Light and the Electromagnetic Spectrum The Electromagnetic Spectrum






















- Slides: 34

Light and the Electromagnetic Spectrum

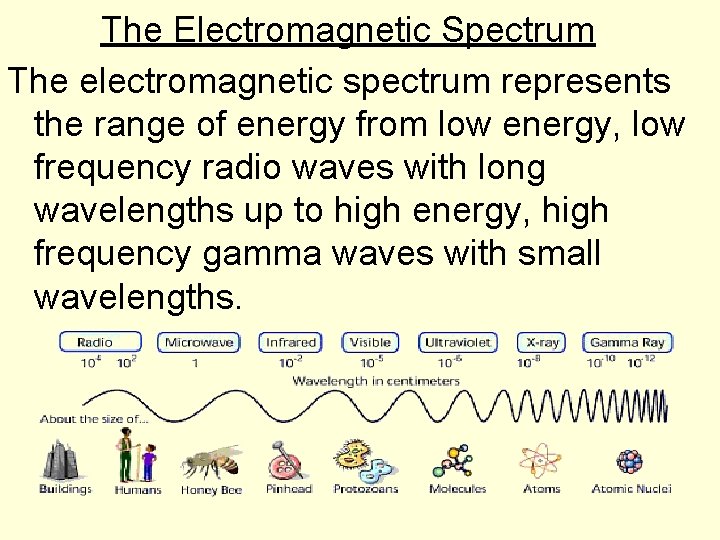
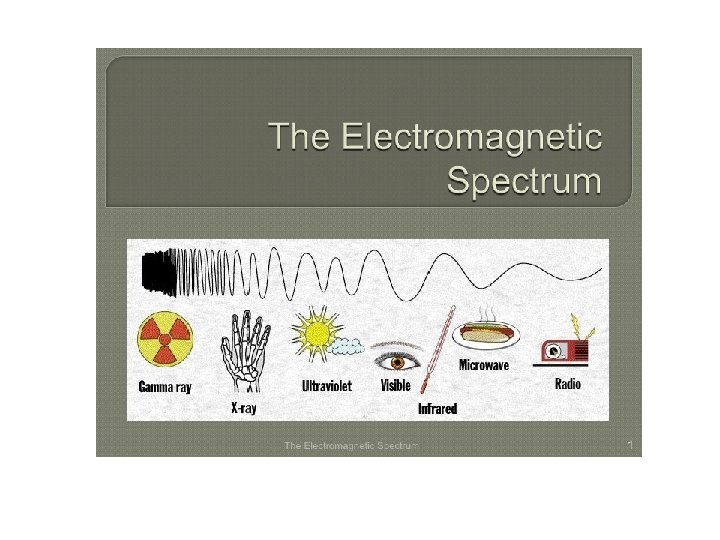
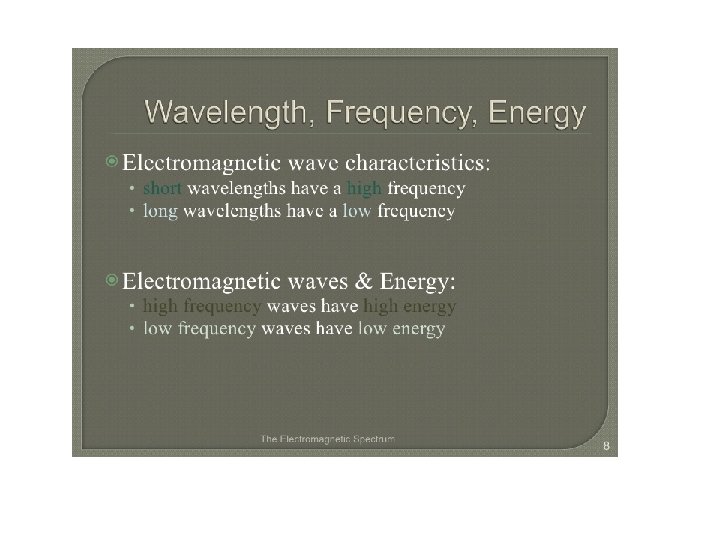
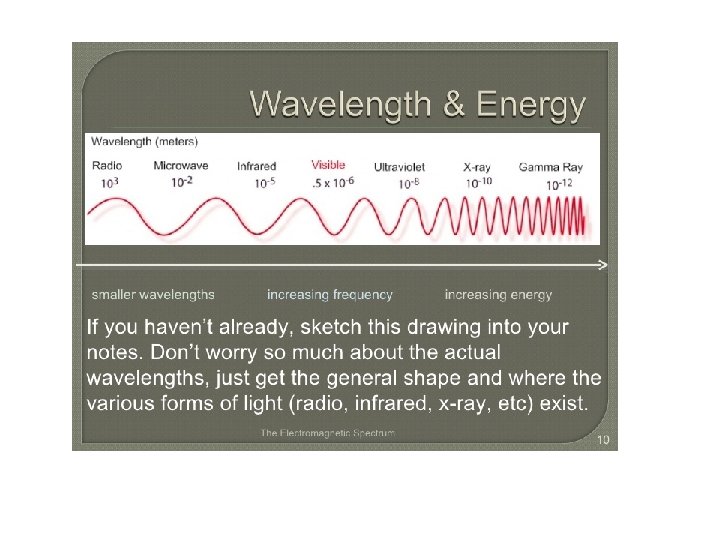
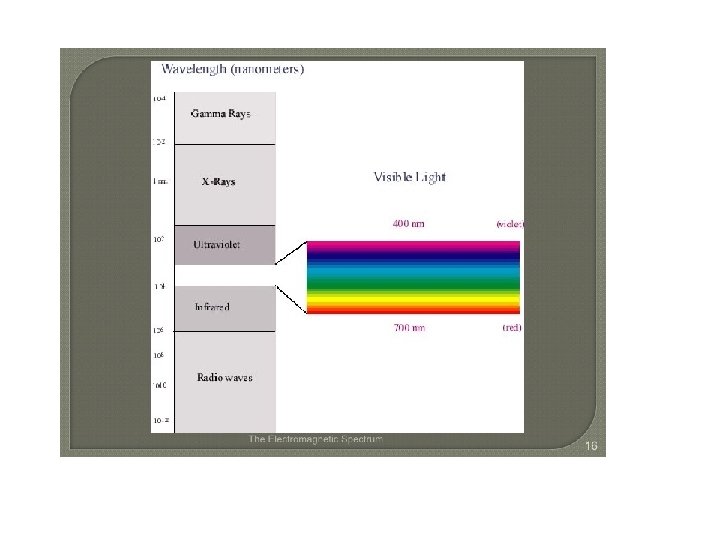
The Electromagnetic Spectrum The electromagnetic spectrum represents the range of energy from low energy, low frequency radio waves with long wavelengths up to high energy, high frequency gamma waves with small wavelengths.

