Electron Configuration The Electromagnetic Spectrum Know what is






- Slides: 66

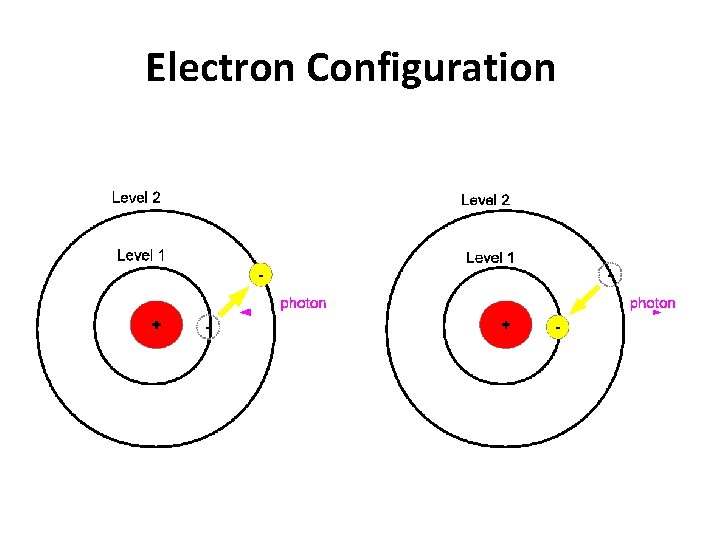
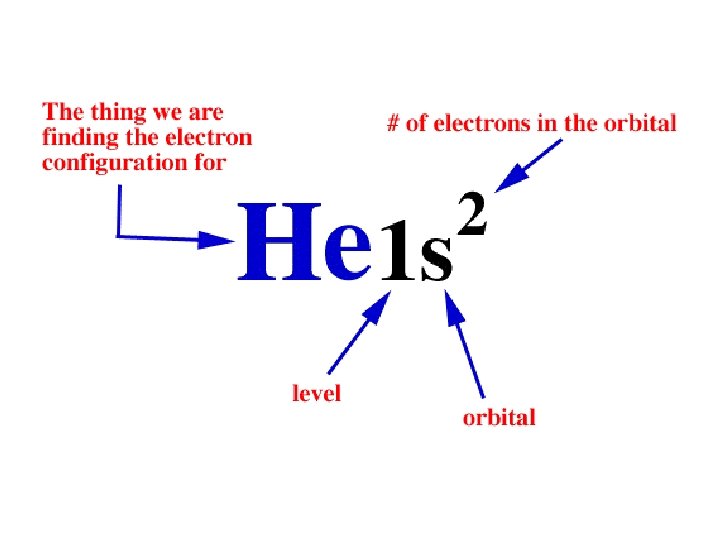
Electron Configuration


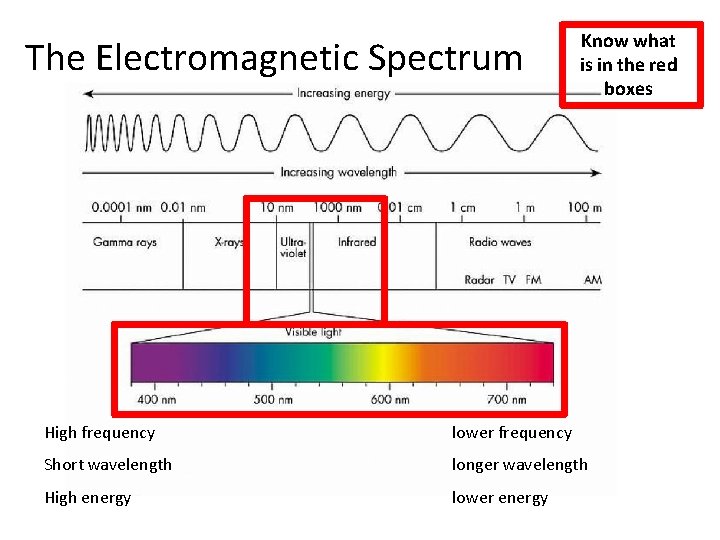
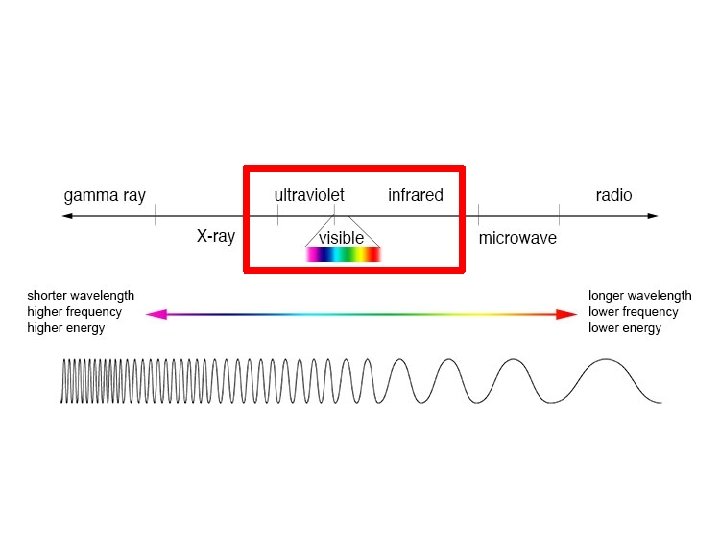
The Electromagnetic Spectrum Know what is in the red boxes High frequency lower frequency Short wavelength longer wavelength High energy lower energy


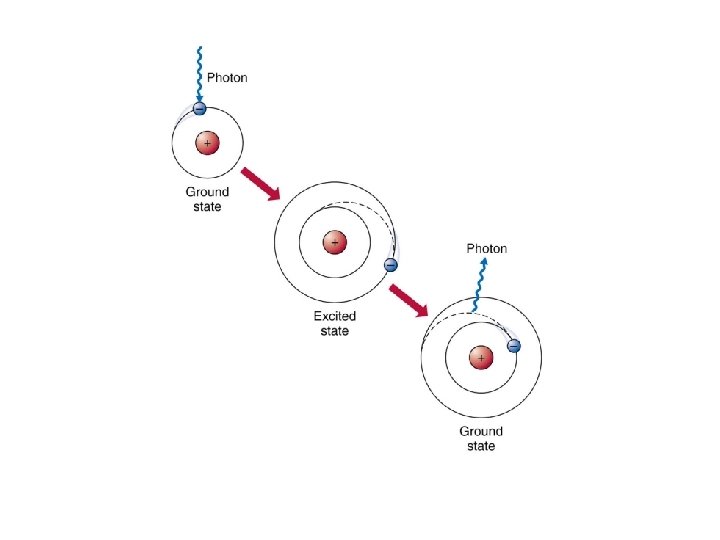
Jumping Electrons • normally electrons exist in the ground state, meaning they are as close to the nucleus as possible • when an electron is excited by adding energy to an atom, the electron will absorb energy and "jump" to a higher energy level – heating a chemical with a Bunsen burner is enough energy to do this

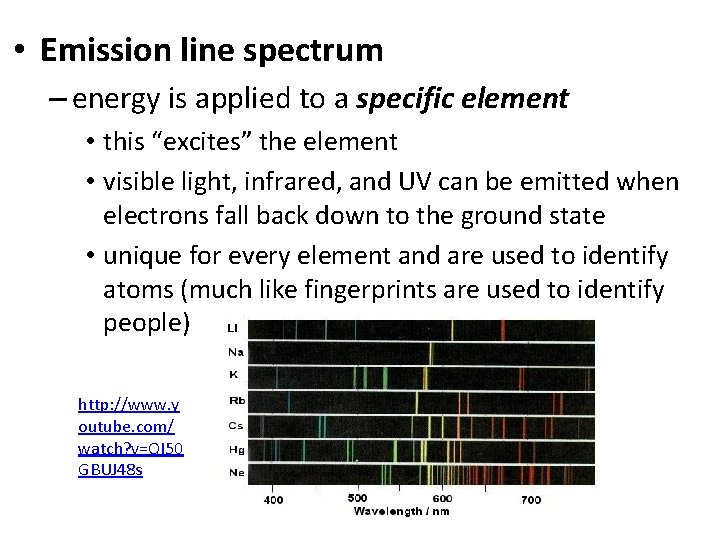
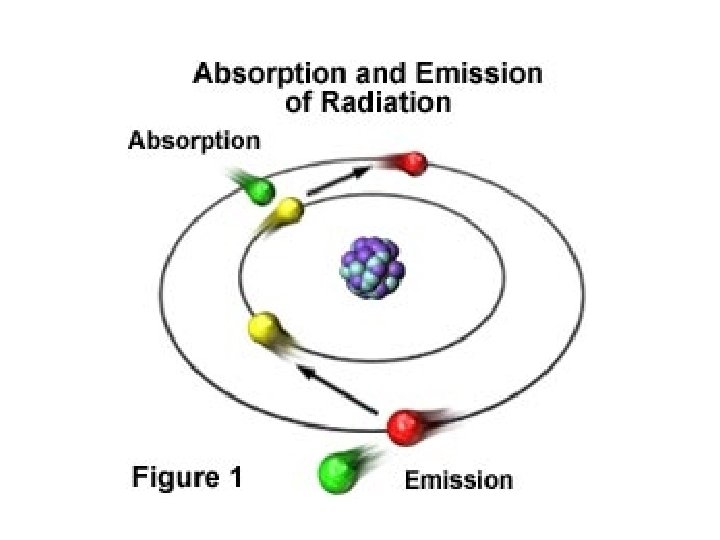
• Emission line spectrum – energy is applied to a specific element • this “excites” the element • visible light, infrared, and UV can be emitted when electrons fall back down to the ground state • unique for every element and are used to identify atoms (much like fingerprints are used to identify people) http: //www. y outube. com/ watch? v=QI 50 GBUJ 48 s

