Light and Quantized Energy Electromagnetic Radiation l A







- Slides: 23

Light and Quantized Energy

Electromagnetic Radiation l A form of energy that exhibits wavelike behavior as it travels through space l Examples: Visible light, microwaves, x-rays, radio and television waves l Different forms of electromagnetic radiation can be seen on the electromagnetic spectrum

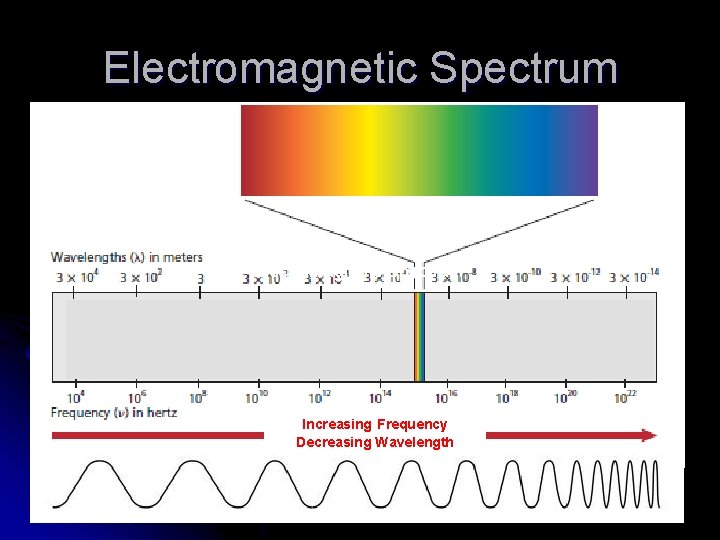
Electromagnetic Spectrum l Encompasses all forms of electromagnetic radiation, showing the differences of wavelength and frequency in the types of radiation

Electromagnetic Spectrum Increasing Frequency Decreasing Wavelength

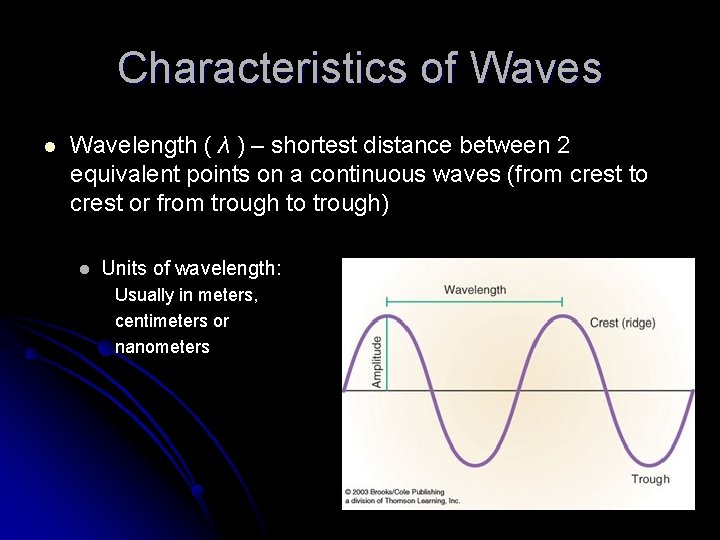
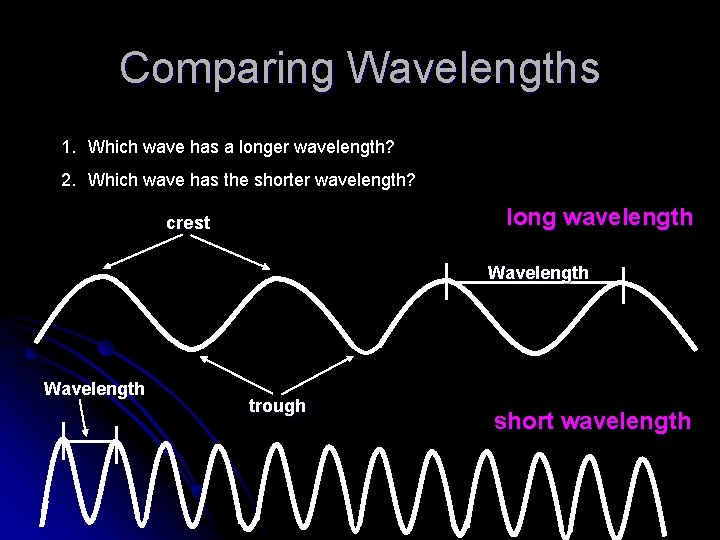
Characteristics of Waves l Wavelength ( λ ) – shortest distance between 2 equivalent points on a continuous waves (from crest to crest or from trough to trough) l Units of wavelength: Usually in meters, centimeters or nanometers

