The Electromagnetic Spectrum and Light 18 1 Electromagnetic










































- Slides: 70

The Electromagnetic Spectrum and Light 18. 1 Electromagnetic Waves

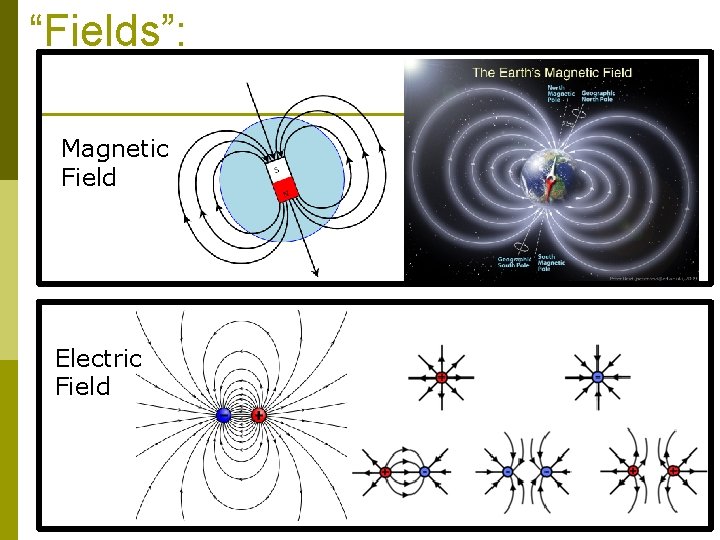
First, Let’s Define Some Terms: p A “Field”: an area in which something can act or operate. n Ex: a football field – an NFL football game occurs on that field, and not on the sidelines or in the stands p Vacuum: Empty space, where there is no particles or other matter p Spectrum: a broad range of possibilities n Ex: Autism Spectrum – not everyone with autism looks and behaves the same way – there are many possibilities.

“Fields”: Magnetic Field Electric Field

18. 1 Electromagnetic (EM) Waves p First, What is “Electromagnetism”? ? n n Abbreviated “EM” The interaction between electricity and magnetism Electricity and Magnetism are related! Ex: Magnetism can cause electricity **This is how turbines and generators work n Many things can be caused by or are types of Electromagnetism p These things can be placed on an “Electromagnetic Spectrum”

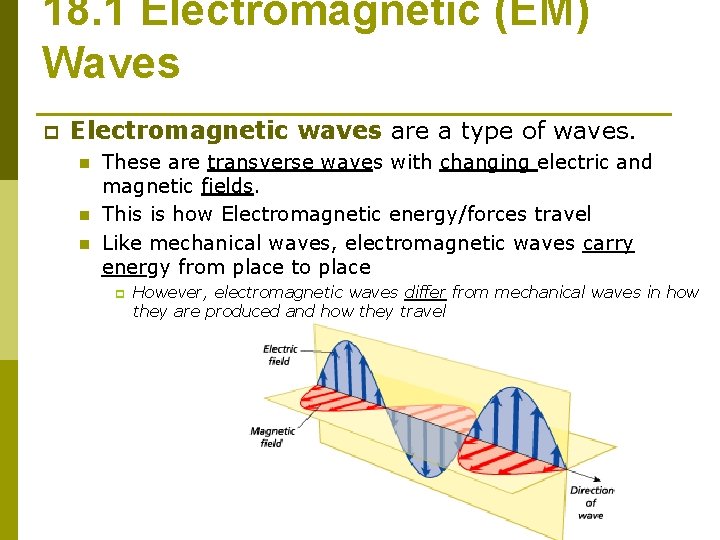
18. 1 Electromagnetic (EM) Waves p Electromagnetic waves are a type of waves. n n n These are transverse waves with changing electric and magnetic fields. This is how Electromagnetic energy/forces travel Like mechanical waves, electromagnetic waves carry energy from place to place p However, electromagnetic waves differ from mechanical waves in how they are produced and how they travel

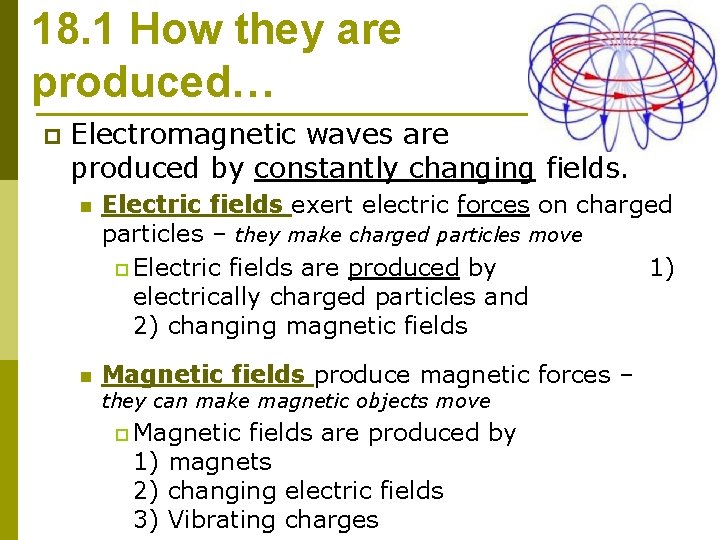
18. 1 How they are produced… p Electromagnetic waves are produced by constantly changing fields. n Electric fields exert electric forces on charged particles – they make charged particles move p Electric fields are produced by 1) electrically charged particles and 2) changing magnetic fields n Magnetic fields produce magnetic forces – they can make magnetic objects move p Magnetic fields are produced by 1) magnets 2) changing electric fields 3) Vibrating charges

18. 1 How EM Waves travel p The fields regenerate each other n n As the fields regenerate, their energy travels in the form of a wave. EM waves can travel through a vacuum, (empty space) as well as through matter. Note: (Mechanical waves can only travel through matter. ) p The transfer of energy by electromagnetic waves traveling through matter or across space is called electromagnetic radiation.