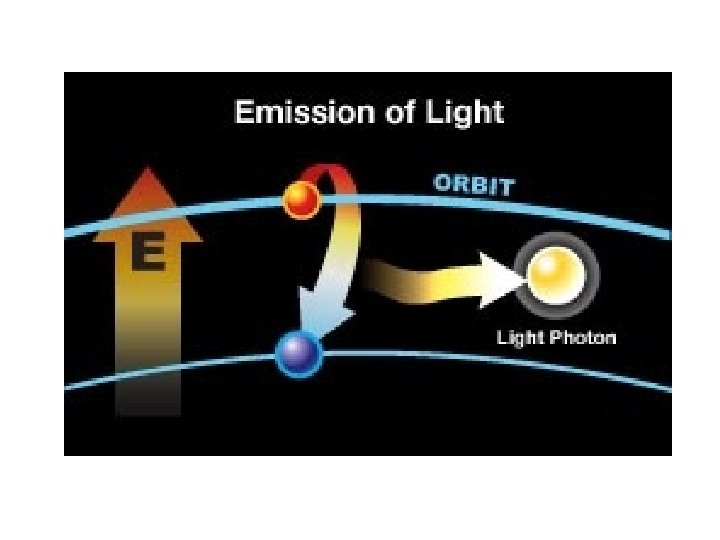
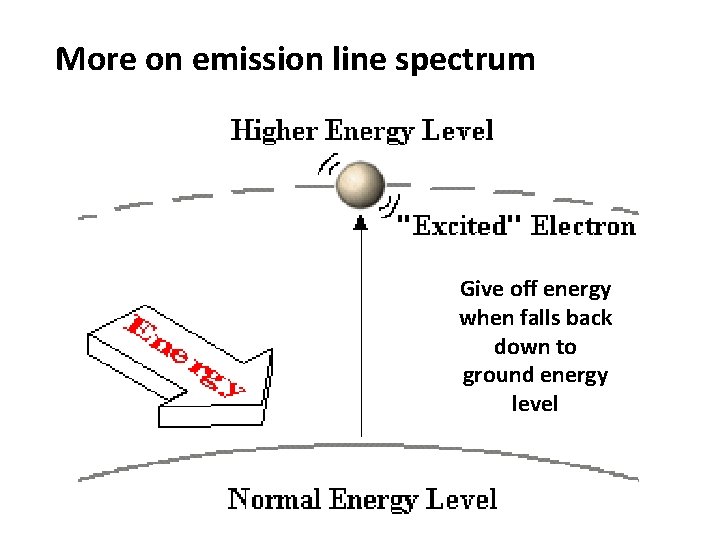
More on emission line spectrum Give off energy when falls back down to ground energy level

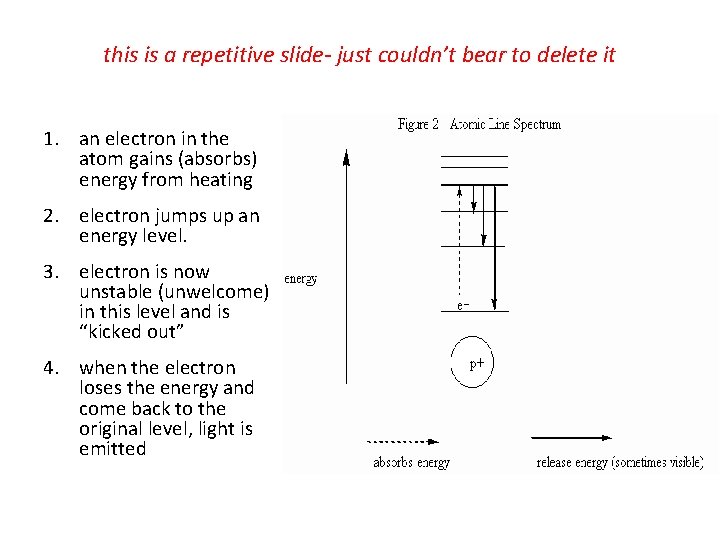
this is a repetitive slide- just couldn’t bear to delete it 1. an electron in the atom gains (absorbs) energy from heating 2. electron jumps up an energy level. 3. electron is now unstable (unwelcome) in this level and is “kicked out” 4. when the electron loses the energy and come back to the original level, light is emitted



Scandium 3 -D video (2: 31) 3 -D Graphic Examples of Atomic Orbitals

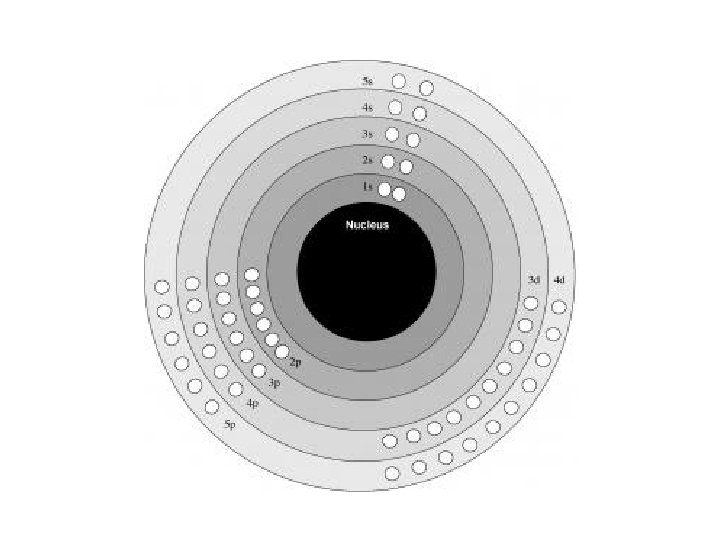
Quantum Numbers (however, actual numbers are often not used) • each electron in an atom is described by four different quantum numbers – think of the 4 quantum numbers as the address of an electron… country > state > city > street • electrons fill low energy orbitals before they fill higher energy ones

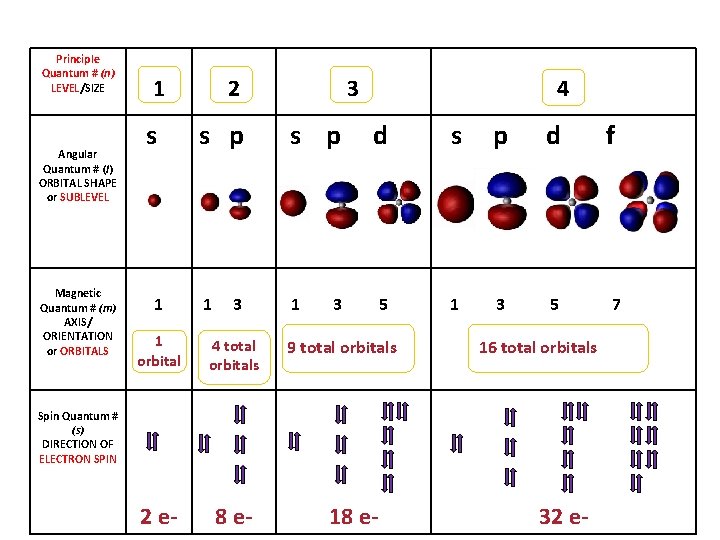
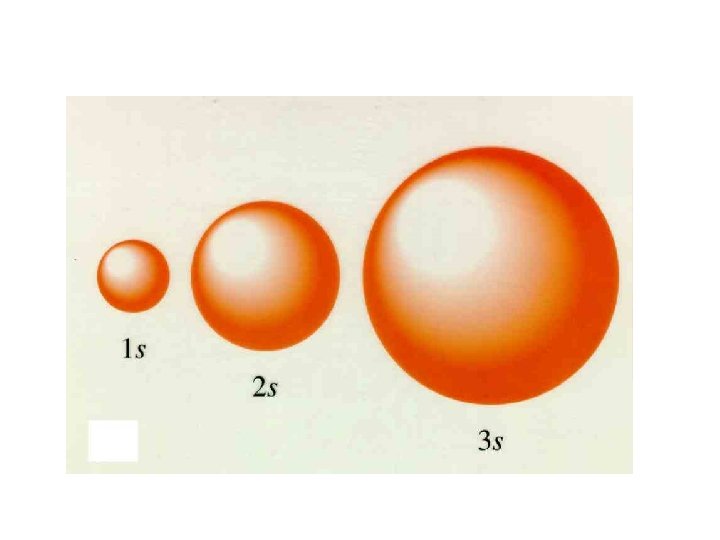
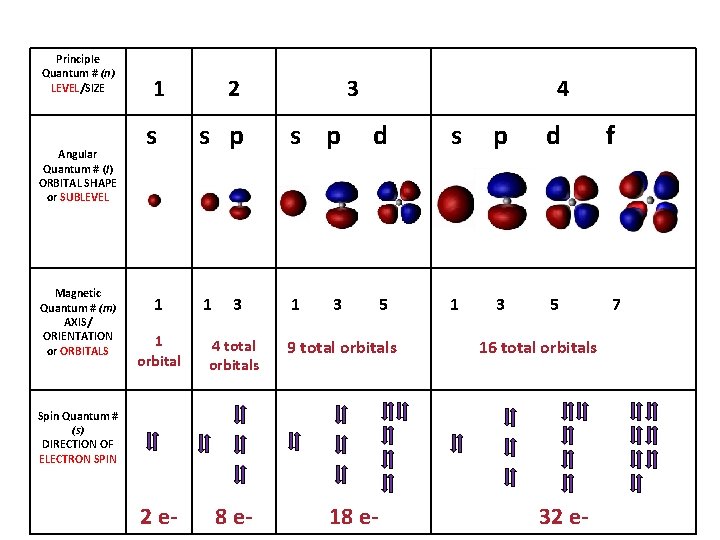
• Principle quantum number (n) Quick intro, more later. – describes the SIZE of the orbital or ENERGY LEVEL (shell) of the atom. • Angular quantum number (l) – a SUB-LEVEL (shell) that describes the type or SHAPE of the orbital • Magnetic quantum number (m) – the NUMBER of orbitals – describes an orbital's ORIENTATION in space • Spin quantum number (s) – describes the SPIN or direction (clockwise or counterclockwise) in which an electron spins