Comparing Wavelengths 1. Which wave has a longer wavelength? 2. Which wave has the shorter wavelength? long wavelength crest Wavelength trough short wavelength

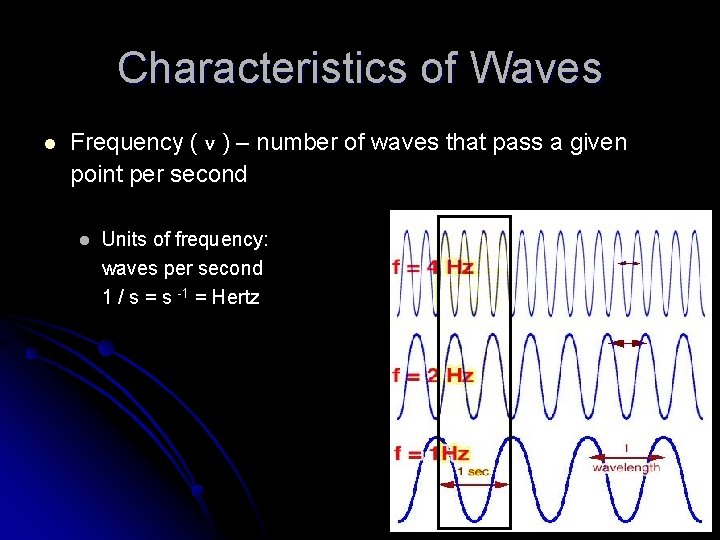
Characteristics of Waves l Frequency ( v ) – number of waves that pass a given point per second l Units of frequency: waves per second 1 / s = s -1 = Hertz

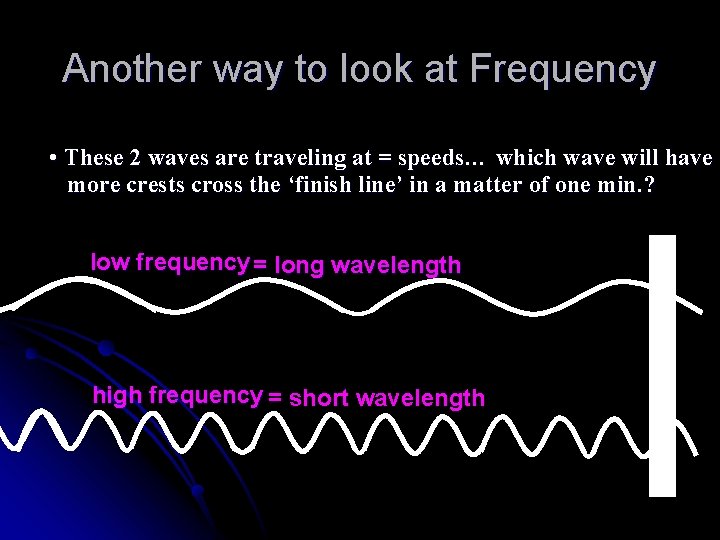
Another way to look at Frequency • These 2 waves are traveling at = speeds… which wave will have more crests cross the ‘finish line’ in a matter of one min. ? low frequency = long wavelength high frequency = short wavelength

