Quantum Theory The Electromagnetic Spectrum Definition The electromagnetic






- Slides: 19

Quantum Theory The Electromagnetic Spectrum

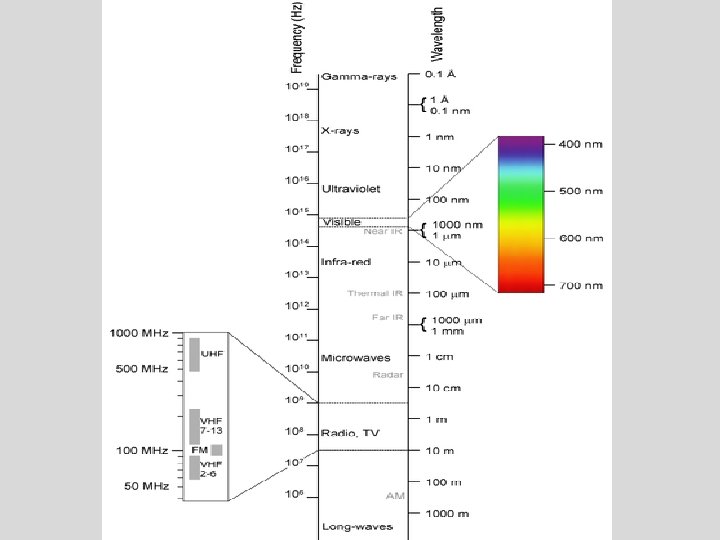
Definition: • The electromagnetic spectrum is a collection of all of the types of electromagnetic radiation. • These are forms of energy that move from point to point as waves – not requiring the presence of matter to transfer that energy.