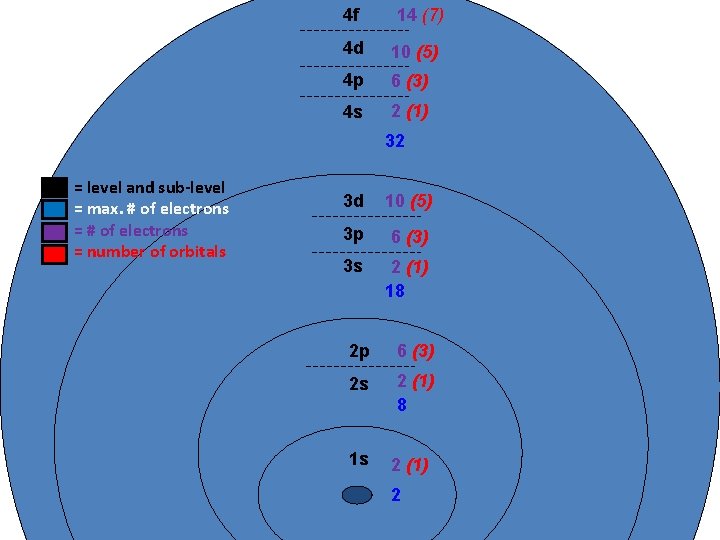
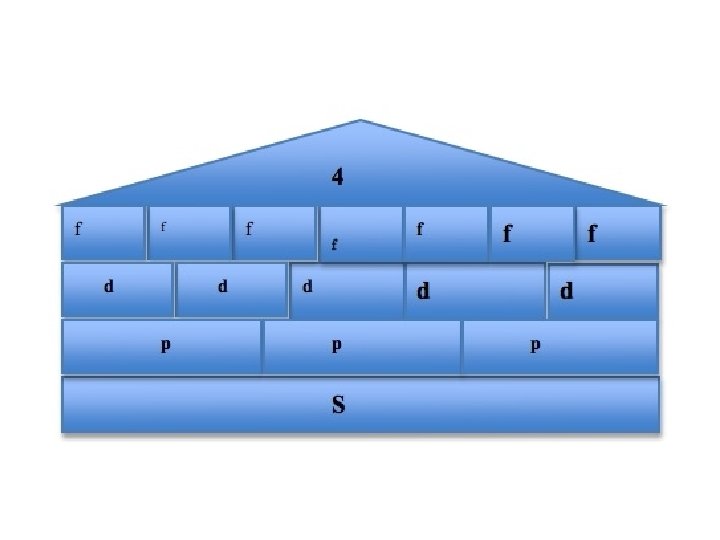
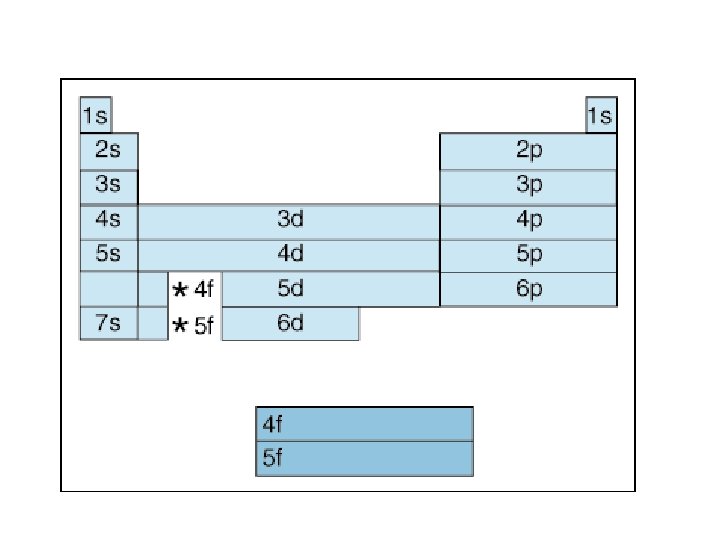
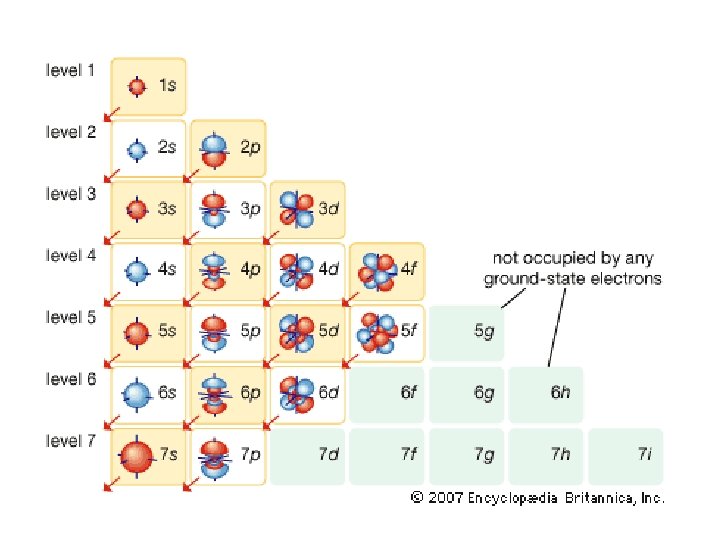
Principle Quantum # (n) LEVEL/SIZE Angular Quantum # (l) ORBITAL SHAPE or SUBLEVEL Magnetic Quantum # (m) AXIS/ ORIENTATION or ORBITALS 1 s 1 2 s p 1 3 1 orbital 4 total orbitals 2 e- 8 e- 3 s p 1 4 d s p d 5 1 3 5 3 9 total orbitals 16 total orbitals Spin Quantum # (s) DIRECTION OF ELECTRON SPIN 18 e- 32 e- f 7

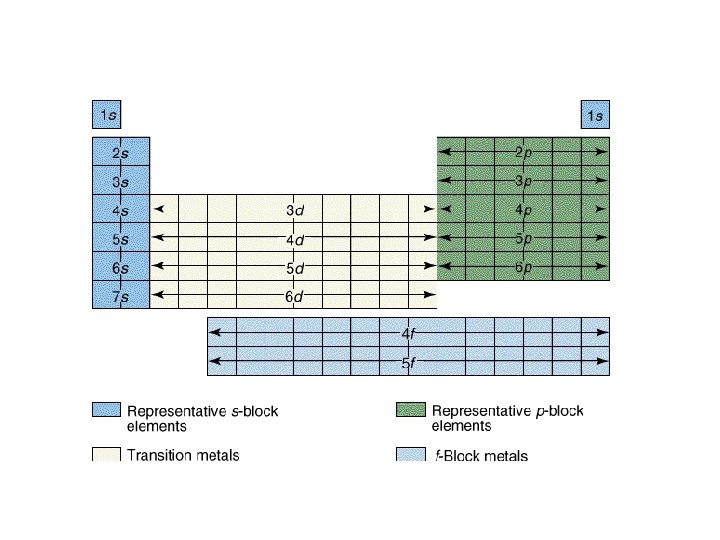
4 f 14 (7) 4 d 10 (5) 4 p 6 (3) 4 s 2 (1) 32 = level and sub-level = max. # of electrons = number of orbitals 3 d 10 (5) 3 p 6 (3) 3 s 2 (1) 18 2 p 6 (3) 2 s 2 (1) 8 1 s 2 (1) 2

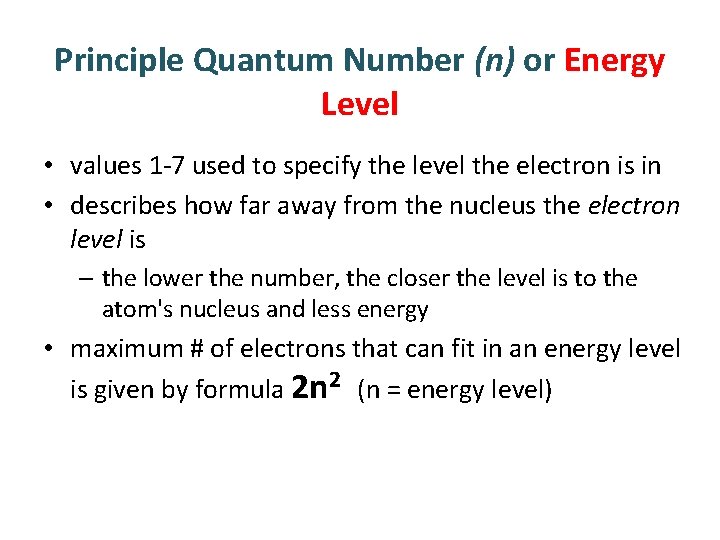
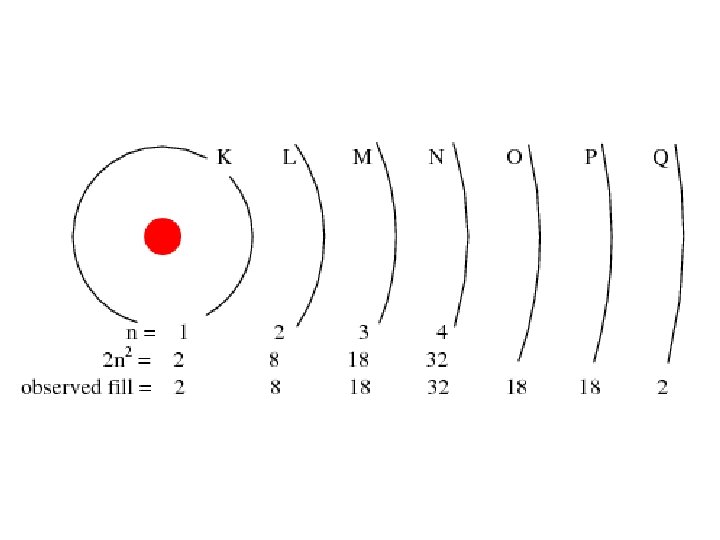
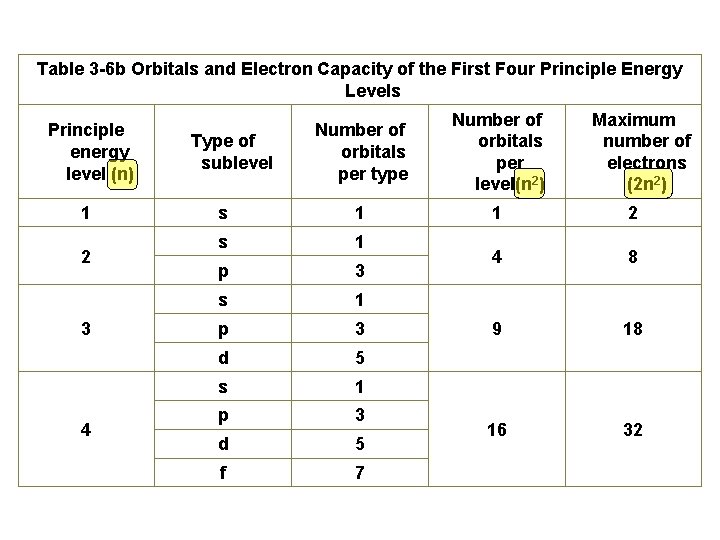
Principle Quantum Number (n) or Energy Level • values 1 -7 used to specify the level the electron is in • describes how far away from the nucleus the electron level is – the lower the number, the closer the level is to the atom's nucleus and less energy • maximum # of electrons that can fit in an energy level is given by formula 2 n 2 (n = energy level)