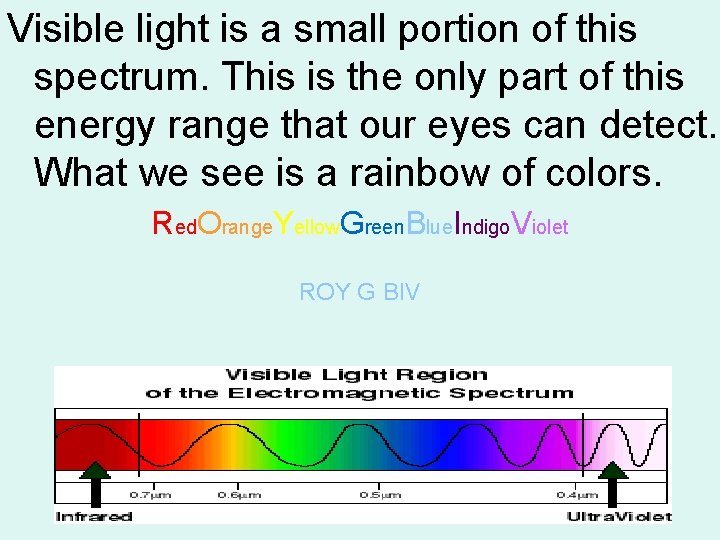
Visible light is a small portion of this spectrum. This is the only part of this energy range that our eyes can detect. What we see is a rainbow of colors. Red. Orange. Yellow. Green. Blue. Indigo. Violet ROY G BIV