Agenda 5/18 p Pick up Final Exam Review Packet n p Instructions Finish Section 18. 1 n n Speed of EM Waves Speed of Light Solving for speed of light Do EM waves have particles? Quiz Monday on 18. 1 p HW: Final Exam Review #1 -8 p

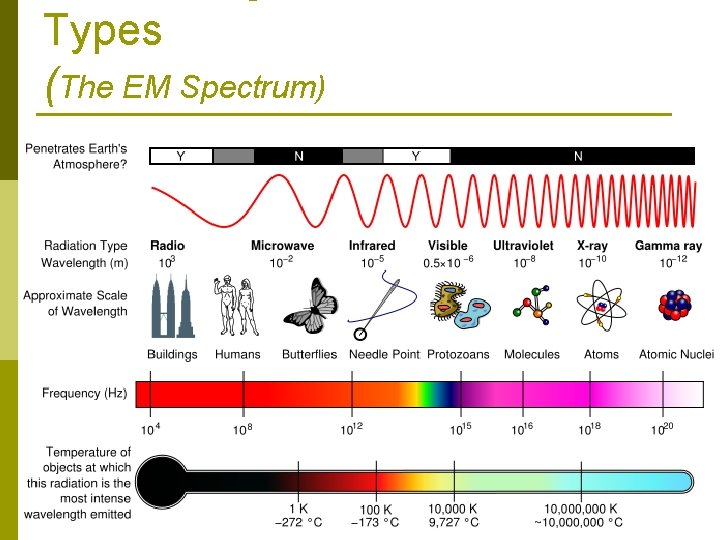
Types (The EM Spectrum)

The Speed of Electromagnetic Waves p Hmmm…How p fast do EM waves travel? ? Michelson's Experiment § 1926, the American physicist Albert Michelson (1852– 1931) measured the speed of light more accurately than ever before.

The Speed of Light p Light and all electromagnetic waves travel at the same speed when in a vacuum, regardless of the observer's motion p The speed of light in a vacuum, c, is 3. 00 × 108 m/s Actually… Nothing can travel faster than light Well, nothing that we’ve discovered so far…

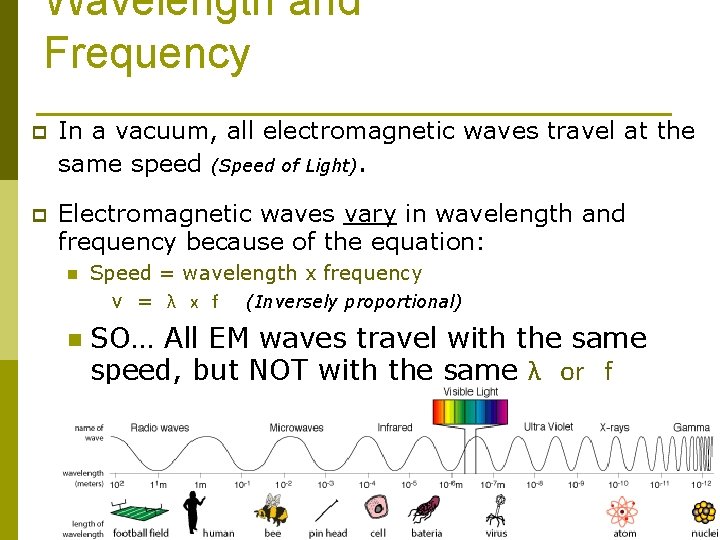
Wavelength and Frequency p In a vacuum, all electromagnetic waves travel at the same speed (Speed of Light). p Electromagnetic waves vary in wavelength and frequency because of the equation: n n Speed = wavelength x frequency v = λ x f (Inversely proportional) SO… All EM waves travel with the same speed, but NOT with the same λ or f

Let’s practice some math!!! p Convert 6. 45 X 10^5 millihertz to hertz p Convert. 0489 kilometers to meters p Convert 344 MHz to Hz

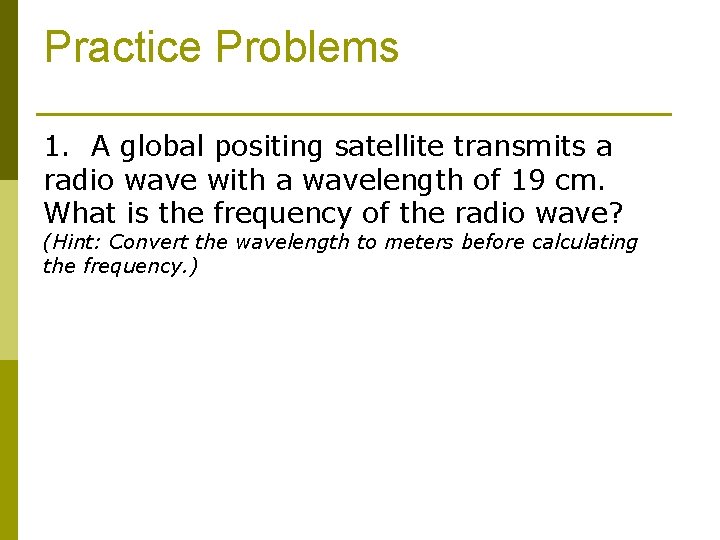
Practice Problems 1. A global positing satellite transmits a radio wave with a wavelength of 19 cm. What is the frequency of the radio wave? (Hint: Convert the wavelength to meters before calculating the frequency. )

Practice Problems 2. The radio waves of a particular AM radio station vibrate 680, 000 times per second. What is the wavelength of the wave?