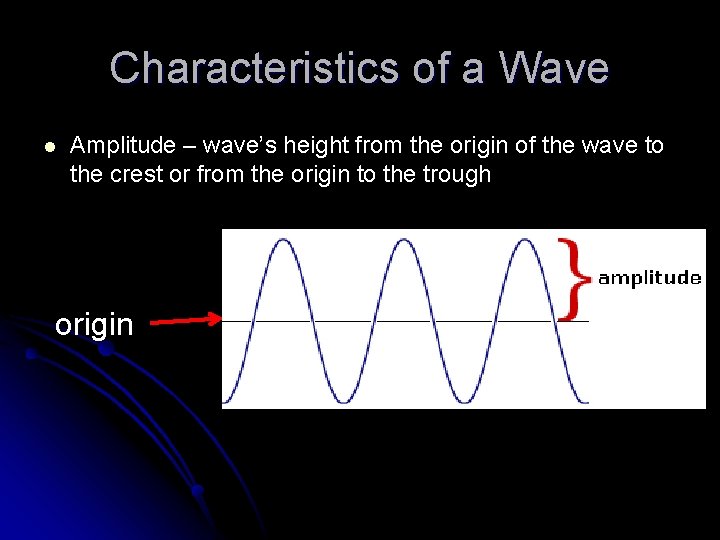
Characteristics of a Wave l Amplitude – wave’s height from the origin of the wave to the crest or from the origin to the trough origin

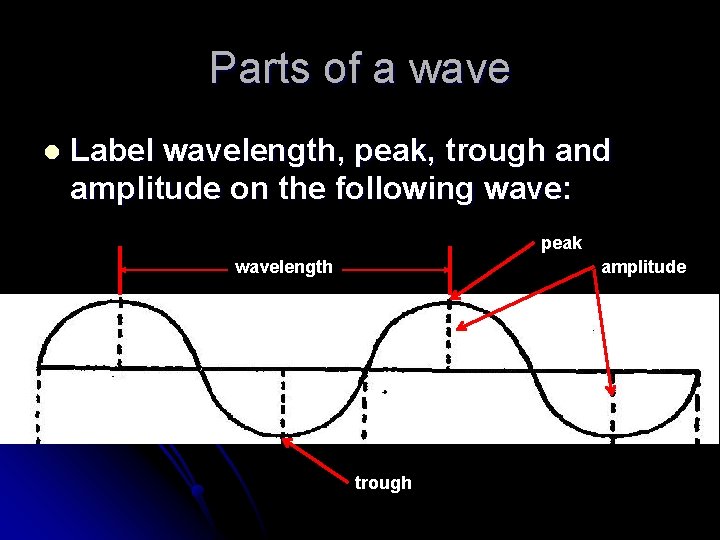
Parts of a wave l Label wavelength, peak, trough and amplitude on the following wave: peak wavelength amplitude trough

Picking back up l l l Electromagnetic radiation - a form of energy that exhibits wavelike behavior as it travels through space Electromagnetic Spectrum - Encompasses all forms of electromagnetic radiation, showing the differences of wavelength and frequency in the types of radiation Wavelength ( λ ) – shortest distance between 2 equivalent points on a continuous waves Frequency ( v ) – number of waves that pass a given point per second Amplitude – wave’s height from the origin of the wave to the crest or from the origin to the trough

Let’s Practice! l Study Guide page 25, #1 -8. 1. 2. 3. 4. 5. 6. 7. 8. Energy Wave Light Speed Wavelength Amplitude Frequency Hertz

Can we do some more? Yes, we can! l Page 25, questions 9 -11. 9. 10. 11. A and C B 2 hertz

