Light is an electromagnetic wave Electromagnetic wave a











- Slides: 43


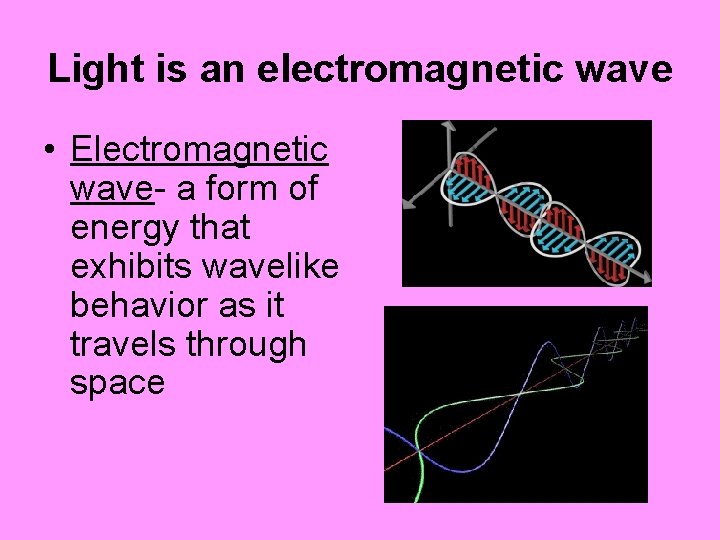
Light is an electromagnetic wave • Electromagnetic wave- a form of energy that exhibits wavelike behavior as it travels through space

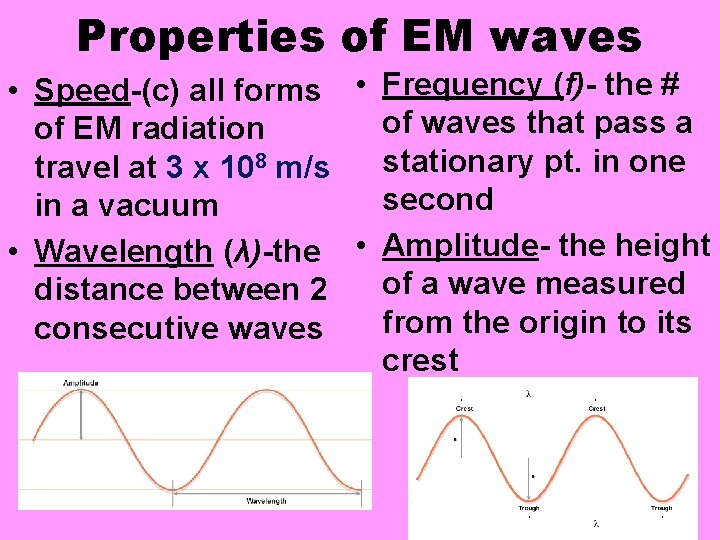
Properties of EM waves • Speed-(c) all forms • Frequency (f)- the # of waves that pass a of EM radiation stationary pt. in one travel at 3 x 108 m/s second in a vacuum • Wavelength (λ)-the • Amplitude- the height of a wave measured distance between 2 from the origin to its consecutive waves crest

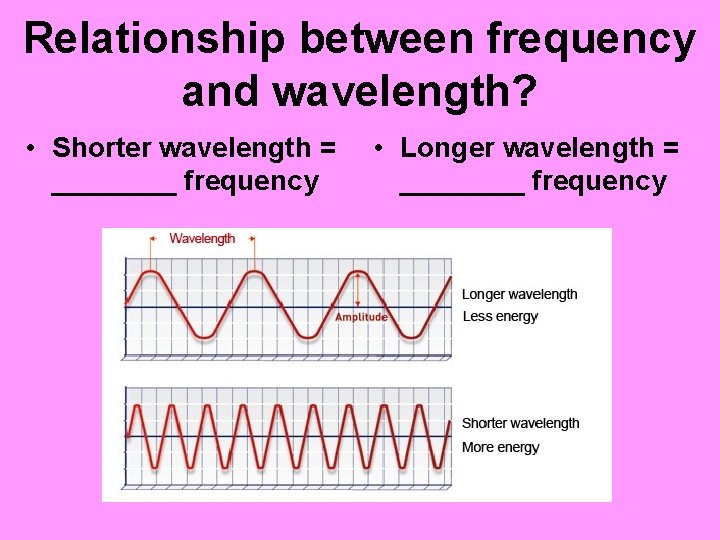
Relationship between frequency and wavelength? • Shorter wavelength = ____ frequency • Longer wavelength = ____ frequency

Frequency and Wavelength… • …are mathematically related c=λv Where : c = speed of light λ = wavelength v = frequency