Practice Problems 3. Radio waves that vibrate 1. 6 x 108 times per second are used on some train lines for communications. If radio waves that vibrate half as many times per second were used instead, how would the wavelength change?

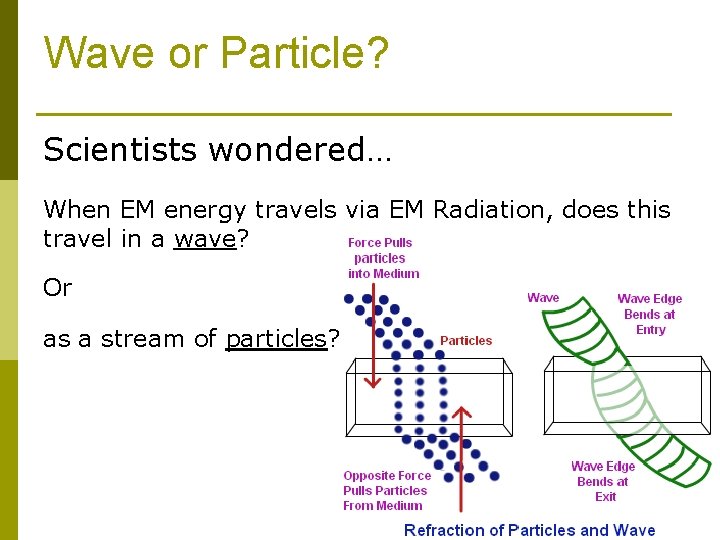
Wave or Particle? Scientists wondered… When EM energy travels via EM Radiation, does this travel in a wave? Or as a stream of particles?

Wave or Particle? p Scientists know EM radiation travels as a wave p They also have evidence that electromagnetic radiation behaves like a stream of particles p So…Electromagnetic radiation behaves sometimes like a wave and sometimes like a stream of particles p How do they know all this? !? Well…….

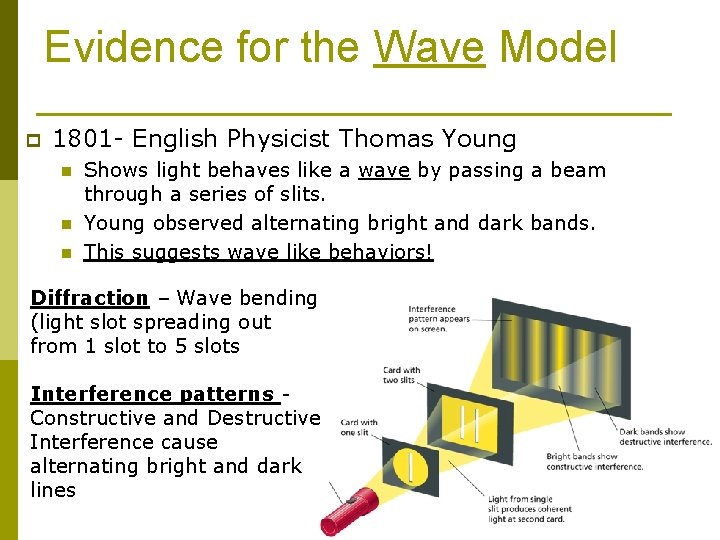
Evidence for the Wave Model p 1801 - English Physicist Thomas Young n n n Shows light behaves like a wave by passing a beam through a series of slits. Young observed alternating bright and dark bands. This suggests wave like behaviors! Diffraction – Wave bending (light slot spreading out from 1 slot to 5 slots Interference patterns Constructive and Destructive Interference cause alternating bright and dark lines

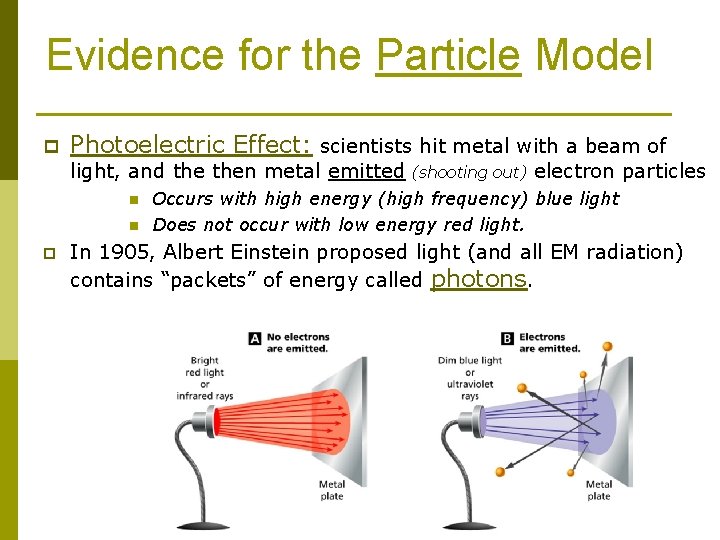
Evidence for the Particle Model p Photoelectric Effect: scientists hit metal with a beam of light, and then metal emitted n n p (shooting out) electron particles Occurs with high energy (high frequency) blue light Does not occur with low energy red light. In 1905, Albert Einstein proposed light (and all EM radiation) contains “packets” of energy called photons.

Waves or Particles? What’s the Verdict? p We accept that waves can be describes by BOTH waves and particles.