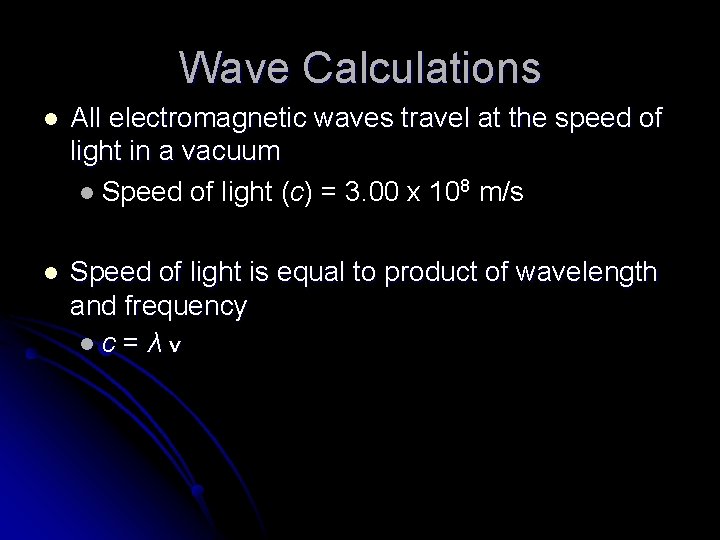
Wave Calculations l All electromagnetic waves travel at the speed of light in a vacuum l Speed of light (c) = 3. 00 x 108 m/s l Speed of light is equal to product of wavelength and frequency lc=λv

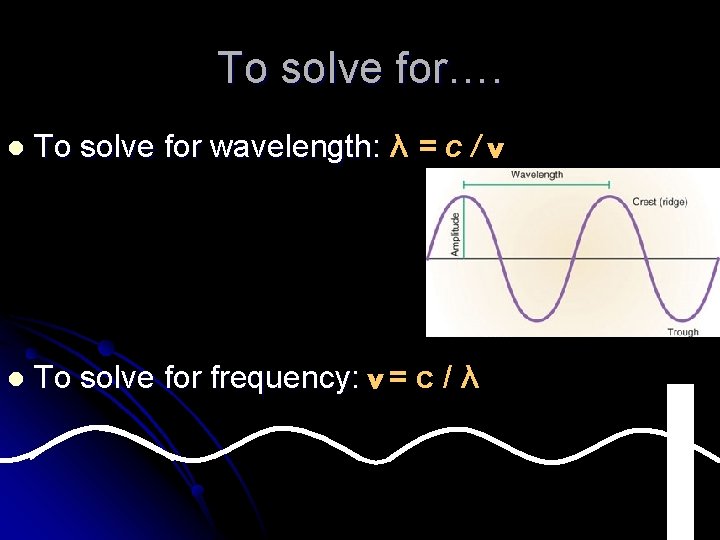
To solve for…. l To solve for wavelength: λ = c / v l To solve for frequency: v = c / λ

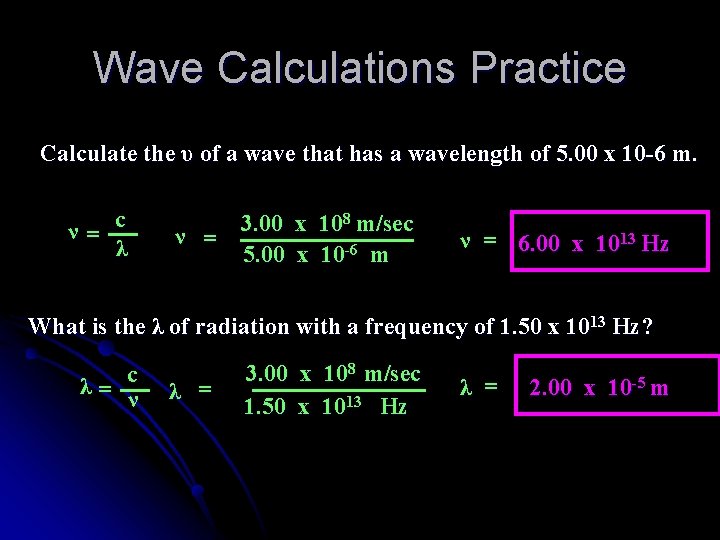
Wave Calculations Practice Calculate the υ of a wave that has a wavelength of 5. 00 x 10 -6 m. ν= c λ 3. 00 x 108 m/sec ν = 5. 00 x 10 -6 m ν = 6. 00 x 1013 Hz What is the λ of radiation with a frequency of 1. 50 x 1013 Hz? λ= c ν λ = 3. 00 x 108 m/sec 1. 50 x 1013 Hz λ = 2. 00 x 10 -5 m