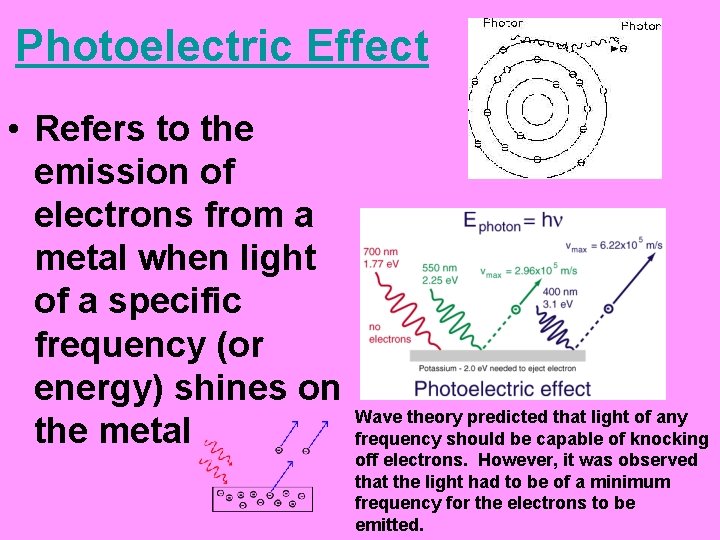
Photoelectric Effect • Refers to the emission of electrons from a metal when light of a specific frequency (or energy) shines on the metal Wave theory predicted that light of any frequency should be capable of knocking off electrons. However, it was observed that the light had to be of a minimum frequency for the electrons to be emitted.

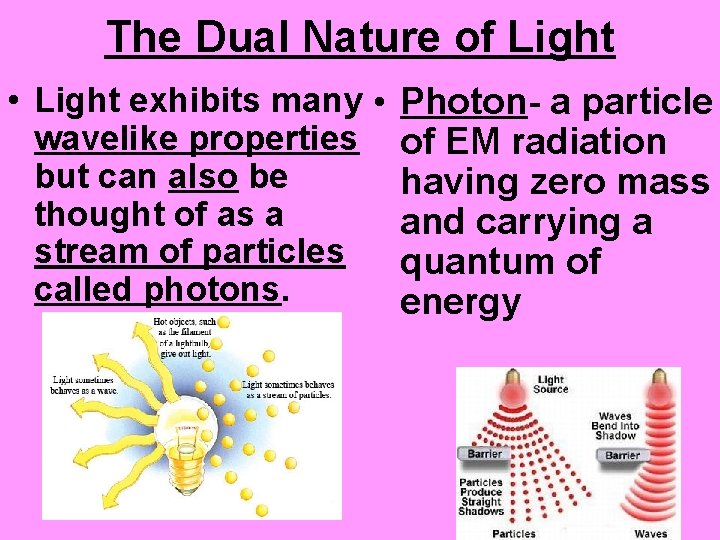
The Dual Nature of Light • Light exhibits many • Photon- a particle wavelike properties of EM radiation but can also be having zero mass thought of as a and carrying a stream of particles quantum of called photons. energy

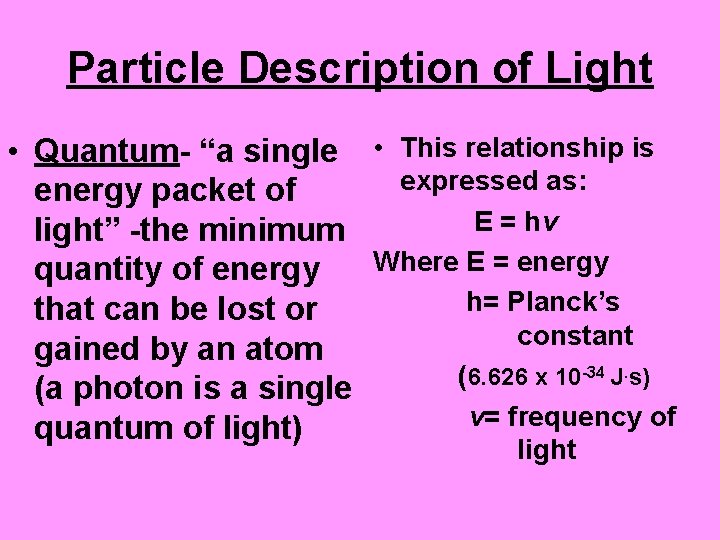
Particle Description of Light • Quantum- “a single • This relationship is expressed as: energy packet of E = hv light” -the minimum Where E = energy quantity of energy h= Planck’s that can be lost or constant gained by an atom ( 6. 626 x 10 -34 J. s) (a photon is a single v= frequency of quantum of light) light

De. Broglie’s Idea • Suggested that electrons could also have a wave nature much like light. This was based on the fact that: v Electrons can be diffracted (bending) v Electrons exhibit interference (overlapping that results in a reduction of energy)

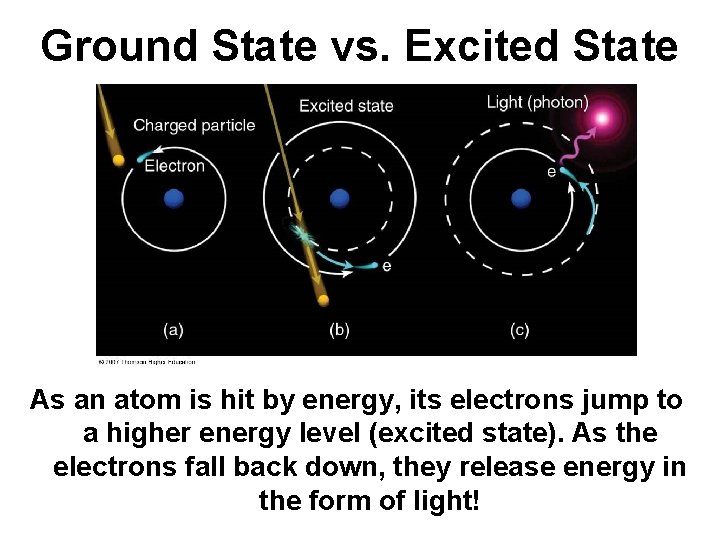
Line Emission Spectra • The lowest energy state of an electron is the ground state. • A state in which that atom has a higher energy potential is called an excited state. • As the excited electron falls back to its ground state, it releases EM radiation of an energy that corresponds to the amount of energy gained to reach the excited state.