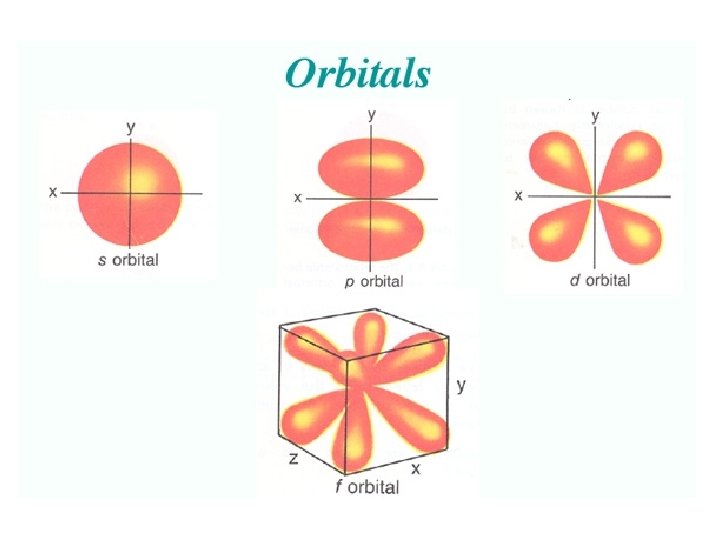
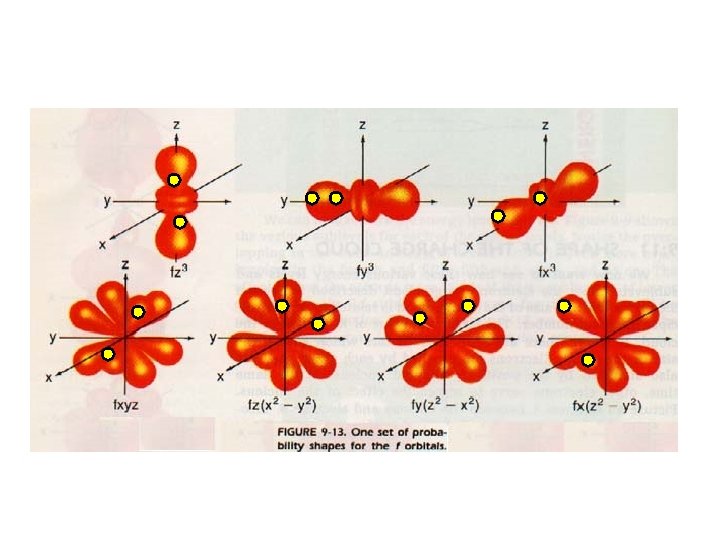
Angular Quantum Number (l) or SUB-LEVELS • determines the shape of the sub-level • number of sub-levels equal the level number – ex. the second level has two sub-levels, the third has three and so on… • they are numbered but are also given letters referring to the sub-level type – l=0 refers to the s sub-level – l=1 refers to the p sub-level just know this – l=2 refers to the d sub-level – l=3 refers to the f sub-level



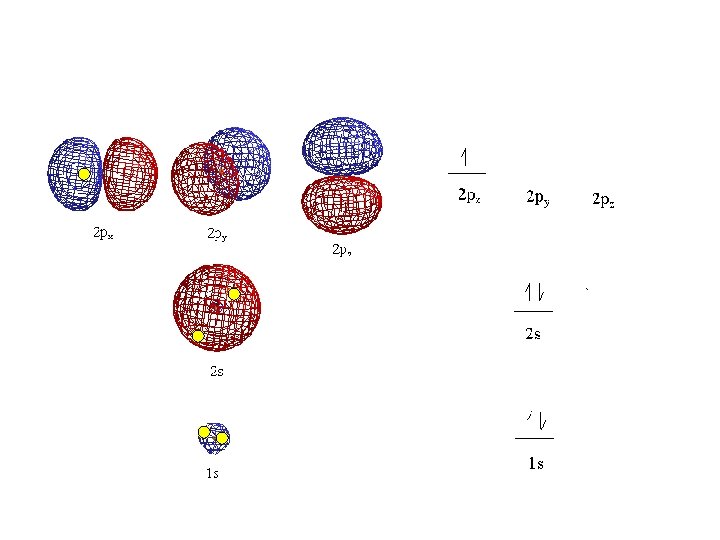
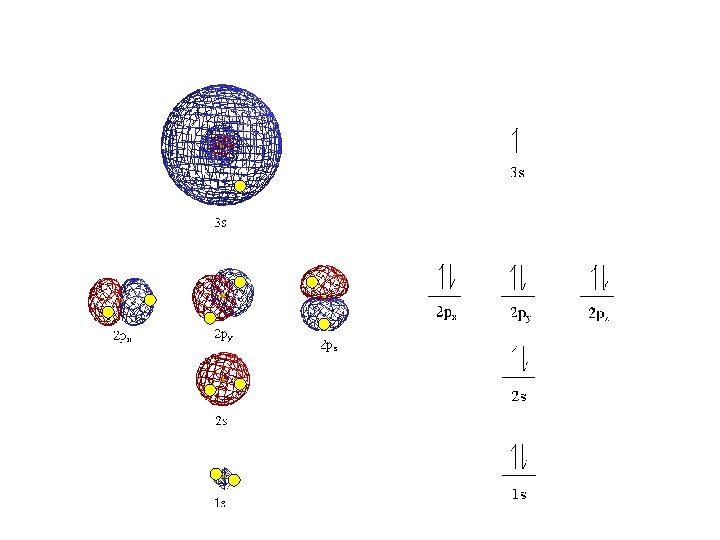
Magnetic quantum number (m) or ORBITALS • Electron Orbitals You. Tube 1: 37 • the third of a set of quantum numbers • tells us how many sub-levels there are of a particular type and their orientation in space of a particular sub-level • only two electrons can fit in an orbital • = electron

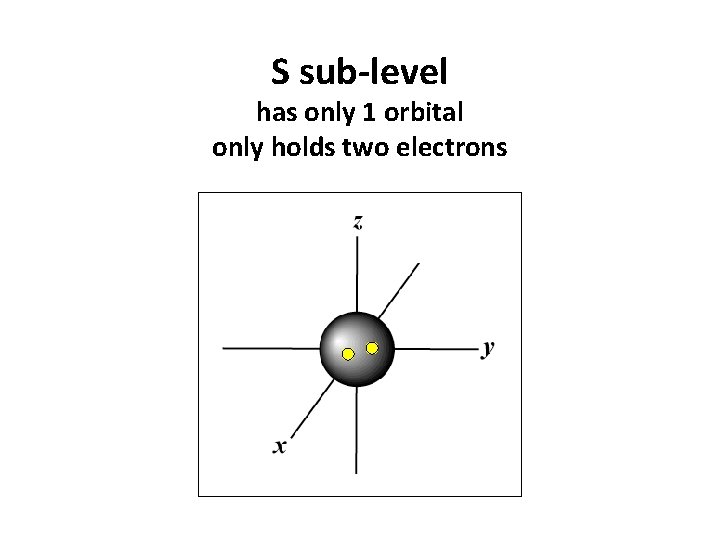
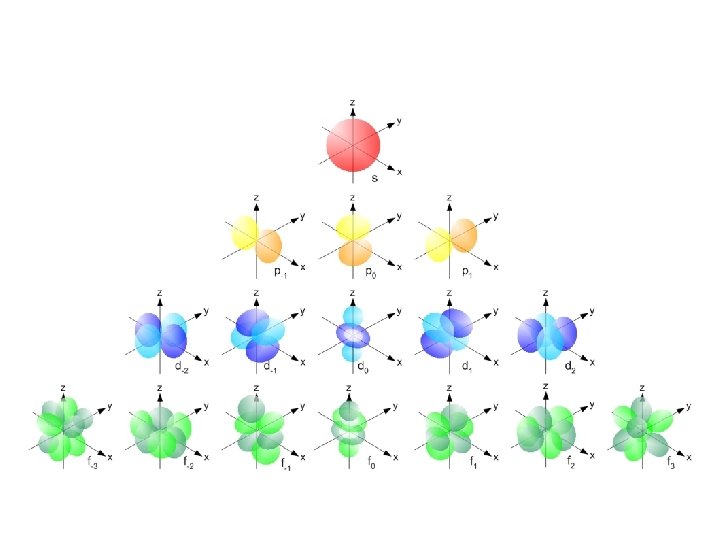
S sub-level has only 1 orbital only holds two electrons