Frequency Ranges of Visible Light Red light has a frequency of roughly 4. 3 × 1014 Hz, and a wavelength of about 7. 0 × 10 7 m (700 nm). Violet light, at the other end of the visible range, has nearly double the frequency — 7. 5 × 1014 Hz—and (since the speed of light is the same in either case) just over half the wavelength— 4. 0 × 10 7 m (400 nm).

The radiation to which our eyes are most sensitive has a wavelength near the middle of this range, at about 5. 5 x 10 7 m (550 nm), in the yellow green region of the spectrum.

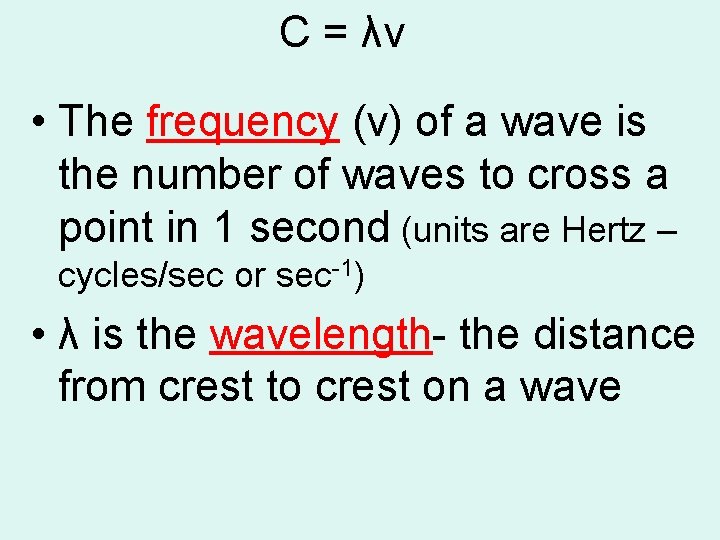
C = λν • The frequency (v) of a wave is the number of waves to cross a point in 1 second (units are Hertz – cycles/sec or sec 1) • λ is the wavelength the distance from crest to crest on a wave

• The product of wavelength and frequency always equals the speed of light. C = λν • Why does this make sense? • NOTE: c is a constant value= 3. 00 x 108 m/s

PROBLEMS • Calculate the wavelength of yellow light emitted from a sodium lamp if the frequency is 5. 10 x 1014 Hz (5. 10 x 1014 s 1) List the known info List the unknown c = 3. 00 x 1010 cm/s wavelength (λ) = ? cm Frequency (v) = 5. 10 x 1014 s 1 C = λv λ=c v λ = 3. 00 x 1010 cm/s = 5. 88 x 10 5 cm 5. 10 x 1014 s 1

YOUR TURN 1 What is the wavelength of radiation with a frequency of 1. 50 x 1013 s 1? 2 What frequency is radiation with a wavelength of 5. 00 x 10 6 cm? In what region of the electromagnetic spectrum is this radiation?


Flame Test Debrief © 2000 Microsoft Clip Gallery • Light Energy – Atoms • As atoms absorb energy, electrons jump out to a higher energy level. • Electrons release light when falling down to the lower energy level. – Photons bundles/packets of energy released when the electrons fall. • Light: Stream of Photons © 2000 Microsoft Clip Gallery