Ground State vs. Excited State As an atom is hit by energy, its electrons jump to a higher energy level (excited state). As the electrons fall back down, they release energy in the form of light!

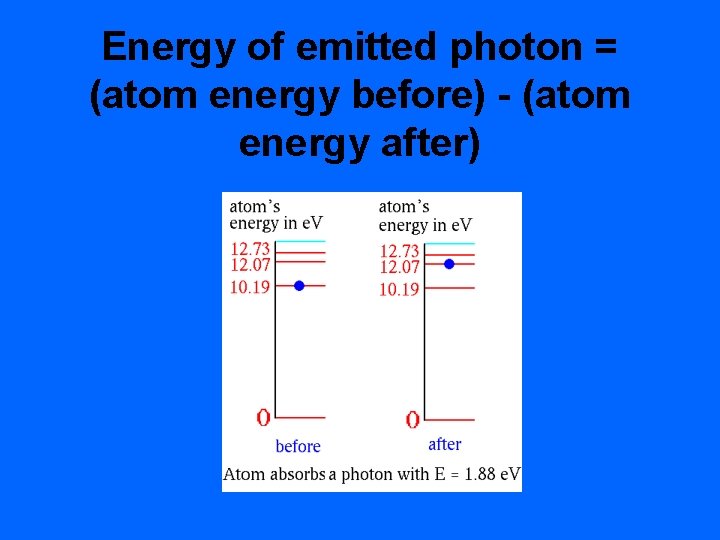
Energy of emitted photon = (atom energy before) - (atom energy after)

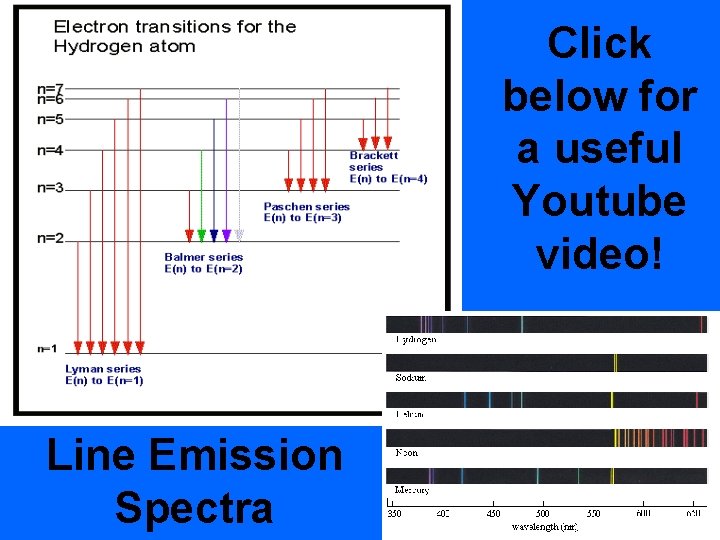
hyperlink • When the EM radiation released is passed through a prism or diffraction grating- it is separated into a series of specific frequencies of visible light. • This is referred to as a line emission spectra

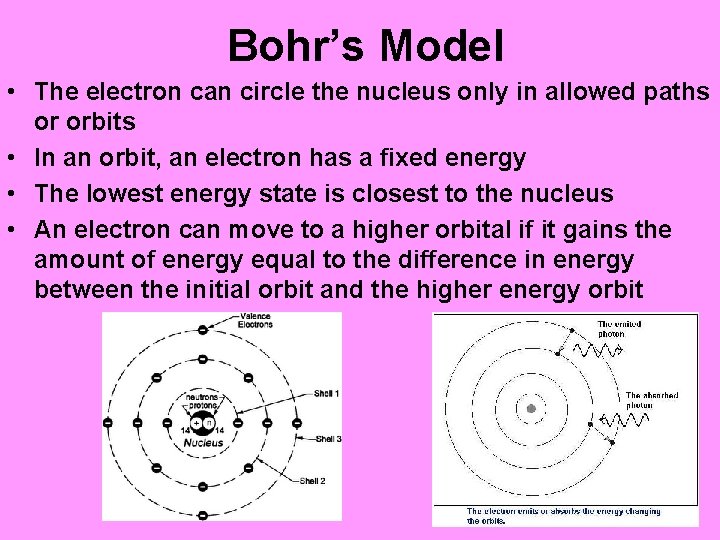
Bohr’s Model • The electron can circle the nucleus only in allowed paths or orbits • In an orbit, an electron has a fixed energy • The lowest energy state is closest to the nucleus • An electron can move to a higher orbital if it gains the amount of energy equal to the difference in energy between the initial orbit and the higher energy orbit

Click below for a useful Youtube video! Line Emission Spectra

• It is impossible to determine simultaneously both the position and velocity of an electron.