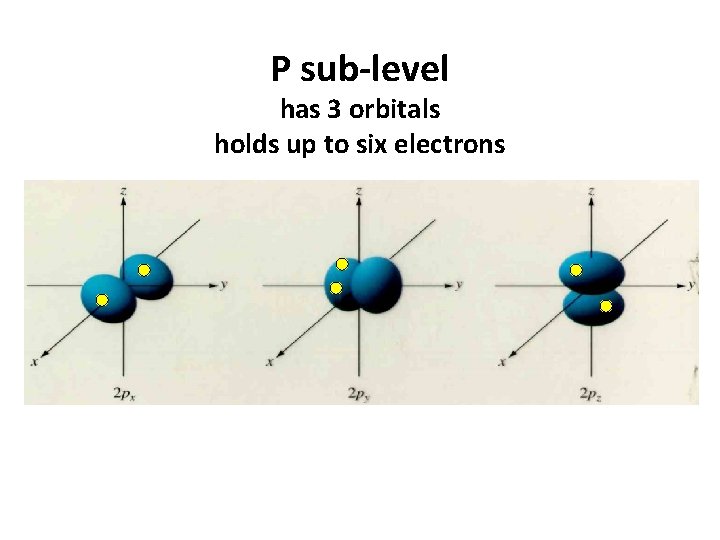
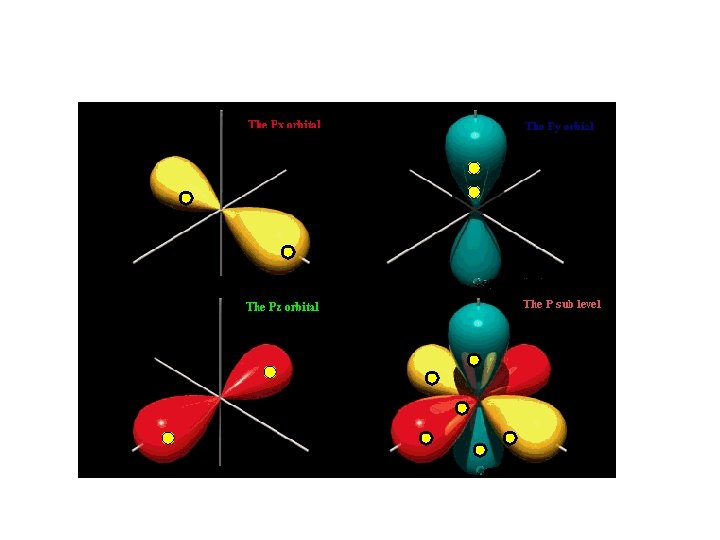
P sub-level has 3 orbitals holds up to six electrons


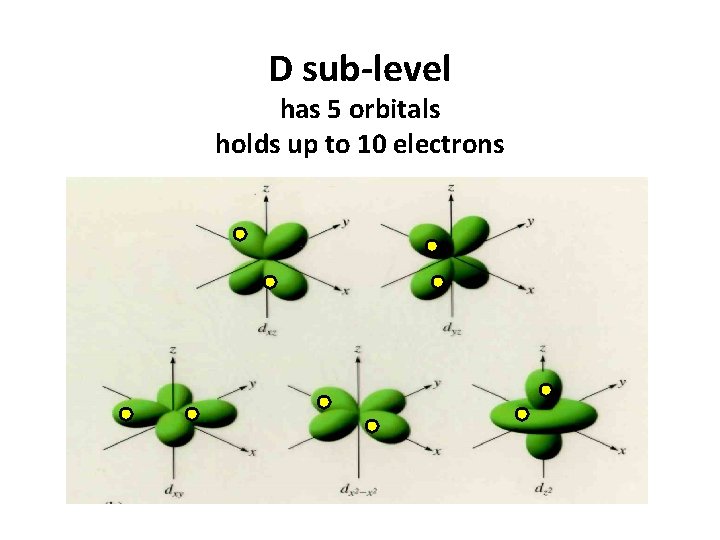
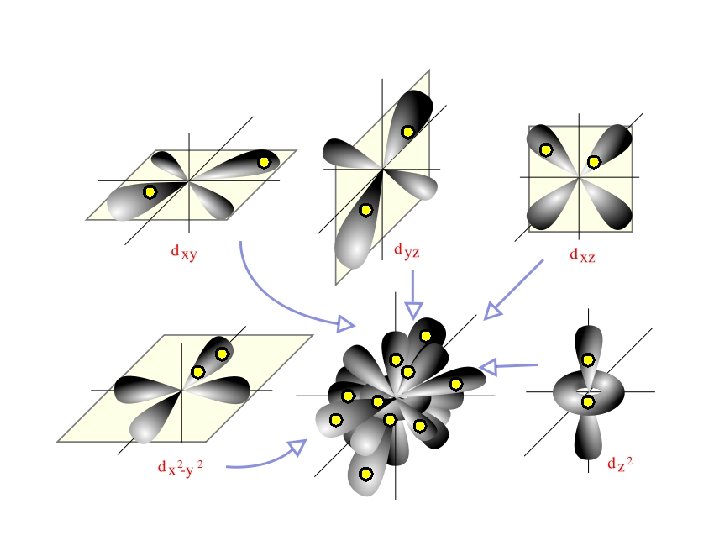
D sub-level has 5 orbitals holds up to 10 electrons


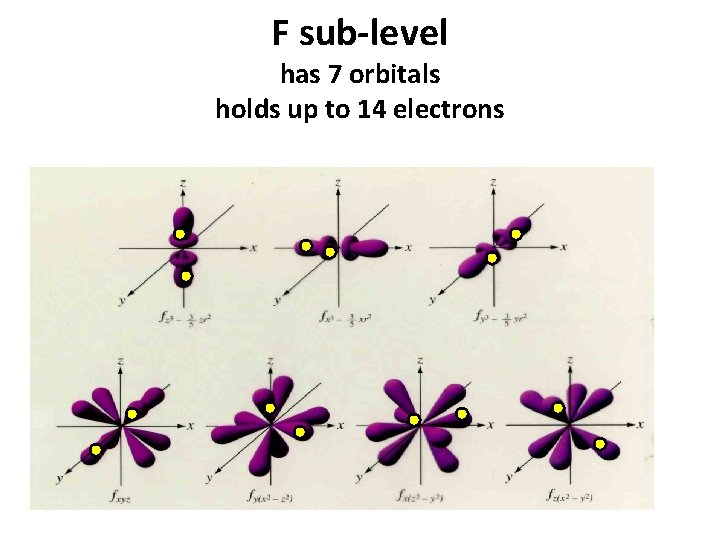
F sub-level has 7 orbitals holds up to 14 electrons



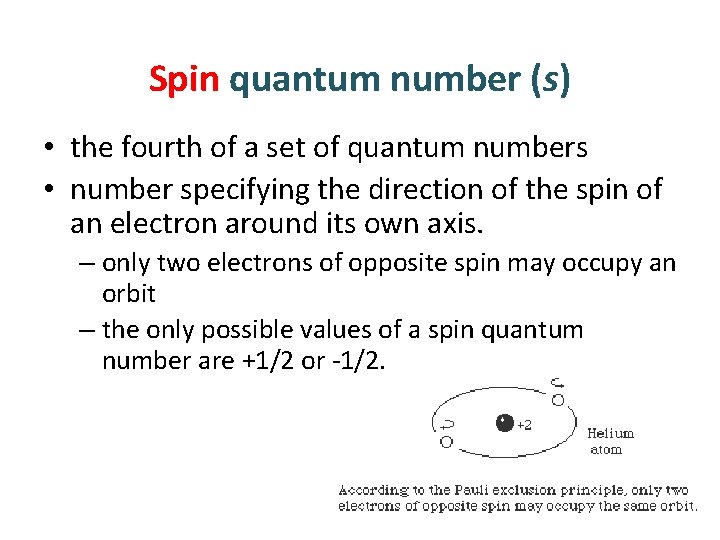
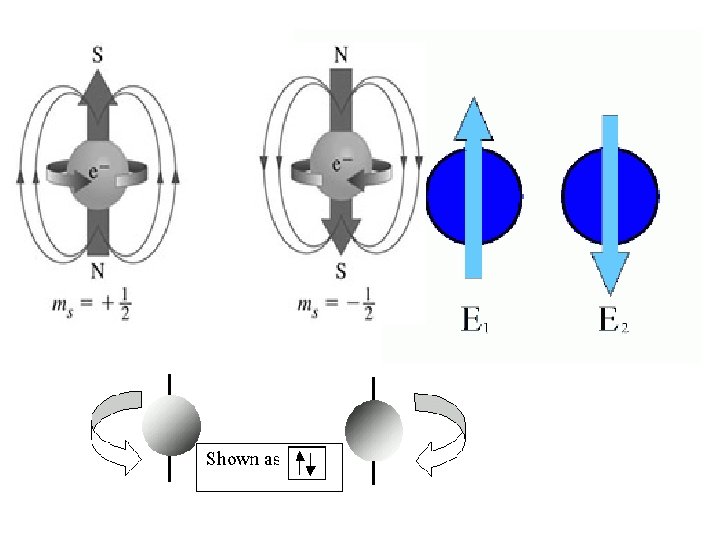
Spin quantum number (s) • the fourth of a set of quantum numbers • number specifying the direction of the spin of an electron around its own axis. – only two electrons of opposite spin may occupy an orbit – the only possible values of a spin quantum number are +1/2 or -1/2.


