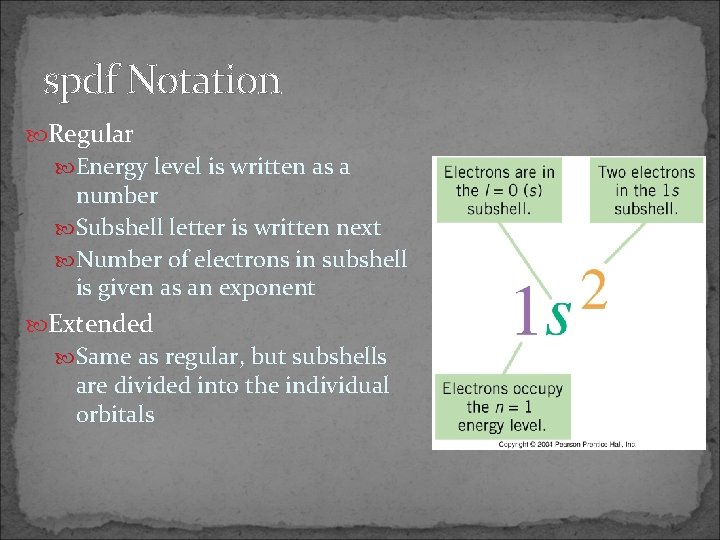
spdf Notation Regular Energy level is written as a number Subshell letter is written next Number of electrons in subshell is given as an exponent Extended Same as regular, but subshells are divided into the individual orbitals

spdf Notation Examples Regular He _____ O _______ Cl ____________ Extended He _____ O ___________ Cl ________________

Noble-gas-core Abbreviated Electron Configuration Same as spdf configuration but it starts from the noble gas of the previous period Examples Be ________ K ________ Br ________

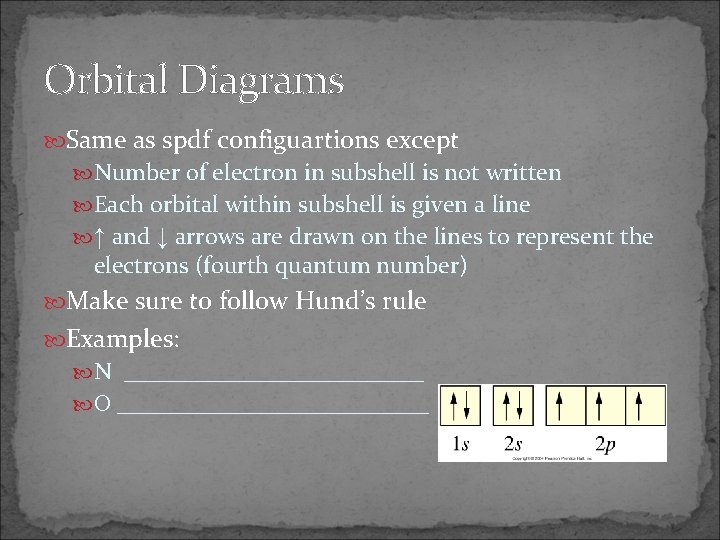
Orbital Diagrams Same as spdf configuartions except Number of electron in subshell is not written Each orbital within subshell is given a line ↑ and ↓ arrows are drawn on the lines to represent the electrons (fourth quantum number) Make sure to follow Hund’s rule Examples: N _____________ O _____________

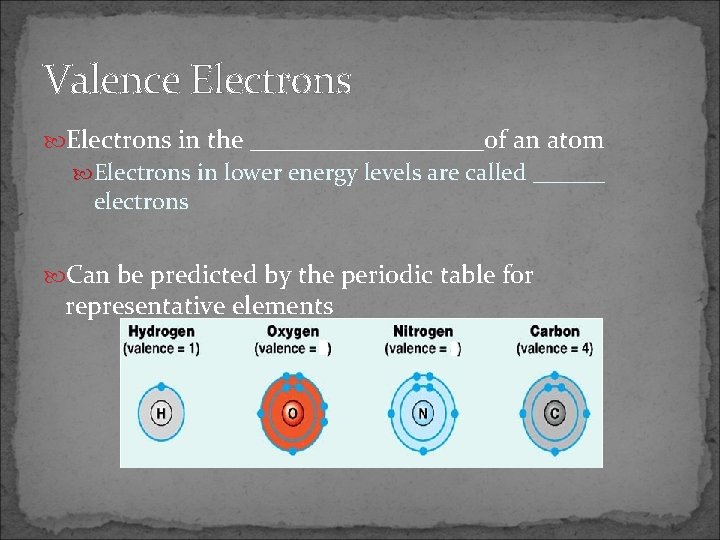
Valence Electrons in the _________of an atom Electrons in lower energy levels are called ______ electrons Can be predicted by the periodic table for representative elements