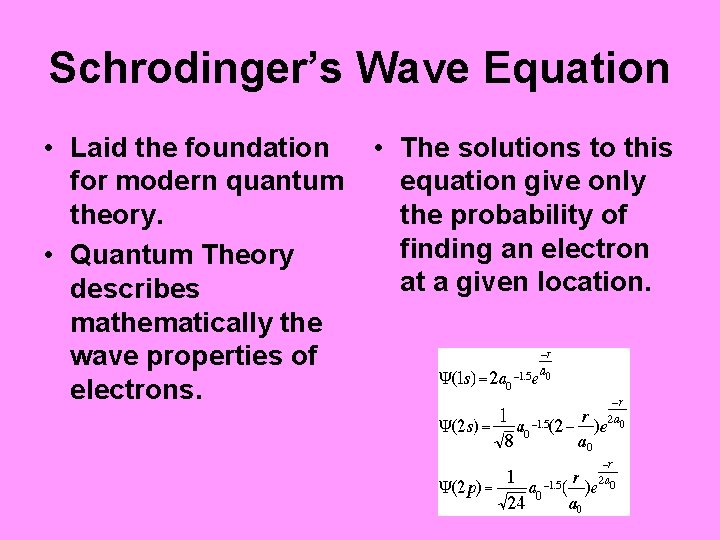
Schrodinger’s Wave Equation • Laid the foundation for modern quantum theory. • Quantum Theory describes mathematically the wave properties of electrons. • The solutions to this equation give only the probability of finding an electron at a given location.

• Electrons do not travel in neat orbits • They exist in threedimensional regions called orbits that indicate the probable location of an electron.

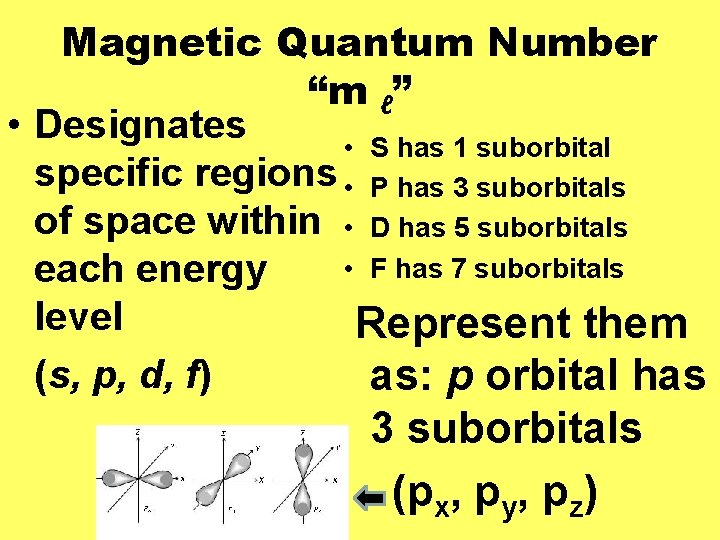
The location of electrons within the atom can be described using quantum numbers: v. Principle v. Orbital (Angular Momentum) v. Magnetic v. Spin

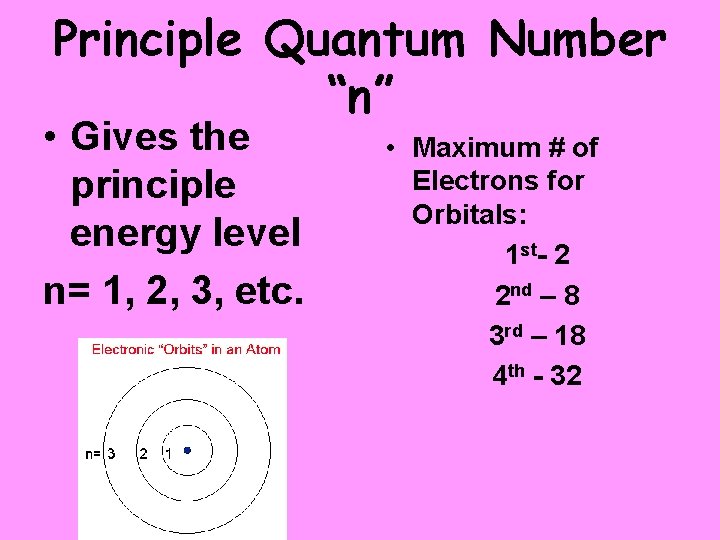
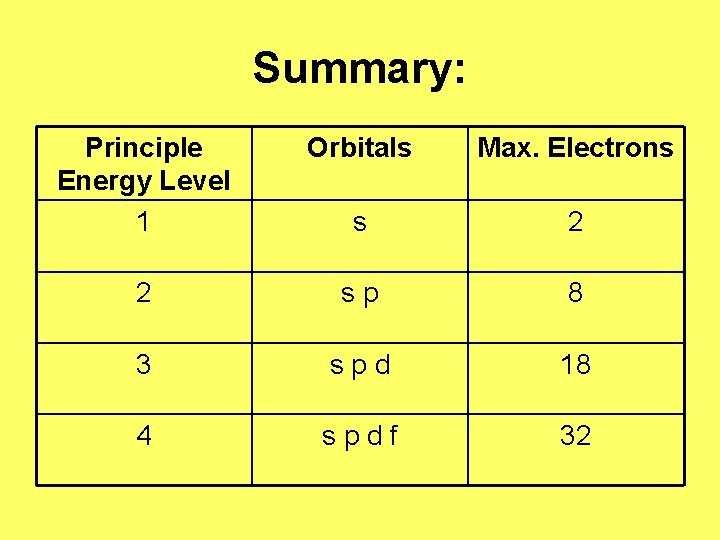
Principle Quantum Number “n” • Gives the principle energy level n= 1, 2, 3, etc. • Maximum # of Electrons for Orbitals: 1 st- 2 2 nd – 8 3 rd – 18 4 th - 32