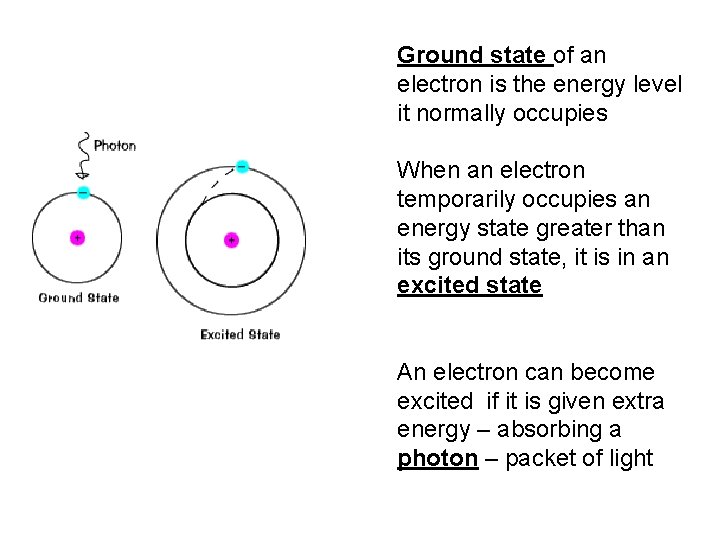
Ground state of an electron is the energy level it normally occupies When an electron temporarily occupies an energy state greater than its ground state, it is in an excited state An electron can become excited if it is given extra energy – absorbing a photon – packet of light

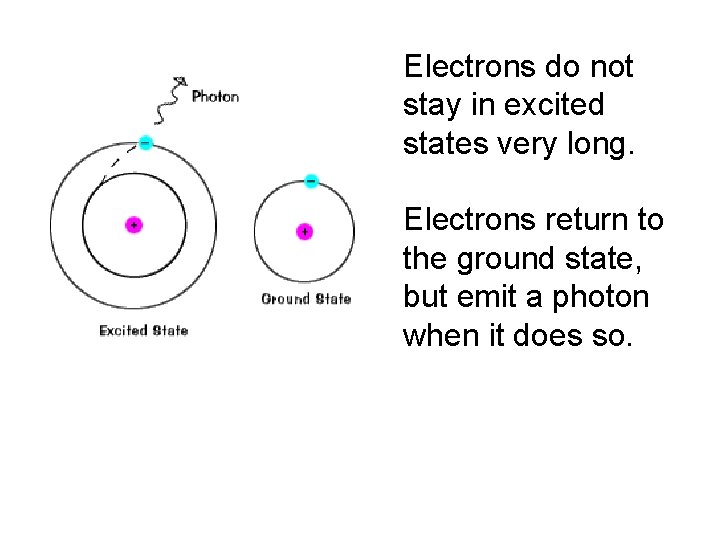
Electrons do not stay in excited states very long. Electrons return to the ground state, but emit a photon when it does so.

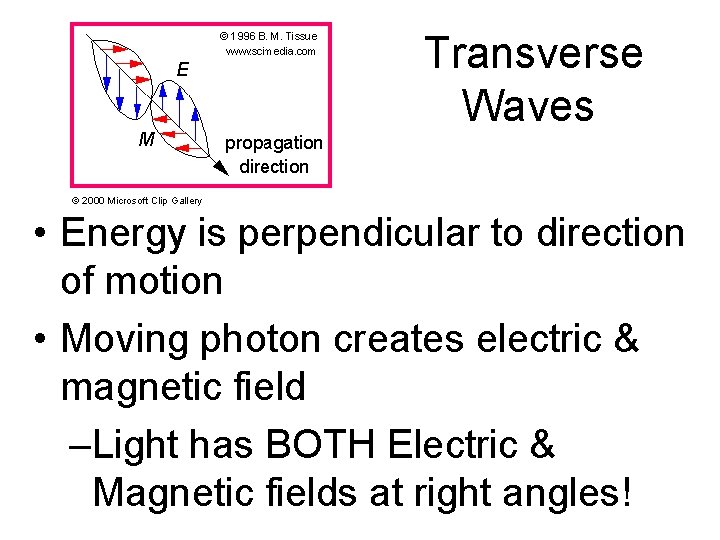
Transverse Waves © 2000 Microsoft Clip Gallery • Energy is perpendicular to direction of motion • Moving photon creates electric & magnetic field –Light has BOTH Electric & Magnetic fields at right angles!



