Diagram of a Wave

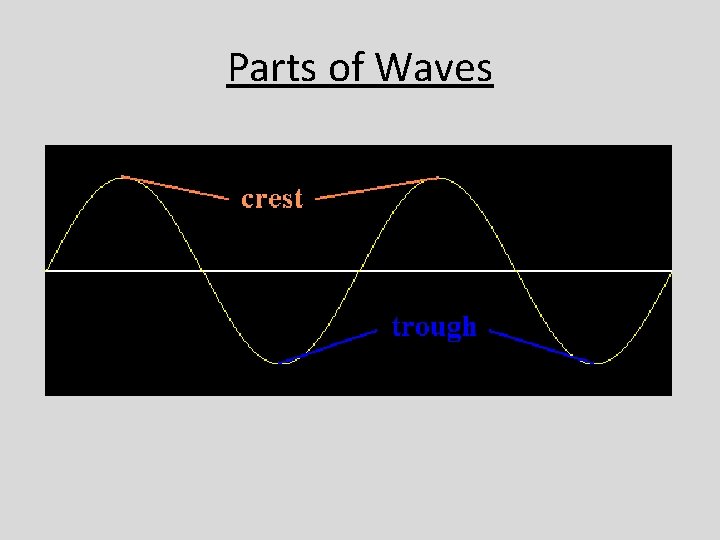
Parts of Waves

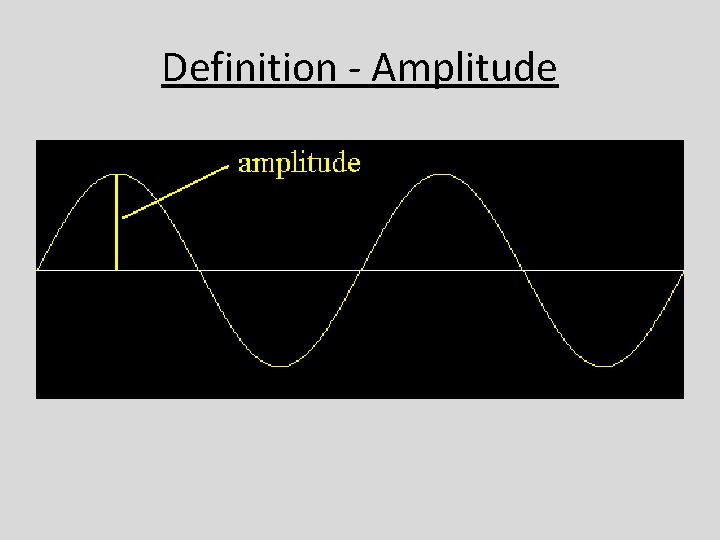
Definition - Amplitude

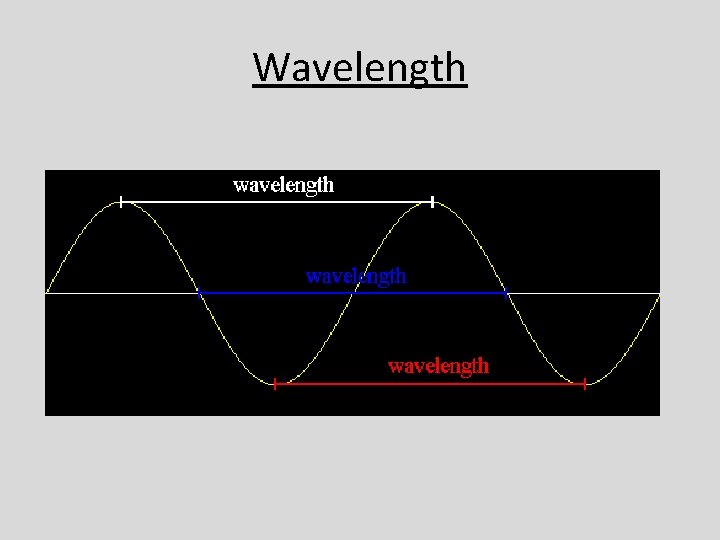
Wavelength

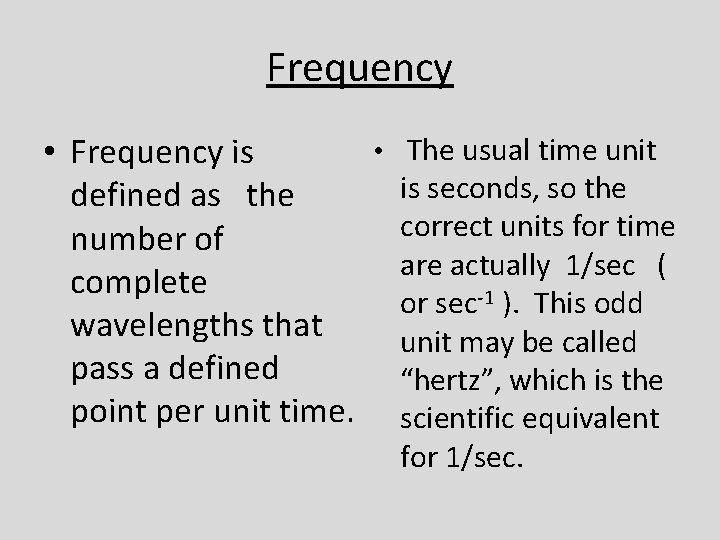
Frequency • Frequency is defined as the number of complete wavelengths that pass a defined point per unit time. • The usual time unit is seconds, so the correct units for time are actually 1/sec ( or sec-1 ). This odd unit may be called “hertz”, which is the scientific equivalent for 1/sec.

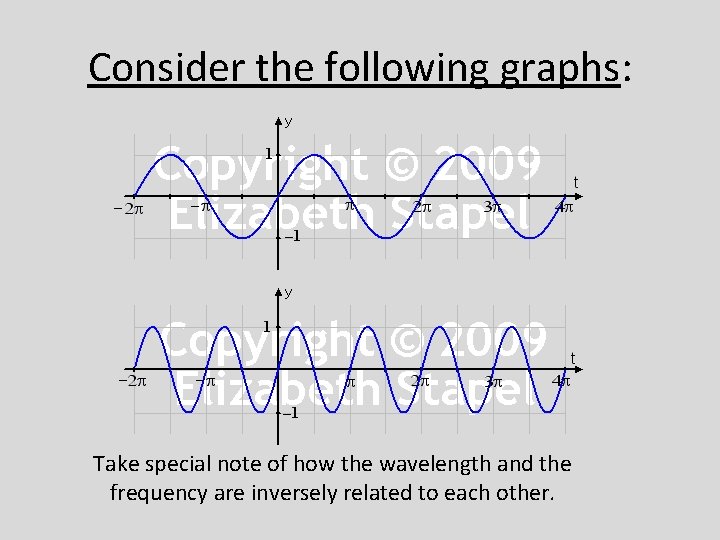
Consider the following graphs: Take special note of how the wavelength and the frequency are inversely related to each other.