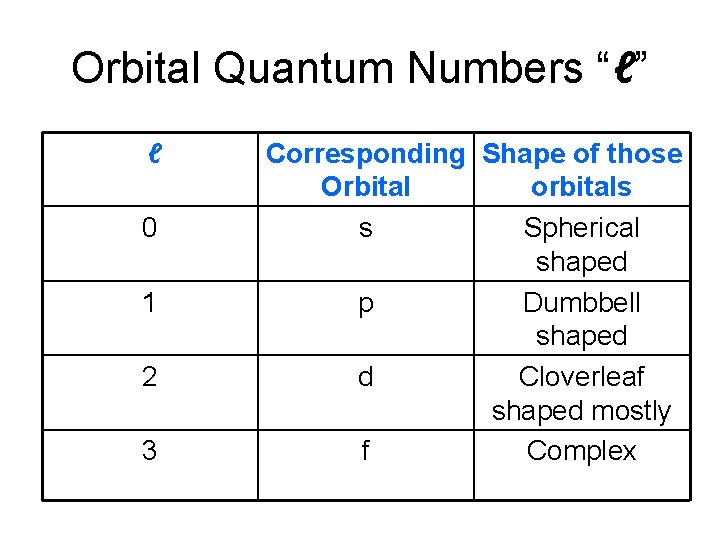
Orbital Quantum Number (“ℓ”) • Tells the shape or type of orbital • s orbital is a spherical shape • p orbital is dumbbell shaped

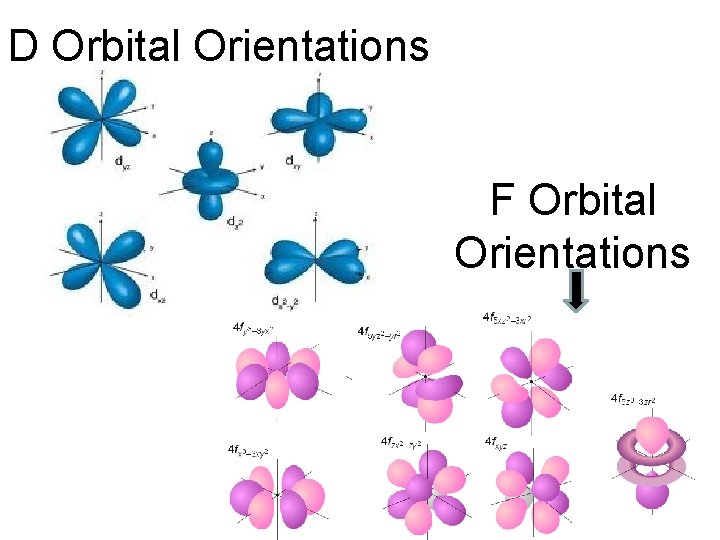
Orbital Quantum Numbers “ℓ” ℓ 0 1 2 3 Corresponding Shape of those Orbital orbitals s Spherical shaped p Dumbbell shaped d Cloverleaf shaped mostly f Complex

D Orbital Orientations F Orbital Orientations

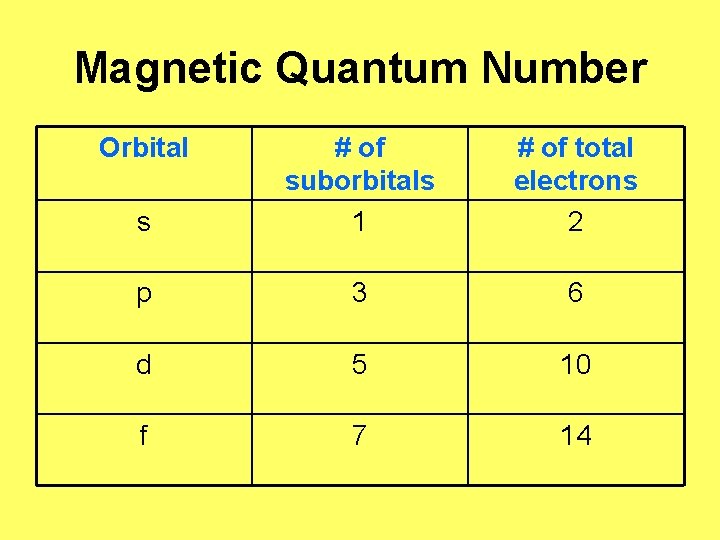
Magnetic Quantum Number “m ℓ” • Designates • S has 1 suborbital specific regions • P has 3 suborbitals of space within • D has 5 suborbitals • F has 7 suborbitals each energy level Represent them (s, p, d, f) as: p orbital has 3 suborbitals (px, py, pz)