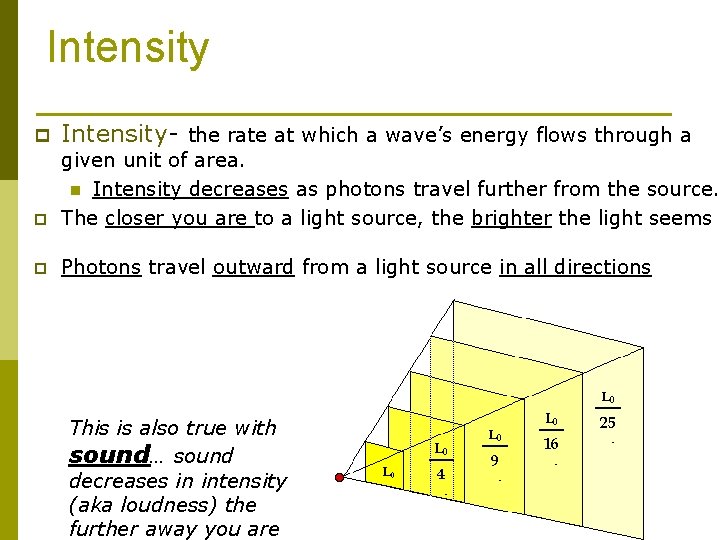
Intensity p Intensity- the rate at which a wave’s energy flows through a p given unit of area. n Intensity decreases as photons travel further from the source. The closer you are to a light source, the brighter the light seems p Photons travel outward from a light source in all directions This is also true with sound… sound decreases in intensity (aka loudness) the further away you are


Agenda 5/21 Section 18. 1 Quiz MOVED to Wed p Section 18. 2 p n n Herschel’s Discovery Exploring the EM Spectrum Quiz Wednesday on 18. 1 and 18. 2 p HW: 1) Next 2 sections of Final Exam Review 2) HW Packet Pgs 1 -5 p

Spectrum William Herschel’s Discovery n He used a prism to separate sunlight n Herschel noticed the temperature was lower at the blue end and higher towards the red end n Temperature was even higher just beyond the red end n Cosmos Video Clip Via Netflix: Episode 5 16: 00 – 22: 00 41: 30 - end

Herschel’s Conclusion p Temperature was even high just beyond the red end. p Herschel concluded there must be invisible radiation beyond the red end of the color band.

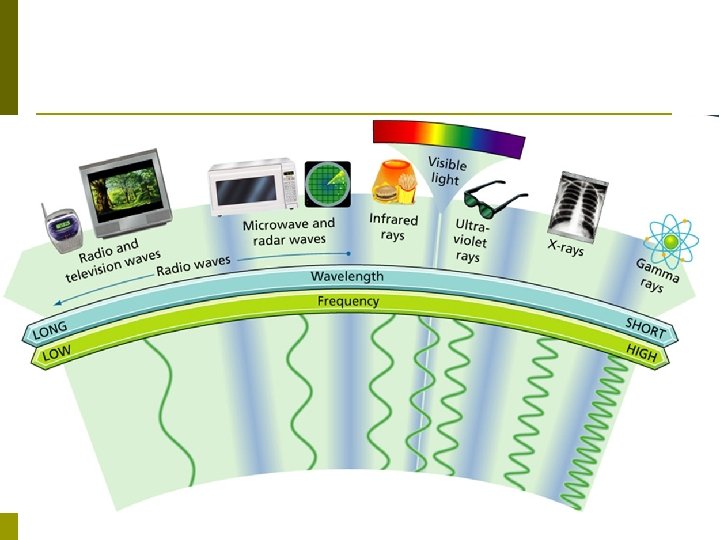
Electromagnetic Radiation p Today, radiation beyond the red end of the color band is called infrared radiation. …And there are MORE types of EM radiation p Electromagnetic spectrum- full range of frequencies of electromagnetic radiation. p It includes: n Radio waves § Ultraviolet rays n Infrared rays § X-rays n Visible light § Gamma rays p


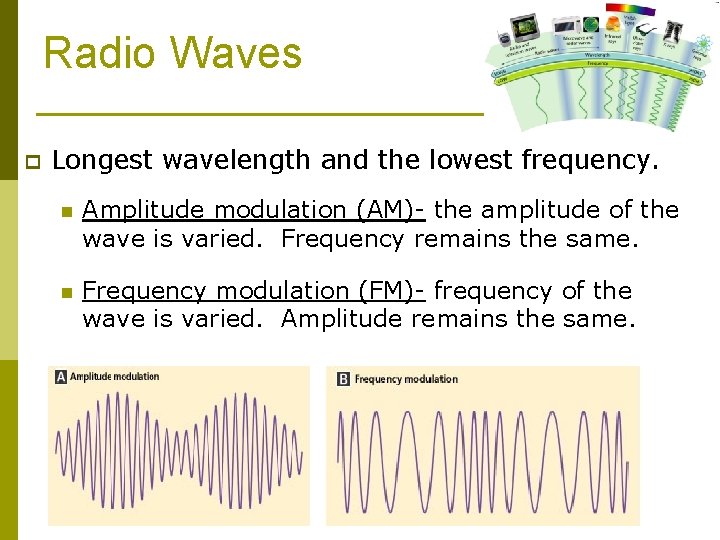
Radio Waves p Longest wavelength and the lowest frequency. n Amplitude modulation (AM)- the amplitude of the wave is varied. Frequency remains the same. n Frequency modulation (FM)- frequency of the wave is varied. Amplitude remains the same.