Principle Quantum # (n) LEVEL/SIZE Angular Quantum # (l) ORBITAL SHAPE or SUBLEVEL Magnetic Quantum # (m) AXIS/ ORIENTATION or ORBITALS 1 s 1 2 s p 1 3 1 orbital 4 total orbitals 2 e- 8 e- 3 s p 1 4 d s p d 5 1 3 5 3 9 total orbitals 16 total orbitals Spin Quantum # (s) DIRECTION OF ELECTRON SPIN 18 e- 32 e- f 7

Table 3 -6 b Orbitals and Electron Capacity of the First Four Principle Energy Levels Principle energy level (n) 1 2 3 4 Number of orbitals per type Number of orbitals per level(n 2) s 1 1 2 s 1 p 3 4 8 s 1 p 3 9 18 d 5 s 1 p 3 d 5 16 32 f 7 Type of sublevel Maximum number of electrons (2 n 2)


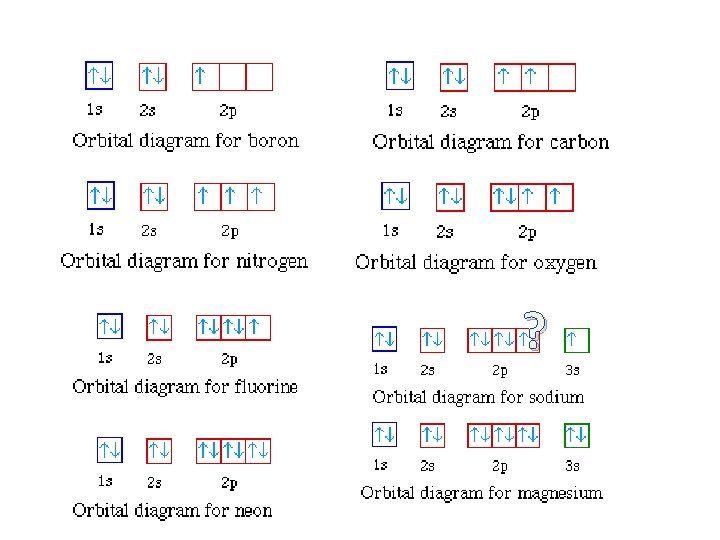
“Rules” for Writing Electron Configurations • a method of writing where electrons are found in various orbitals around the nuclei of atoms. – three rules in order to determine this: 1. Aufbau principle 2. Pauli exclusion principle 3. Hund’s rule

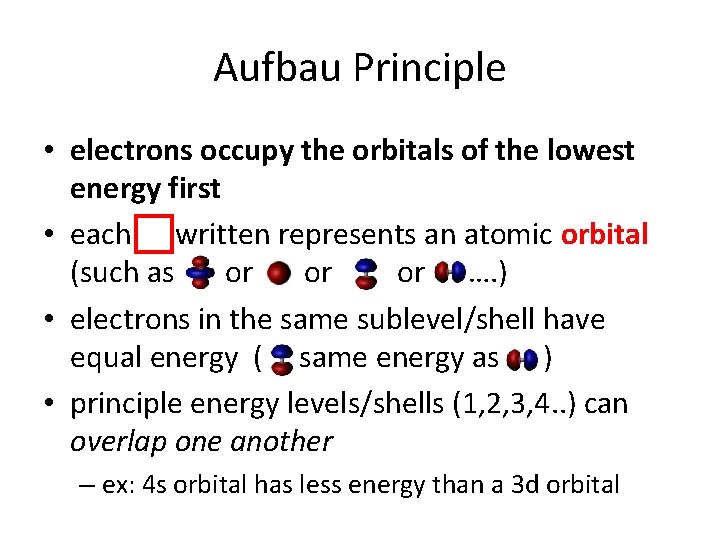
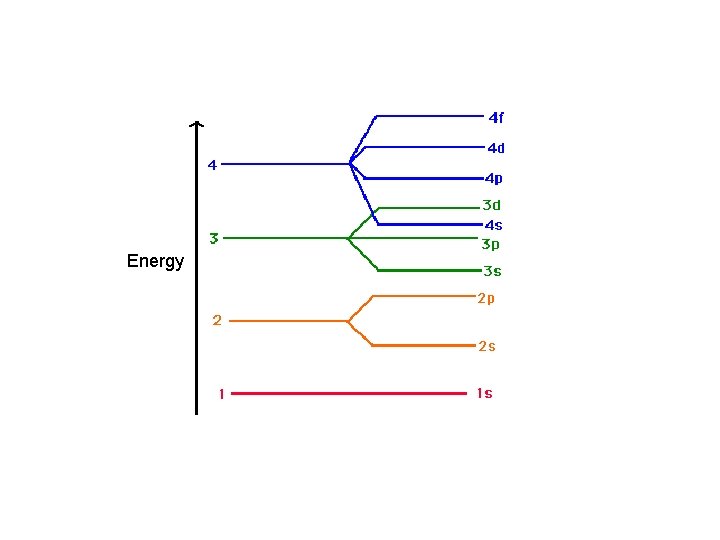
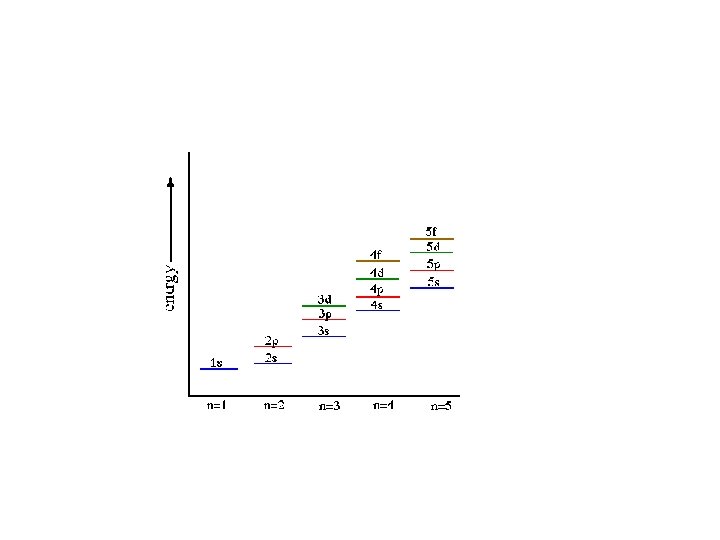
Aufbau Principle • electrons occupy the orbitals of the lowest energy first • each written represents an atomic orbital (such as or …. ) • electrons in the same sublevel/shell have equal energy ( same energy as ) • principle energy levels/shells (1, 2, 3, 4. . ) can overlap one another – ex: 4 s orbital has less energy than a 3 d orbital

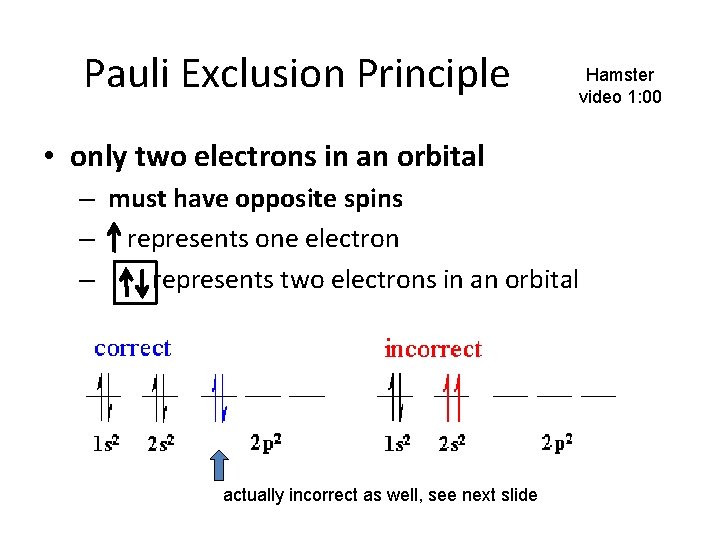
Pauli Exclusion Principle Hamster video 1: 00 • only two electrons in an orbital – must have opposite spins – represents one electron – represents two electrons in an orbital actually incorrect as well, see next slide

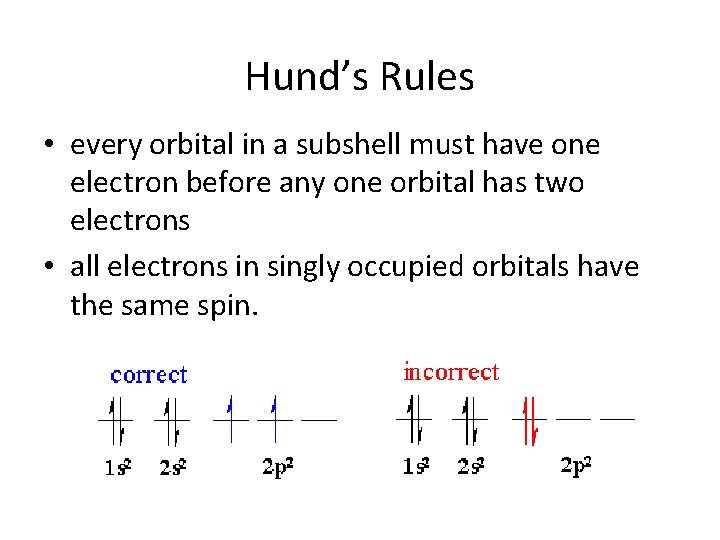
Hund’s Rules • every orbital in a subshell must have one electron before any one orbital has two electrons • all electrons in singly occupied orbitals have the same spin.

Writing Orbital Diagrams

Energy




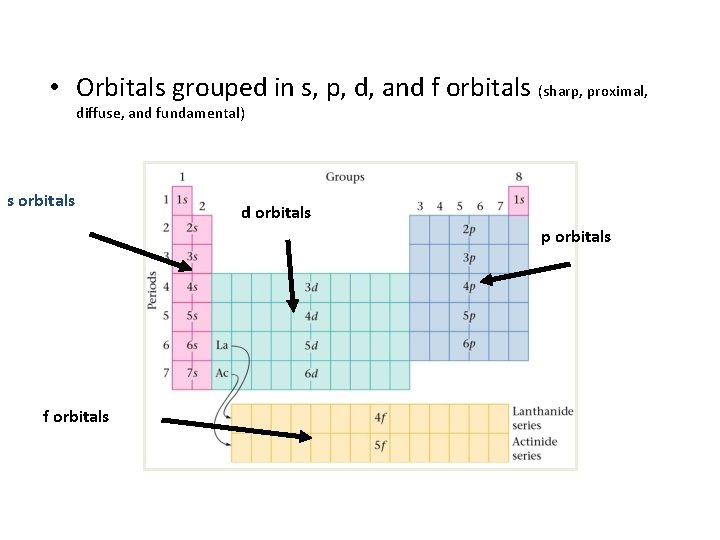
• Orbitals grouped in s, p, d, and f orbitals (sharp, proximal, diffuse, and fundamental) s orbitals d orbitals p orbitals f orbitals