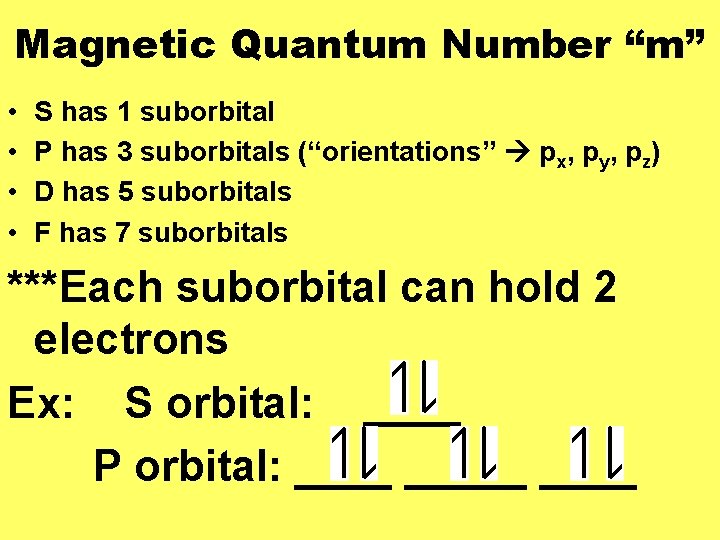
Magnetic Quantum Number “m” • • S has 1 suborbital P has 3 suborbitals (“orientations” px, py, pz) D has 5 suborbitals F has 7 suborbitals ***Each suborbital can hold 2 electrons Ex: S orbital: ____ P orbital: _____

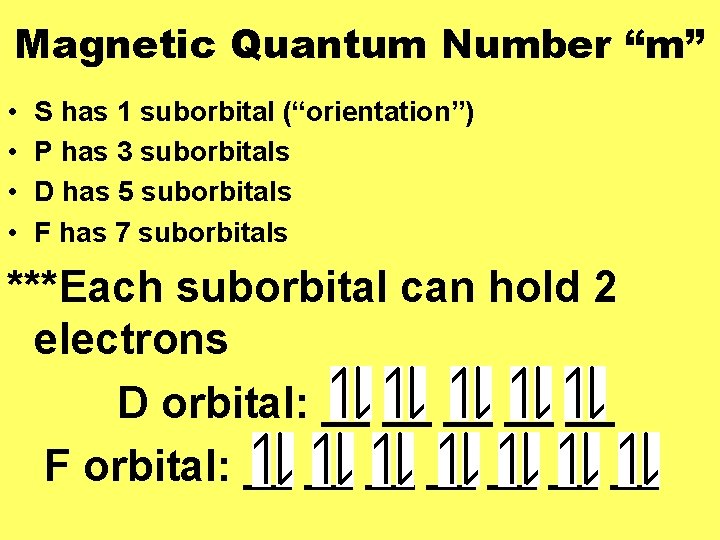
Magnetic Quantum Number “m” • • S has 1 suborbital (“orientation”) P has 3 suborbitals D has 5 suborbitals F has 7 suborbitals ***Each suborbital can hold 2 electrons D orbital: __ __ __ F orbital: __ __

Magnetic Quantum Number Orbital s # of suborbitals 1 # of total electrons 2 p 3 6 d 5 10 f 7 14

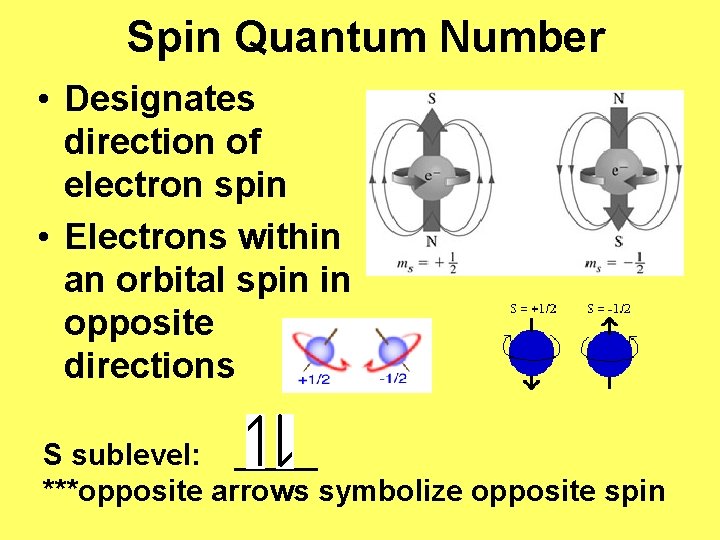
Spin Quantum Number • Designates direction of electron spin • Electrons within an orbital spin in opposite directions S sublevel: _____ ***opposite arrows symbolize opposite spin

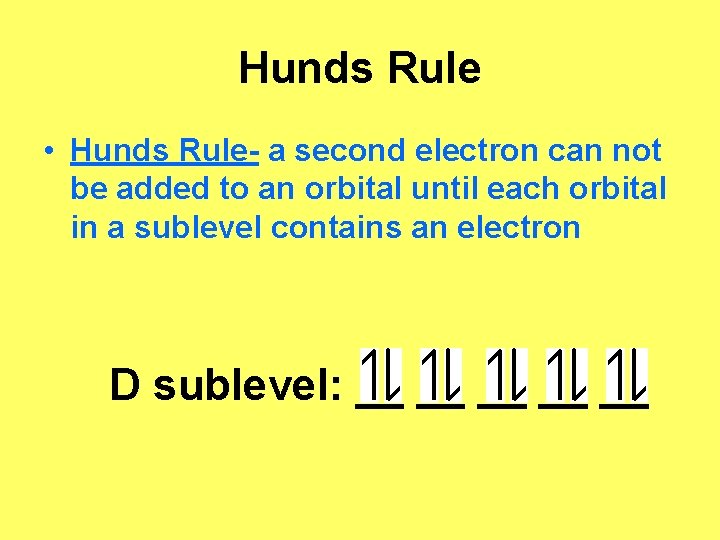
Governing Rules and Principles • Pauli Exclusion Principle- only 2 electrons in each orbital • Aufbau Principle- electrons must occupy lower energy orbitals first • Hunds Rule- a second electron can not be added to an orbital until each orbital in a sublevel contains an electron

Hunds Rule • Hunds Rule- a second electron can not be added to an orbital until each orbital in a sublevel contains an electron D sublevel: __ __ __

Summary: Principle Energy Level 1 Orbitals Max. Electrons s 2 2 s p 8 3 s p d 18 4 s p d f 32

Electron Configuration How do we actually write where the electrons are located for each atom? ?