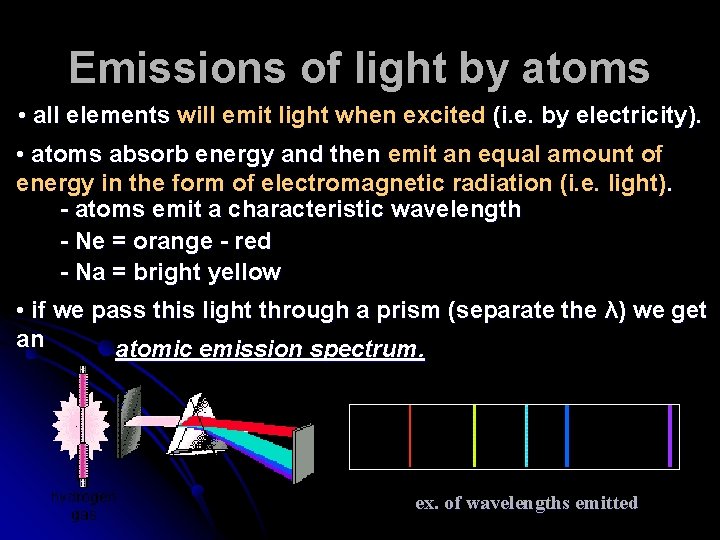
Emissions of light by atoms • all elements will emit light when excited (i. e. by electricity). • atoms absorb energy and then emit an equal amount of energy in the form of electromagnetic radiation (i. e. light). - atoms emit a characteristic wavelength - Ne = orange - red - Na = bright yellow • if we pass this light through a prism (separate the λ) we get an atomic emission spectrum. ex. of wavelengths emitted

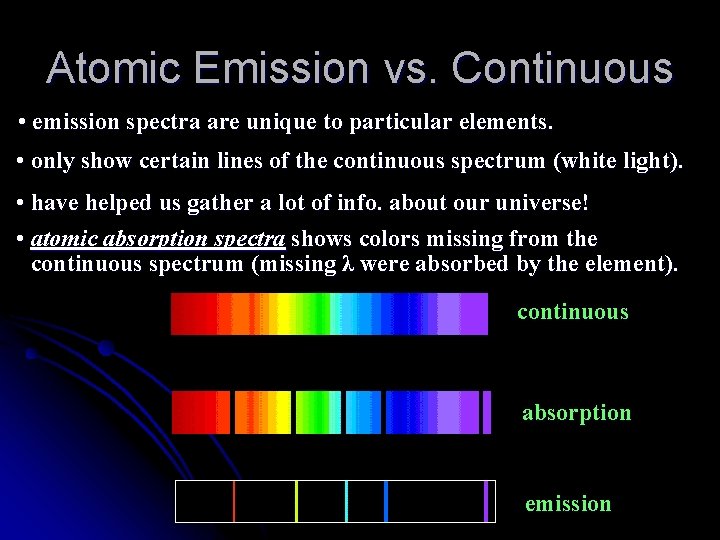
Atomic Emission vs. Continuous • emission spectra are unique to particular elements. • only show certain lines of the continuous spectrum (white light). • have helped us gather a lot of info. about our universe! • atomic absorption spectra shows colors missing from the continuous spectrum (missing λ were absorbed by the element). continuous absorption emission