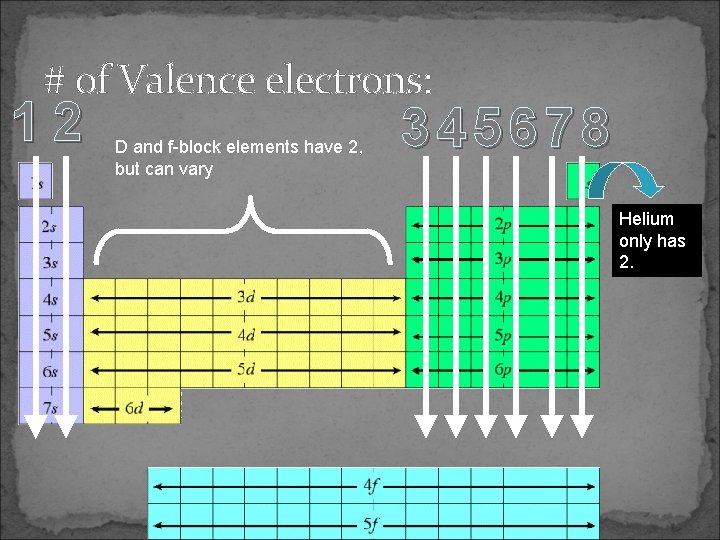
# of Valence electrons: 12 D and f-block elements have 2, but can vary 345678 Helium only has 2.

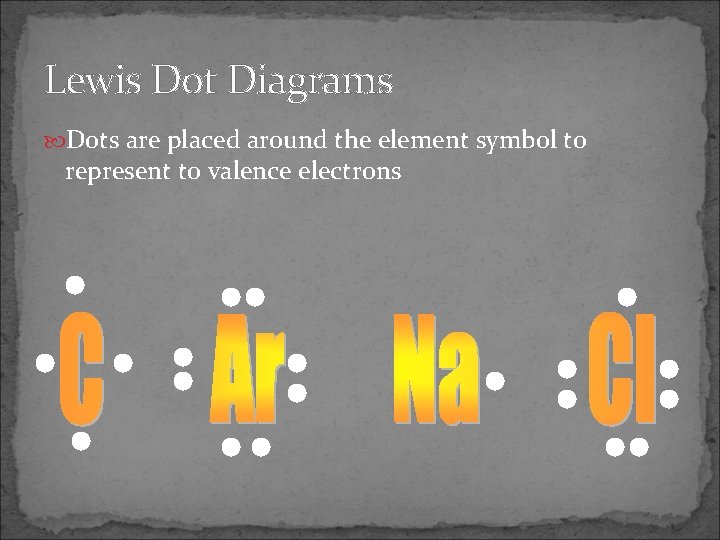
Lewis Dot Diagrams Dots are placed around the element symbol to represent to valence electrons

Octet Rule To be stable, atoms want _____ in their outer shell Noble gases already have 8 and don’t usually react with anyone Others will lose or gain electrons to get to 8 Said to be isoelectronic with the Nobel gas from the last period Ion will have the same electron configuration as this noble gas Atoms that gain or lose electrons to become isoelectronic with Helium follow the duet rule (2 electrons in valence shell)

Common Ions Group 1 - 1 valence electron Lose 1 electron, become +1 cation Group 2 - 2 valence electrons Lose 2 electrons, become +2 cation Group 13 - 3 valence electrons Lose 3 electrons, become +3 cation Group 15 - 5 valence electrons Gain 3 electrons, become -3 anion Group 16 - 6 valence electrons Gain 2 electrons, become -2 anion Group 17 - 7 valence electrons Gain 1 electron, become -1 anion

Ions of Other Elements Transition metals form cations, but the charge cannot be predicted Group 14 does not commonly form ions Group 18 does not form ions