Radio Waves p n FM radio signals do not travel as far as AM signals along Earth’s curved surface. n Particles in Earth’s upper atmosphere reflect the lower frequency radio waves of AM better than the higher frequency FM waves. Radio Waves also include: n n n Microwaves are the shortest wavelength radio waves. They also carry cell phone convos. Radar TV

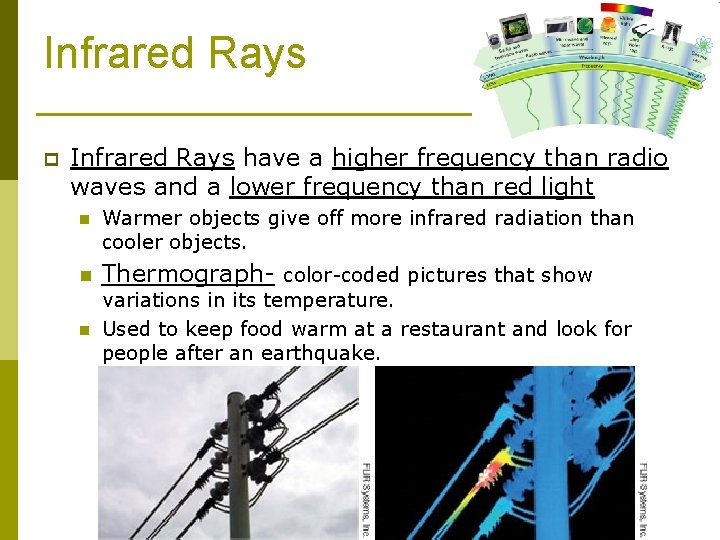
Infrared Rays p Infrared Rays have a higher frequency than radio waves and a lower frequency than red light n Warmer objects give off more infrared radiation than cooler objects. n Thermograph- color-coded pictures that show n variations in its temperature. Used to keep food warm at a restaurant and look for people after an earthquake.

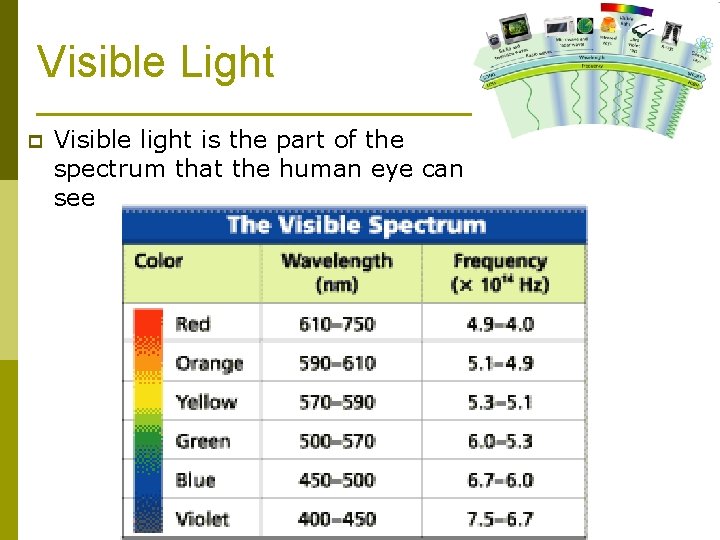
Visible Light p Visible light is the part of the spectrum that the human eye can see

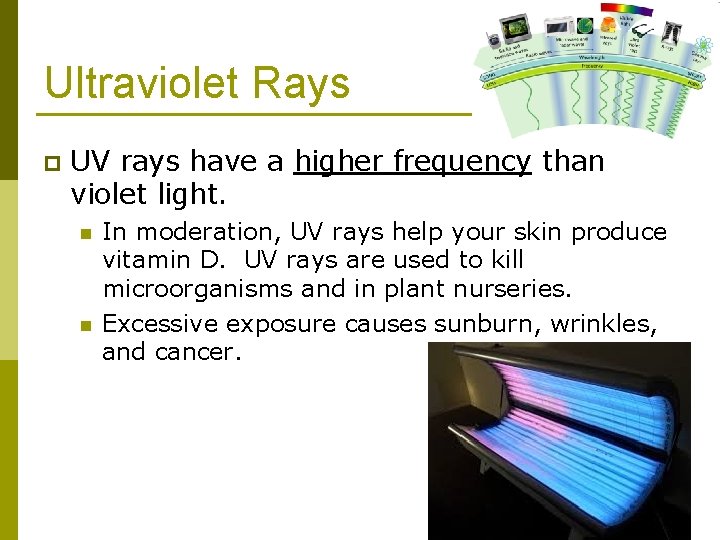
Ultraviolet Rays p UV rays have a higher frequency than violet light. n n In moderation, UV rays help your skin produce vitamin D. UV rays are used to kill microorganisms and in plant nurseries. Excessive exposure causes sunburn, wrinkles, and cancer.

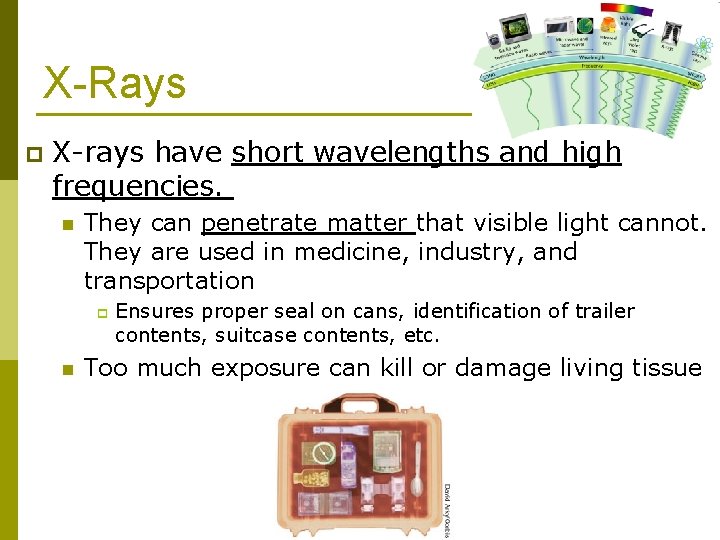
X-Rays p X-rays have short wavelengths and high frequencies. n They can penetrate matter that visible light cannot. They are used in medicine, industry, and transportation p n Ensures proper seal on cans, identification of trailer contents, suitcase contents, etc. Too much exposure can kill or damage living tissue