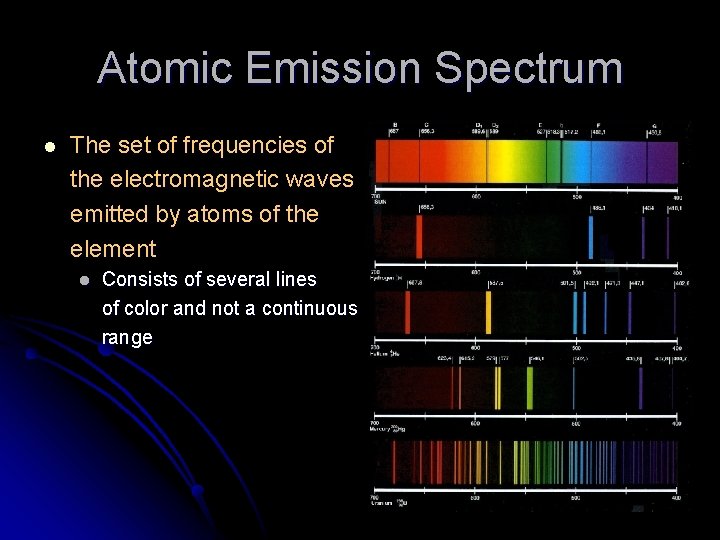
Atomic Emission Spectrum l The set of frequencies of the electromagnetic waves emitted by atoms of the element l Consists of several lines of color and not a continuous range

Time to Practice l Page 26, #16 -22. 16. 17. 18. 19. 20. 21. 22. False True False True

Quantum of Energy • e- are found on certain energy levels (orbitals) around the atom. -there is a maximum of seven energy levels in an atom. -e- on the energy level closest to the nucleus have the lowest energy. -The 7 th energy level has the highest energy. - An e- requires one ‘quanta’ (minimum amount of energy gained or lost by an electron) of energy to jump to the next energy level.

• e- at their lowest energy level are considered to be at the ground state (most stable). • if e- absorb a quantum or more of energy (from electricity), they can jump to higher energy levels (excited state). • e- must lose energy in order to fall from the excited state back to the ground state. - this energy is emitted in the form of electromagnetic radiation (sometimes visible)!

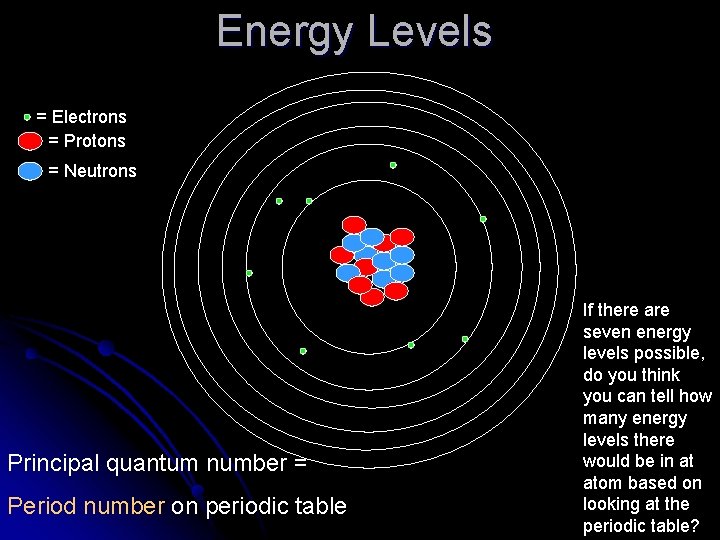
Energy Levels = Electrons = Protons = Neutrons Principal quantum number = Period number on periodic table If there are seven energy levels possible, do you think you can tell how many energy levels there would be in at atom based on looking at the periodic table?