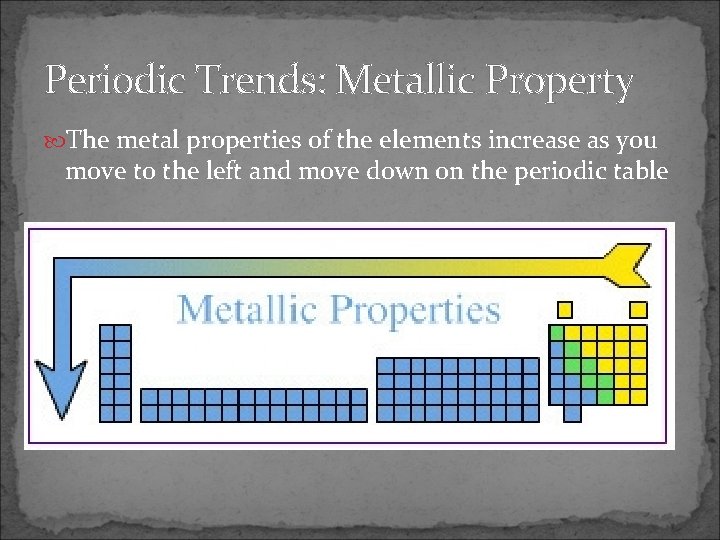
Periodic Trends: Metallic Property The metal properties of the elements increase as you move to the left and move down on the periodic table

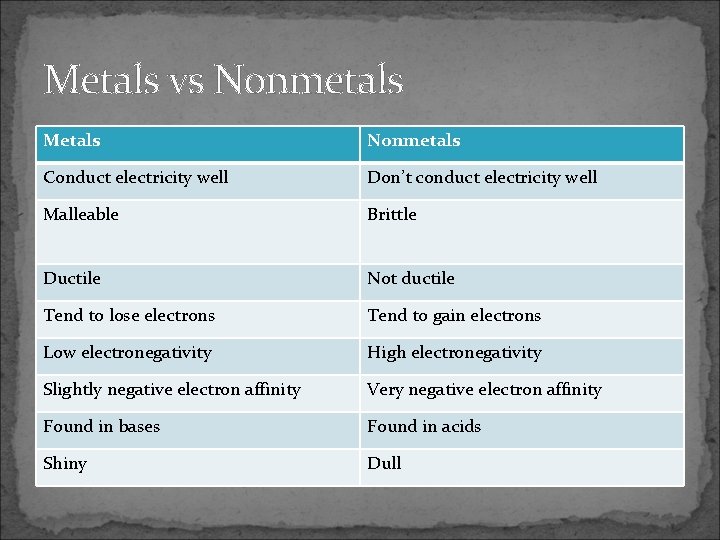
Metals vs Nonmetals Metals Nonmetals Conduct electricity well Don’t conduct electricity well Malleable Brittle Ductile Not ductile Tend to lose electrons Tend to gain electrons Low electronegativity High electronegativity Slightly negative electron affinity Very negative electron affinity Found in bases Found in acids Shiny Dull

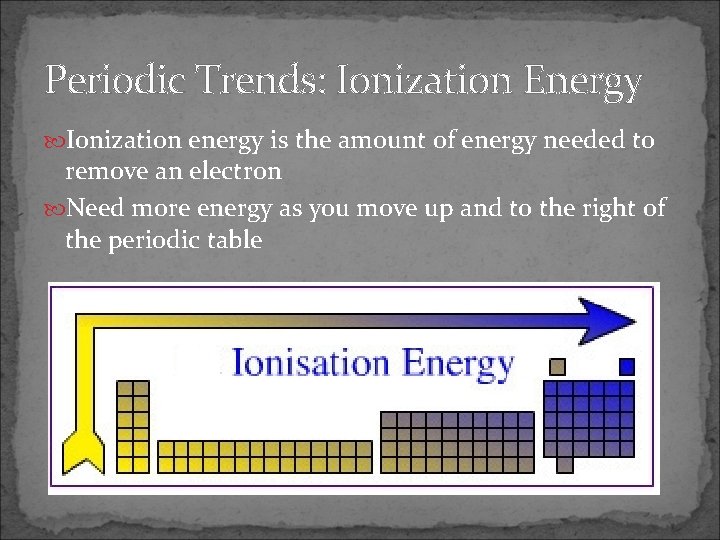
Periodic Trends: Ionization Energy Ionization energy is the amount of energy needed to remove an electron Need more energy as you move up and to the right of the periodic table