Electron Configurations • Electron configurations tells us in which orbitals the electrons for an element are located. • Three rules: (Review) – electrons fill orbitals starting with lowest n and moving upwards; – no two electrons can fill one orbital with the same spin (Pauli); – for degenerate orbitals, electrons fill each orbital singly before any orbital gets a second electron (Hund’s rule).

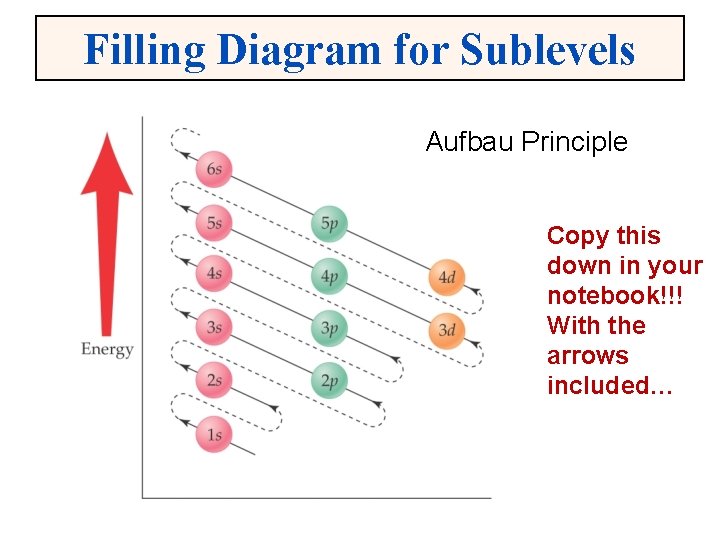
Filling Diagram for Sublevels Aufbau Principle Copy this down in your notebook!!! With the arrows included…

Writing Electron Configurations “Superscript Notation” • First, determine how many electrons are in the atom. Iron has 26 electrons. • Arrange the energy sublevels according to increasing energy: – 1 s 2 s 2 p 3 s 3 p 4 s 3 d … • Fill each sublevel with electrons until you have used all the electrons in the atom: – Fe: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 • The sum of the superscripts equals the atomic number of iron (26)

Write the Superscript Notation… • • Nitrogen (N) Silicon (Si) Chlorine (Cl) Sodium (Na) Beryllium Calcium Carbon Neon

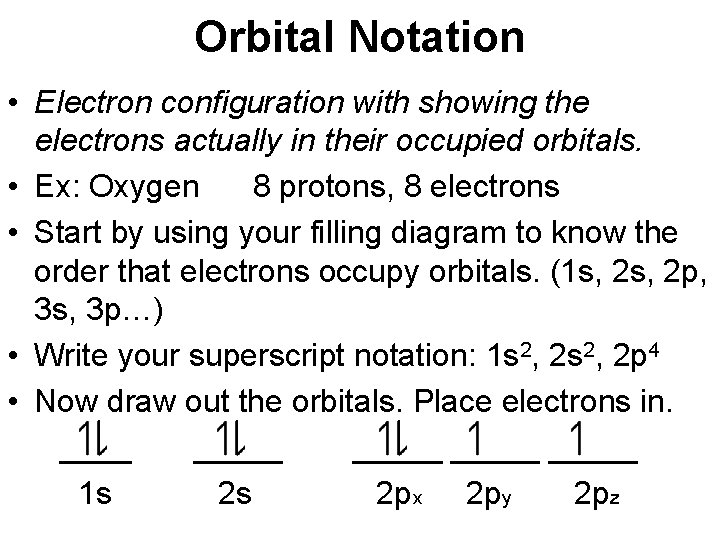
Orbital Notation • Electron configuration with showing the electrons actually in their occupied orbitals. • Ex: Oxygen 8 protons, 8 electrons • Start by using your filling diagram to know the order that electrons occupy orbitals. (1 s, 2 p, 3 s, 3 p…) • Write your superscript notation: 1 s 2, 2 p 4 • Now draw out the orbitals. Place electrons in. _____ _____ 1 s 2 s 2 px 2 py 2 pz