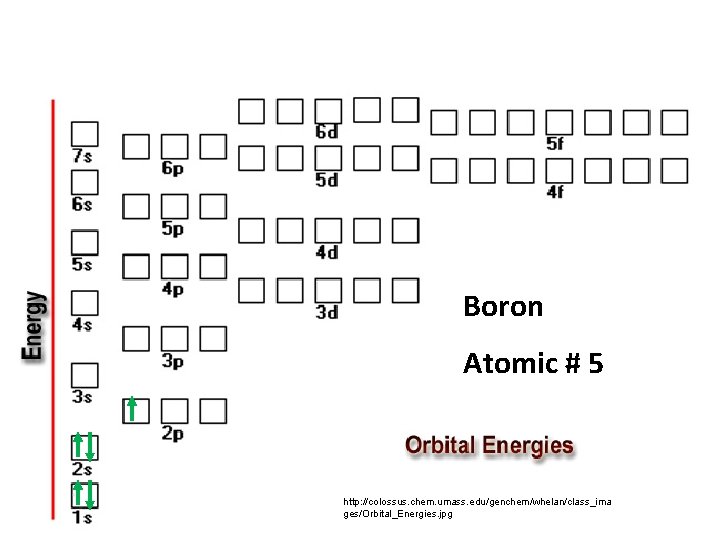
Boron Atomic # 5 http: //colossus. chem. umass. edu/genchem/whelan/class_ima ges/Orbital_Energies. jpg

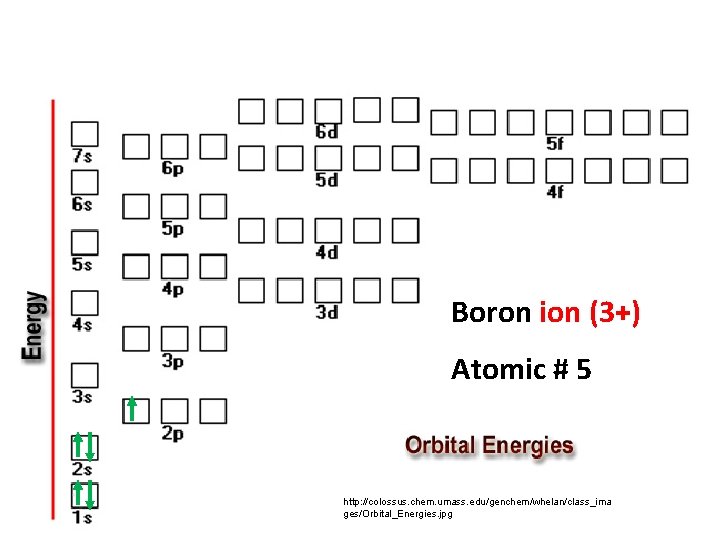
Boron ion (3+) Atomic # 5 http: //colossus. chem. umass. edu/genchem/whelan/class_ima ges/Orbital_Energies. jpg

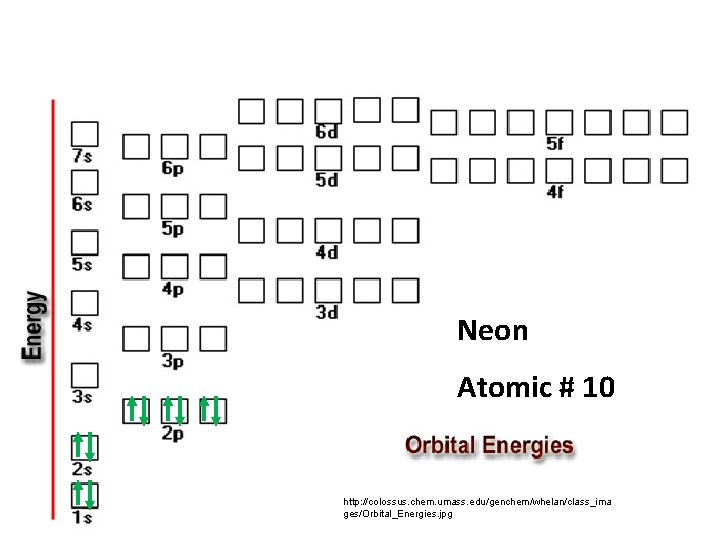
Neon Atomic # 10 http: //colossus. chem. umass. edu/genchem/whelan/class_ima ges/Orbital_Energies. jpg

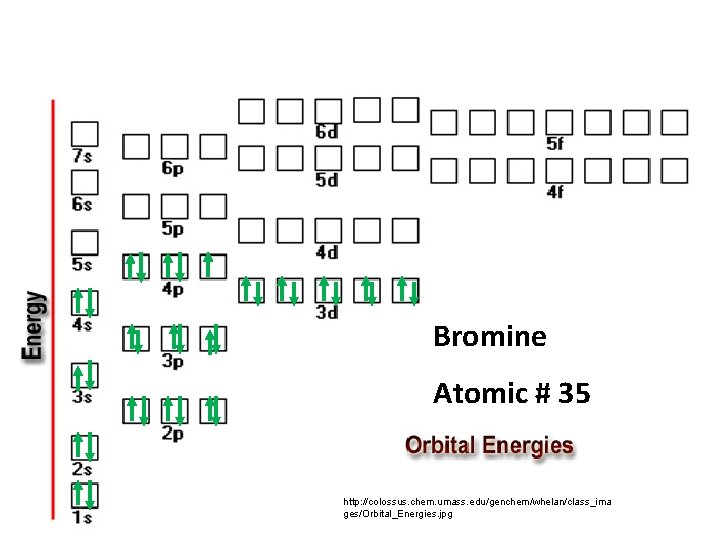
Bromine Atomic # 35 http: //colossus. chem. umass. edu/genchem/whelan/class_ima ges/Orbital_Energies. jpg

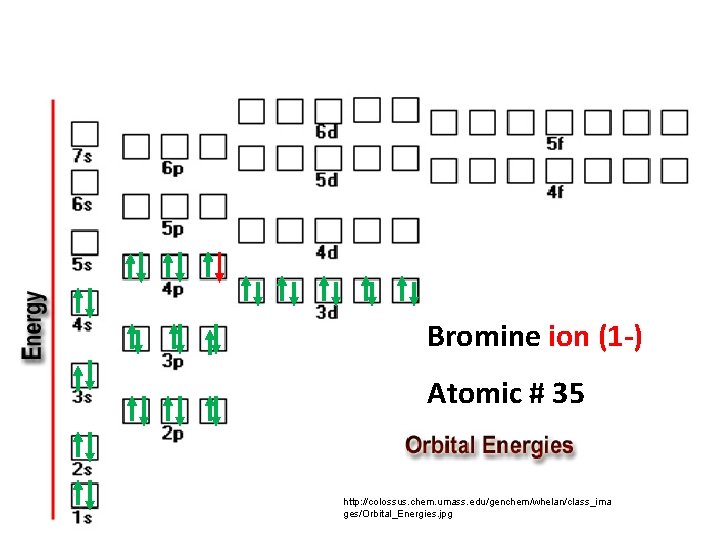
Bromine ion (1 -) Atomic # 35 http: //colossus. chem. umass. edu/genchem/whelan/class_ima ges/Orbital_Energies. jpg



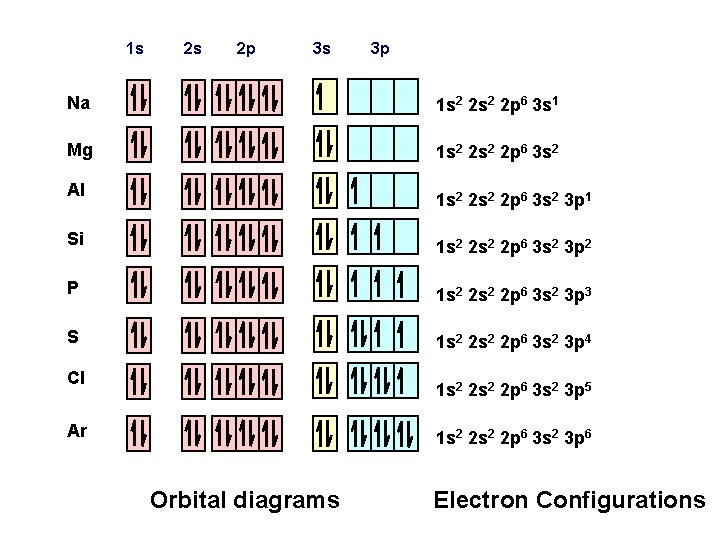
1 s 2 s 2 p 3 s 3 p Na 1 s 2 2 p 6 3 s 1 Mg 1 s 2 2 p 6 3 s 2 Al 1 s 2 2 p 6 3 s 2 3 p 1 Si 1 s 2 2 p 6 3 s 2 3 p 2 P 1 s 2 2 p 6 3 s 2 3 p 3 S 1 s 2 2 p 6 3 s 2 3 p 4 Cl 1 s 2 2 p 6 3 s 2 3 p 5 Ar 1 s 2 2 p 6 3 s 2 3 p 6 Orbital diagrams Electron Configurations

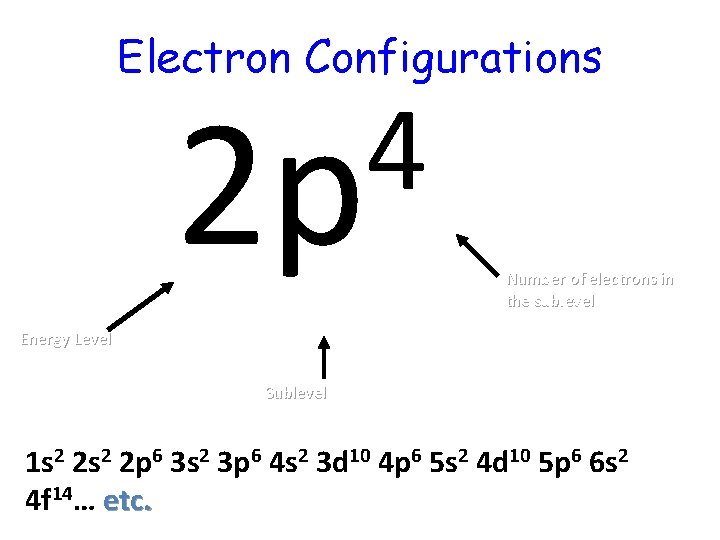
Electron Configurations 4 2 p Number of electrons in the sublevel Energy Level Sublevel 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 14… etc.