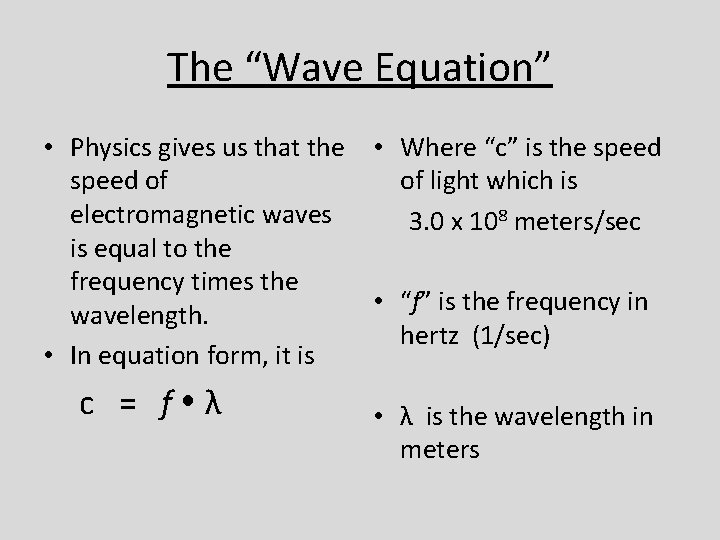
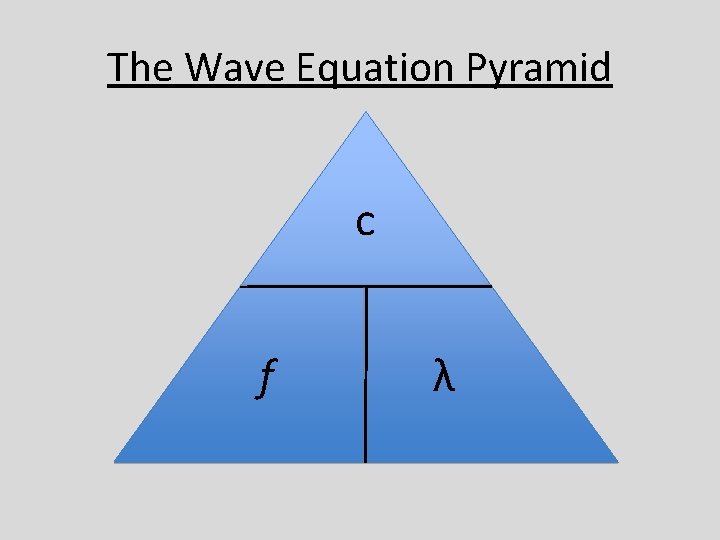
The “Wave Equation” • Physics gives us that the • Where “c” is the speed of of light which is electromagnetic waves 3. 0 x 108 meters/sec is equal to the frequency times the • “f” is the frequency in wavelength. hertz (1/sec) • In equation form, it is c = f λ • λ is the wavelength in meters

The Wave Equation Pyramid c f λ

Electrons and Changing Energy Levels

Think back to Planck’s ladder: Remember that Planck has defined the specific energies that electrons may have. These energies are like the rungs of a ladder – whole number multiples of a specific and basic energy value.

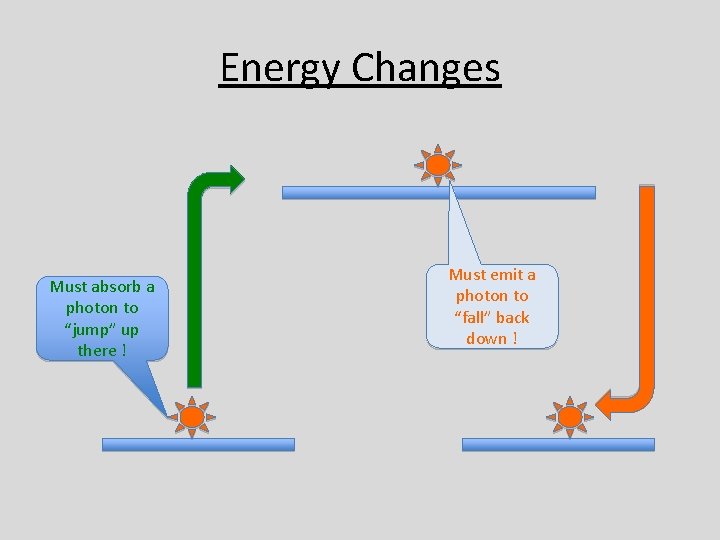
Electron Energy Changes • It is possible for an • It is also possible for an electron at a lower level electron at a higher to gain energy and level to “fall” to lower “jump” to a higher energy level. • When this occurs, the • To do this, the electron must “emit” a must absorb a “photon” photon – this is always – a burst of energy from in the form of some source – quite electromagnetic often electromagnetic radiation.