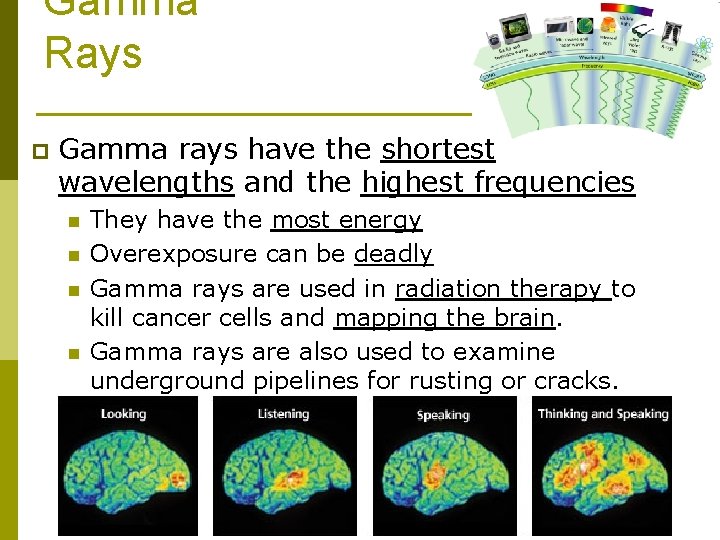
Gamma Rays p Gamma rays have the shortest wavelengths and the highest frequencies n n They have the most energy Overexposure can be deadly Gamma rays are used in radiation therapy to kill cancer cells and mapping the brain. Gamma rays are also used to examine underground pipelines for rusting or cracks.


Do Now 5/23 1. THIS and THIS create the bright and dark lines in Young’s experiment, above 2. THESE waves can pass through your body 3. THESE waves have slightly higher frequencies than the color purple 4. THESE colored waves have the longest wavelength of any color 5. The tiny particles that travel in EM waves are called THIS

Agenda 5/23 Warm Up p Check HW/ Review HW p 18. 1 and 18. 2 Quiz p Section 18. 3: Behavior of light p Quiz 18. 3 Tomorrow! Study vocab of this section overnight p HW: 1) Final Exam Review 2) HW p

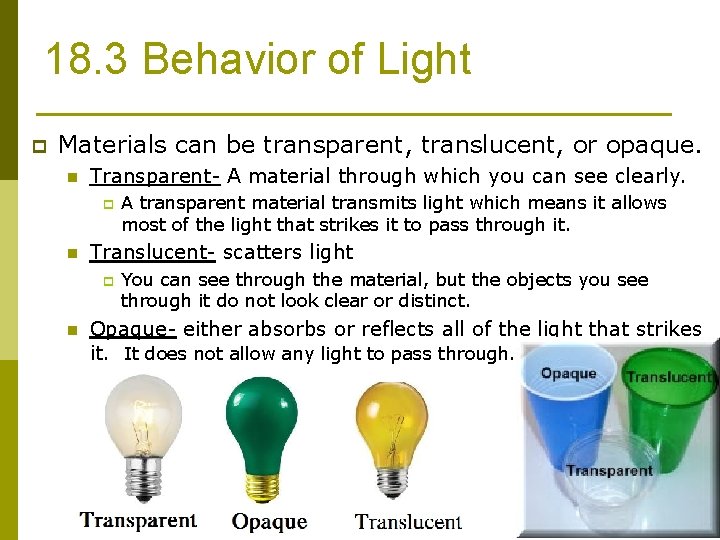
18. 3 Behavior of Light p Materials can be transparent, translucent, or opaque. n Transparent- A material through which you can see clearly. p n Translucent- scatters light p n A transparent material transmits light which means it allows most of the light that strikes it to pass through it. You can see through the material, but the objects you see through it do not look clear or distinct. Opaque- either absorbs or reflects all of the light that strikes it. It does not allow any light to pass through.

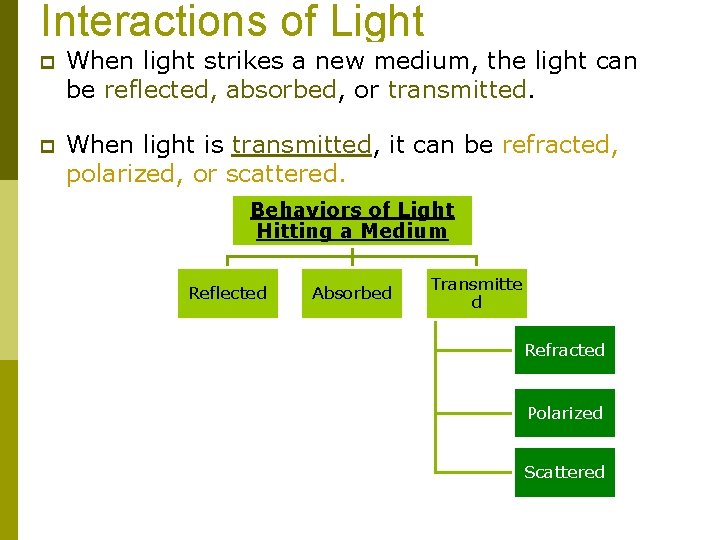
Interactions of Light p When light strikes a new medium, the light can be reflected, absorbed, or transmitted. p When light is transmitted, it can be refracted, polarized, or scattered. Behaviors of Light Hitting a Medium Reflected Absorbed Transmitte d Refracted Polarized Scattered

Reflection p Image- a copy of an object formed by reflected or refracted waves of light. p When light reflects from a smooth surface, you see a clear, sharp image. p When light reflects from a rough surface, you see a blurred reflected image or no image at all.