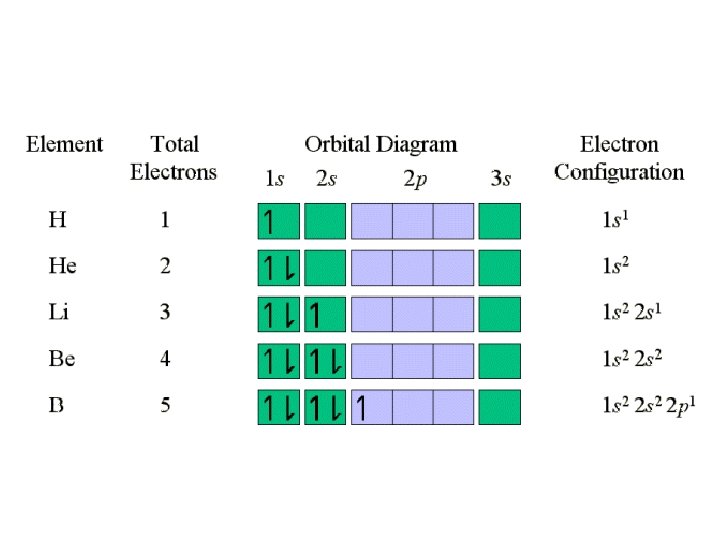
Writing Electron Configurations • To write out the electron configuration of an atom: – use the principal quantum number/energy level (1, 2, 3, or 4…) – use the letter term for each sub-level (s, p, d, or f); • don’t worry about orientation such as x, y, z axis but you do have to be able to draw these for IB – use a superscript number indicates how many electrons are present in each sub-level • hydrogen =1 s 1. • Lithium =1 s 22 s 1. – don’t write anything for spin






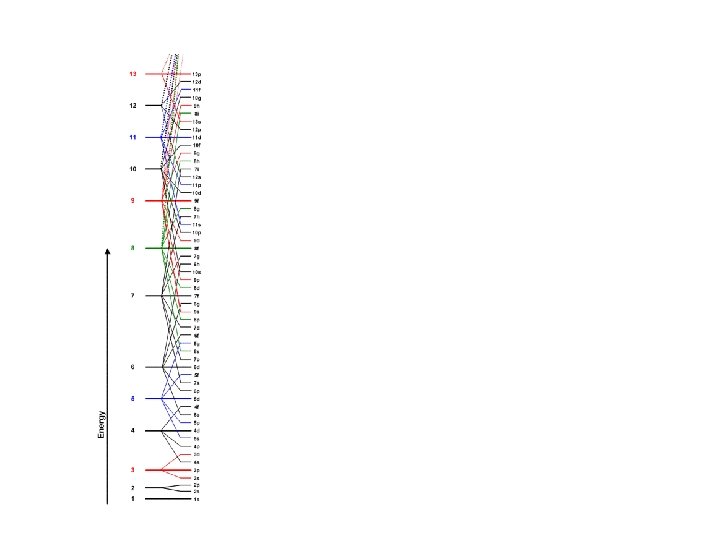
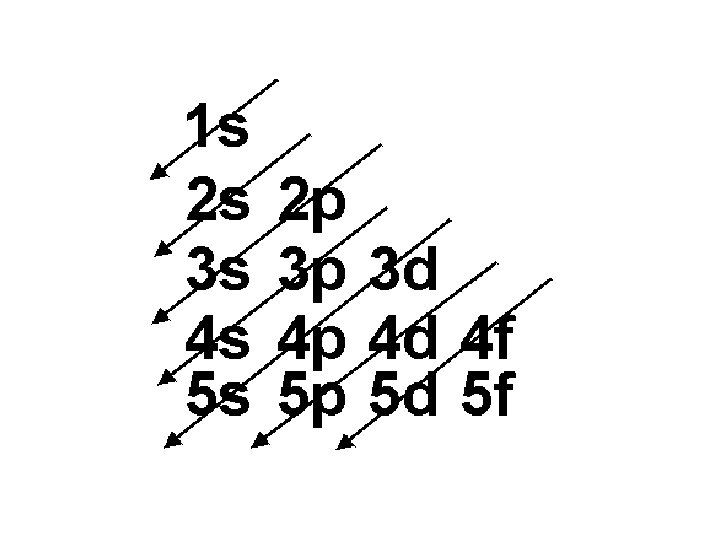
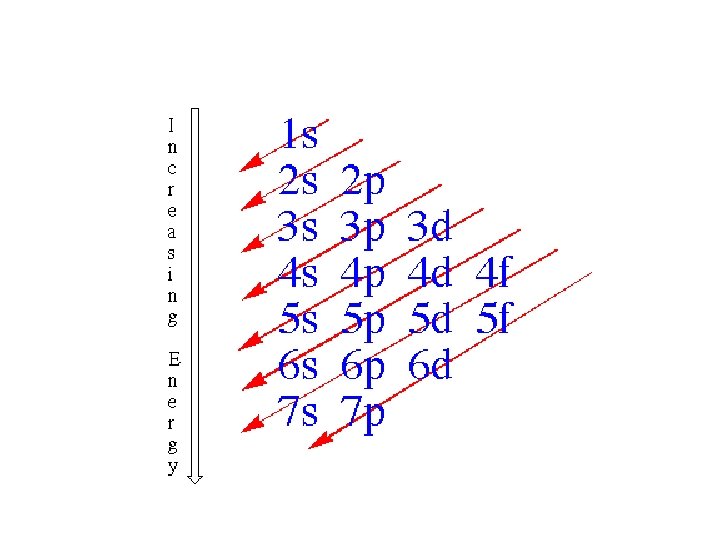
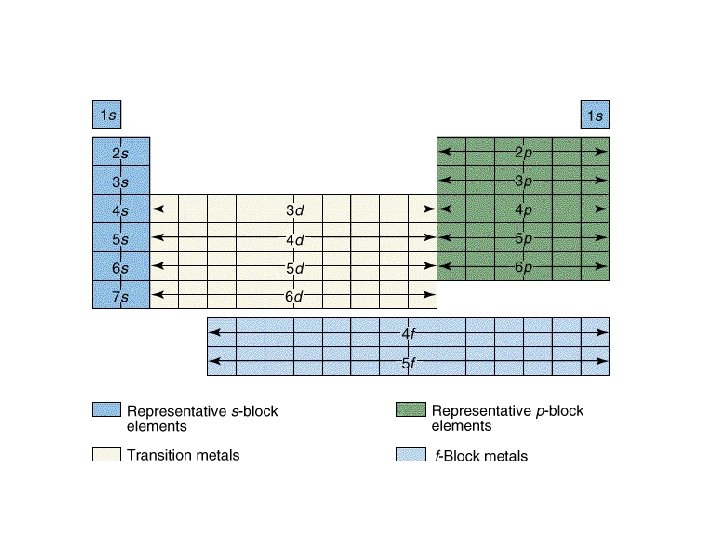
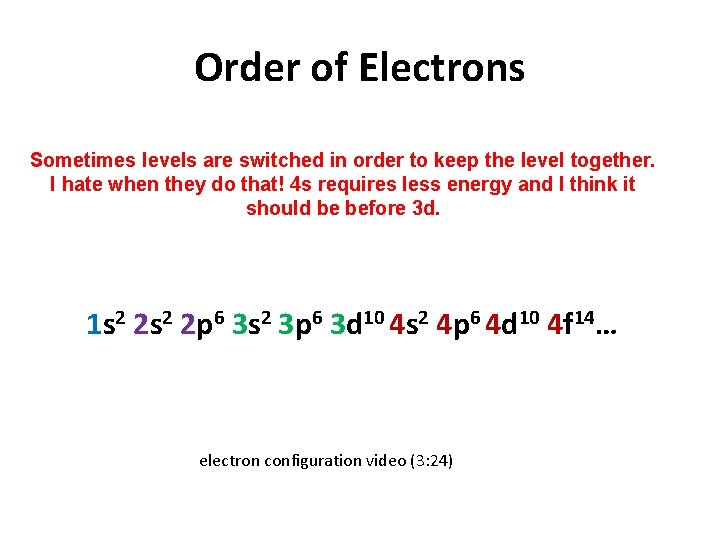
Order of Electrons Sometimes levels are switched in order to keep the level together. I hate when they do that! 4 s requires less energy and I think it should be before 3 d. 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 4 f 14… electron configuration video (3: 24)

• exceptions (don’t need to know this, just be aware that there are exceptions) • orbitals “like” to be empty, half filled, or full • therefore, an electron leaves the 4 s (leaving it half full) and goes to the 3 d in order to make it full Cr we would predict: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 4 but it is actually: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 13 d 5 Cu we would predict: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 9 but it is actually: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10

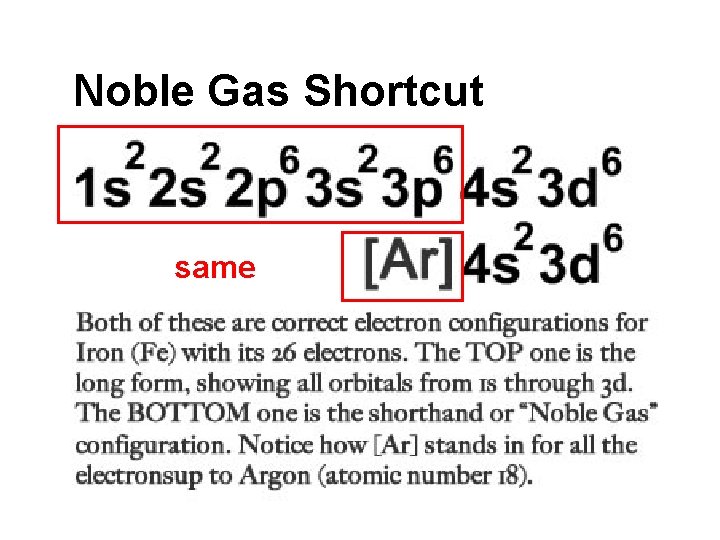
Noble Gas Shortcut same