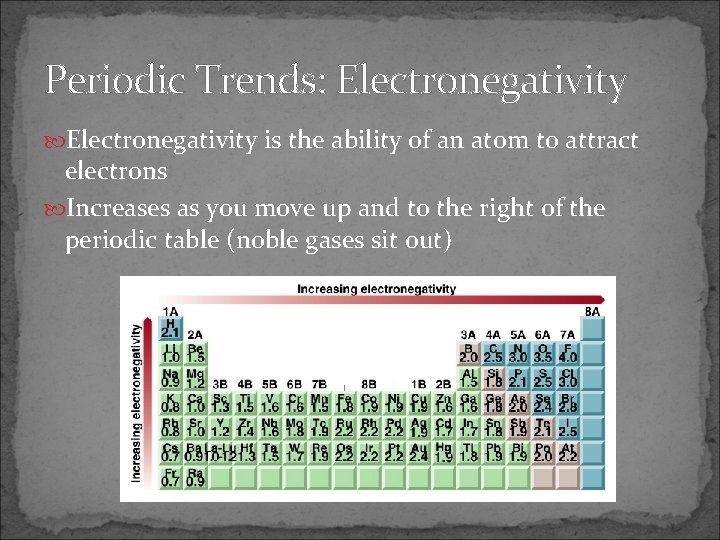
Periodic Trends: Electronegativity is the ability of an atom to attract electrons Increases as you move up and to the right of the periodic table (noble gases sit out)

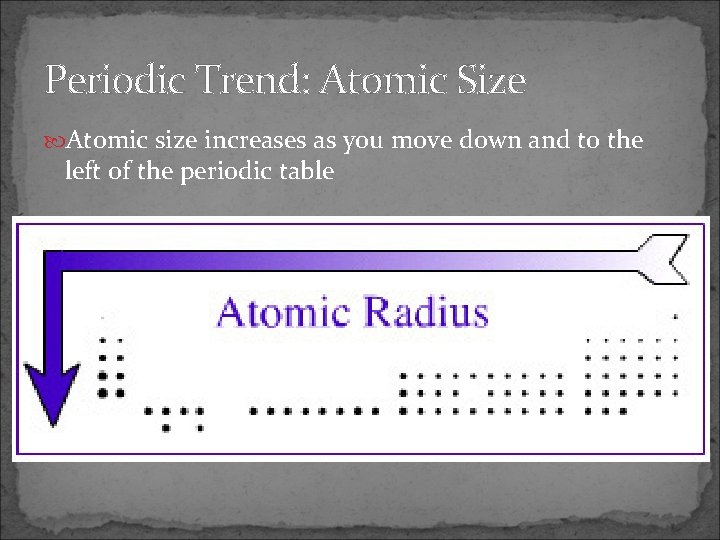
Periodic Trend: Atomic Size Atomic size increases as you move down and to the left of the periodic table

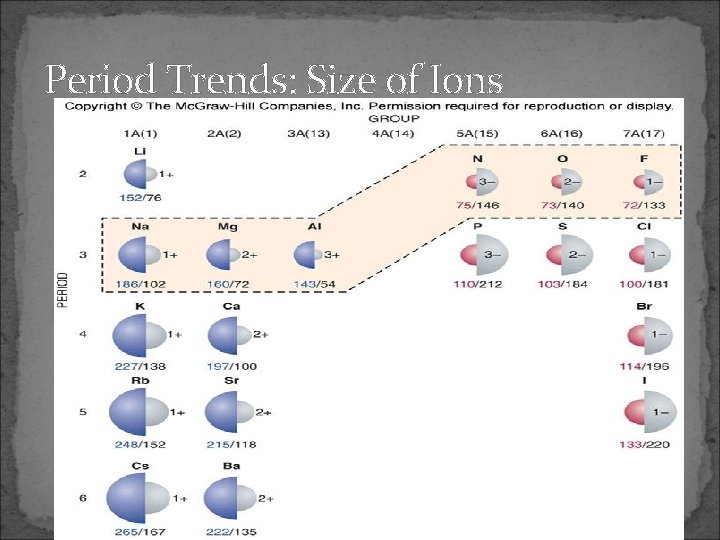
Period Trends: Size of Ions