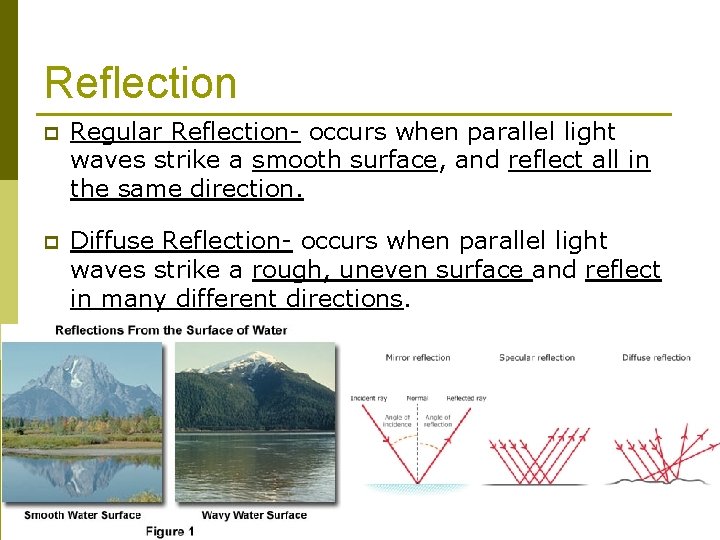
Reflection p Regular Reflection- occurs when parallel light waves strike a smooth surface, and reflect all in the same direction. p Diffuse Reflection- occurs when parallel light waves strike a rough, uneven surface and reflect in many different directions.

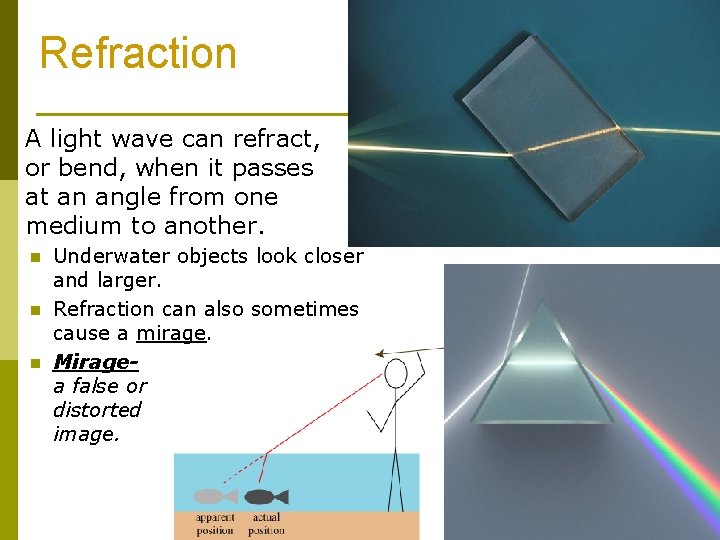
Refraction A light wave can refract, or bend, when it passes at an angle from one medium to another. n n n Underwater objects look closer and larger. Refraction can also sometimes cause a mirage. Miragea false or distorted image.

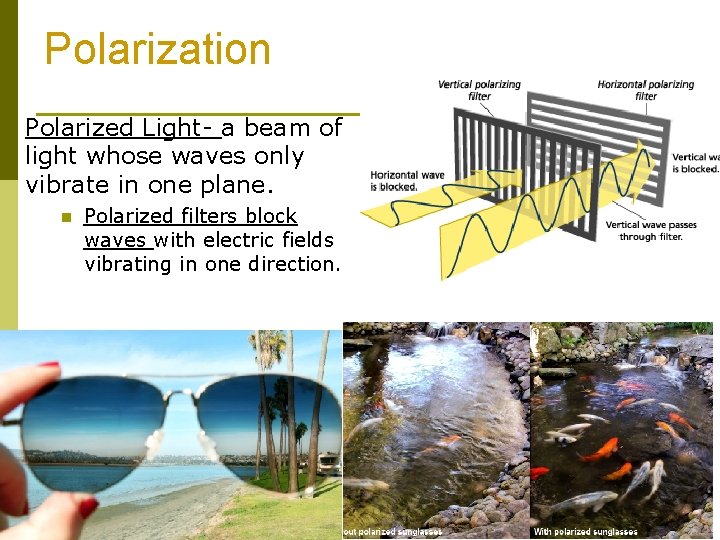
Polarization Polarized Light- a beam of light whose waves only vibrate in one plane. n Polarized filters block waves with electric fields vibrating in one direction.

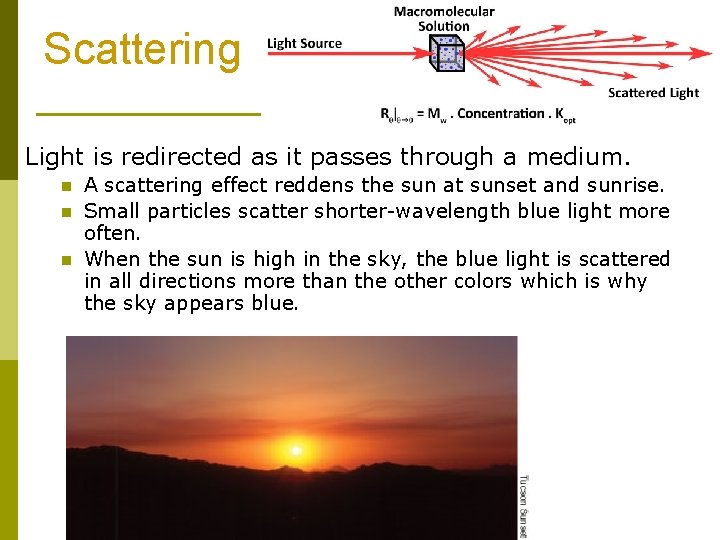
Scattering Light is redirected as it passes through a medium. n n n A scattering effect reddens the sun at sunset and sunrise. Small particles scatter shorter-wavelength blue light more often. When the sun is high in the sky, the blue light is scattered in all directions more than the other colors which is why the sky appears blue.