Energy Changes Must absorb a photon to “jump” up there ! Must emit a photon to “fall” back down !

Important Conclusion! • Since energy is required to cause an electron to “jump” to a higher level… • and energy is given off when an electron “falls” to a lower level… • and both processes involve electromagnetic radiation… • We have to conclude that electromagnetic waves have measureable energies.

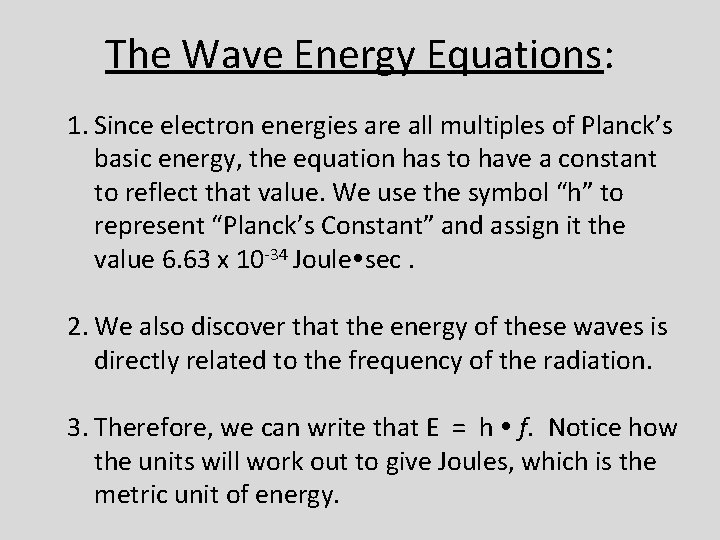
The Wave Energy Equations: 1. Since electron energies are all multiples of Planck’s basic energy, the equation has to have a constant to reflect that value. We use the symbol “h” to represent “Planck’s Constant” and assign it the value 6. 63 x 10 -34 Joule sec. 2. We also discover that the energy of these waves is directly related to the frequency of the radiation. 3. Therefore, we can write that E = h f. Notice how the units will work out to give Joules, which is the metric unit of energy.

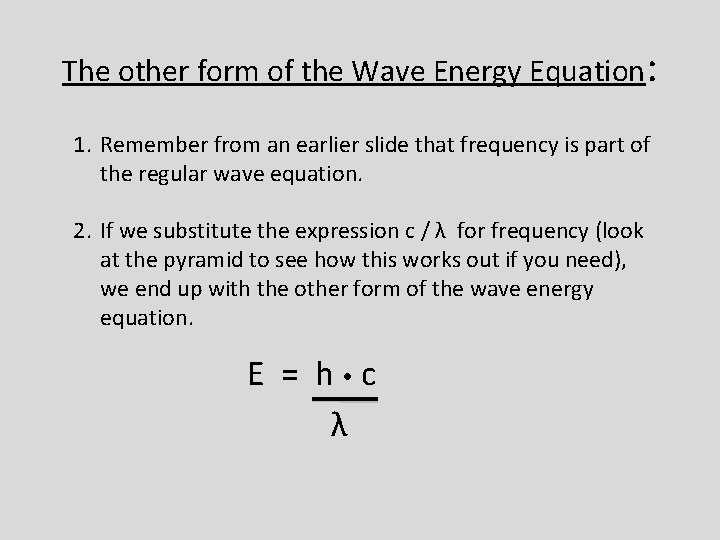
The other form of the Wave Energy Equation: 1. Remember from an earlier slide that frequency is part of the regular wave equation. 2. If we substitute the expression c / λ for frequency (look at the pyramid to see how this works out if you need), we end up with the other form of the wave energy equation. E = h c λ

What does this do for us? • It allows us to calculate the energy of an absorbed photon. • It allows us to calculate the energy of an emitted photon. • It allows us to determine what type of electromagnetic radiation will be given off during a specific electron “fall”. • We can actually predict what color of light will be emitted during an electron “fall”.