Check for Understanding p List the terms Translucent, Transparent, and Opaque in order of the light-transmitting ability p Glass is an object which transmits light. What 3 things can happen to light when it transmits through glass? p Which type of reflection is most useful for us in everyday life?


18. 4 Color p p As white light passes through a prism, shorter wavelengths refract (aka slow down) more than longer wavelengths, and the colors separate Dispersion- The process in which white light separates into colors

The Color of Objects The color of any object depends on what the object is made of and on the color of light that strikes the object. n Red paint mostly reflects red light. Most of the other colors of white light are absorbed. n Objects look black when all light is absorbed.

Mixing Colors of Light *These are different from Art class!* p Primary Colors of Light n p Secondary Colors of Light n p Three specific colors that can be combined to create all possible colors. Primary colors are red, green, and blue. Cyan, yellow and magenta Each secondary color is a combination of two primary colors.

Mixing Colors of Light Any two colors of light that combine to form white light are complementary colors of light. p Complementary colors of pigments combine to form black. p n Pigments are cyan, yellow, and magenta.

Color Lab 5. Get into clusters NOW: Look at your Color Lab worksheet on Pg 3 Send 1 person to get a Manilla envelope Follow instructions on the worksheet Complete the questions 6. When done, finish pages 1 and 2 1. 2. 3. 4.

Sources of Light: Intro 1. Define luminous. Light that we can see with our eyes. Use to describe objects that generate this type of light 2. List several common light sources. Incandescent, fluorescent, lasers, neon lights, tungstenhalogen lights, and sodium-vapor lights Each type of light has pros- and cons- , including expense/cost of the bulb, bulb “life”, eco-friendly, etc

Jig Saw Instructions PART 1: p Stay with your current LETTER groups p Look at your “Sources of Light” Worksheet p Find the topic that corresponds to your group: p p p Use smart phones to research your topic Answer the questions Become an expert so you can teach it to others Group Topic A Incandescent Light B Fluorescent Light C Lasers D Neon Light E Sodium-Vapor Lights F Tungsten-Halogen Lights PART 2: Get into your NUMBER groups. Teach other the information, and answer the Questions

18. 5 Sources of Light p Define luminous. p List several common light sources. p What is incandescent? p Describe an incandescent light bulb p What is fluorescence? p Describe a fluorescent light bulb. p Why do schools and offices use fluorescent light bulbs?

18. 5 p What is a laser? p How is a laser light produced? p What are lasers used for? p How do neon lights emit light? p Do all neon lights contain the element Ne? p What other elements are used in neon lights?

18. 5 Sources of Light p Describe how sodium-vapor lights work. p What are sodium vapor lights used for? p Describe how tungsten-halogen lights work? p What is the advantage of using tungsten-halogen lights? p Why is quartz used instead of glass?


Do Now 5/4 p What do you need for a wave? p Draw a transverse wave and label the parts. p Explain how electromagnetic waves are different from mechanical waves. p Write the speed of light p List the 7 different types of electromagnetic waves.

Agenda 5/5 Do Now p Go over HW p Quiz p Section 18. 2 p p HW: 4, 5, 9, 10, p Quiz Monday! EM Spectrum Vocab p Chapt. 18 Test Wed!

Do Now 5/8 1. 2. What happens to the intensity of light as photons move away from the light source? What is the wavelength of an AM radio wave in a vacuum if its frequency is 810 kilohertz? 3. What is the speed of an x-ray? 4. Which wave has the longest wavelength in the electromagnetic spectrum?

Agenda 5/8 Do Now p Go over quiz p Go over HW p Quiz on EM Spectrum p Section 18. 3: Behavior of Light p p HW: Pgs 6, 7, 11 p Quiz Tomorrow on Sect 18. 3 p Chapt. 18 Test Thurs!

Do Now 5/9 1. THIS is a reflection caused by a rough surface 2. THESE are the primary colors of LIGHT 3. Explain how polarized sunglasses help you while driving a car in the morning during sunrise. 4. What 3 things can light do when it hits a medium? 5. What are the benefits and dangers of gamma rays?

Agenda 5/9 Do Now p Go over HW p Color Lab – Pages 5 & 6 in today’s packet p Finish Pg 12 on the back of your Original HW Packet p Go over quiz p Quiz on Behavior of Light p p p HW: Finish pgs 1 -4 of today’s packet Chapt. 18 Test Thurs!

Do Now 5/10 p Explain how electromagnetic waves are different from mechanical waves. p What 2 things determines the color of an object? p Circle the form of radiation with the longer wavelength. n Green light or Blue light n Microwaves or infared

Agenda 5/10 Do Now p Check HW p Go over quiz p 18. 5 – Sections of Light: Light Bulbs p n Jigsaw Activity p Kahoot – x 2 if we have time p HW: Study!! p Chapt. 18 Test Tomorrow!

Exit Ticket 1. What are four common uses for lasers? 2. Compare and contrast incandescent and tungsten-halogen light bulbs. 3. Throw back! What color does green and blue give you?

Agenda 5/10 p Do Now & HW check (have HW open!) p Go over Do Now p Color Lab – 10 mins p Section 18. 5 Jig Saw “Sources of Light!” p HW: Packet pages p Chapt. 18 Test Thursday!

Do Now 5/11 PLEASE HAVE HW OUT AND OPEN! 1. THIS is the major advantage of tungsten-halogen bulbs over incandescent bulbs. 2. Describe a fluorescent light bulb. 3. How is a laser light produced? 4. What are lasers used for?