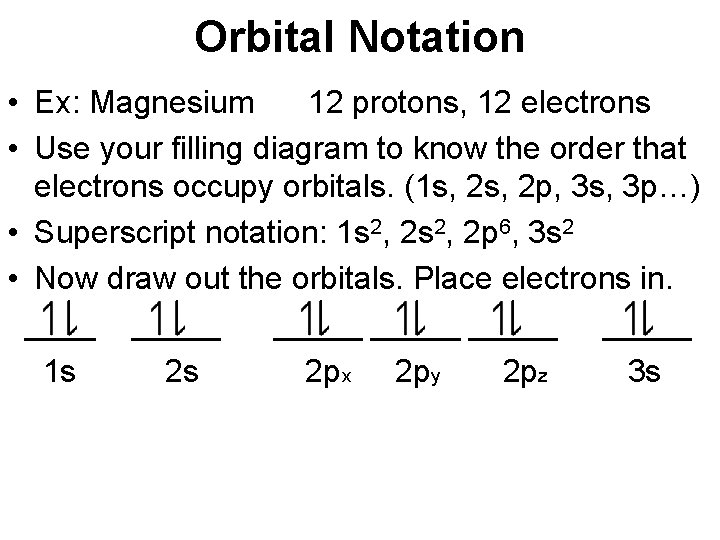
Orbital Notation • Ex: Magnesium 12 protons, 12 electrons • Use your filling diagram to know the order that electrons occupy orbitals. (1 s, 2 p, 3 s, 3 p…) • Superscript notation: 1 s 2, 2 p 6, 3 s 2 • Now draw out the orbitals. Place electrons in. _____ _____ 1 s 2 s 2 px 2 py 2 pz 3 s

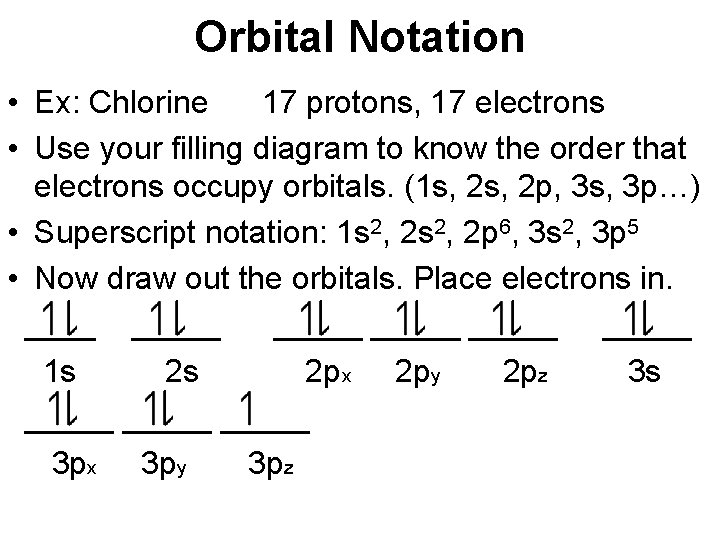
Orbital Notation • Ex: Chlorine 17 protons, 17 electrons • Use your filling diagram to know the order that electrons occupy orbitals. (1 s, 2 p, 3 s, 3 p…) • Superscript notation: 1 s 2, 2 p 6, 3 s 2, 3 p 5 • Now draw out the orbitals. Place electrons in. _____ _____ 1 s 2 s 2 px 2 py 2 pz 3 s _____ 3 px 3 py 3 pz

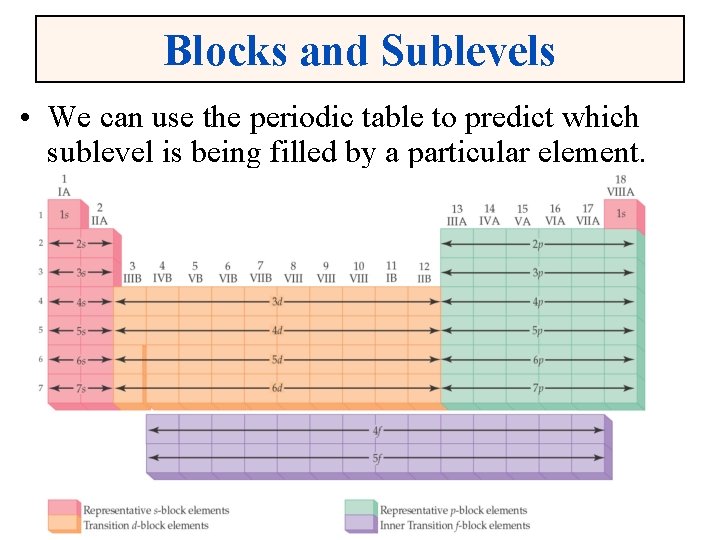
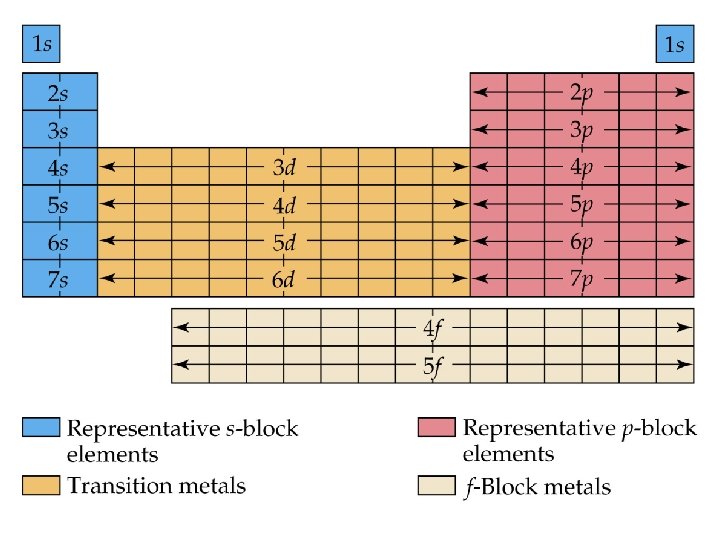
Blocks and Sublevels • We can use the periodic table to predict which sublevel is being filled by a particular element.


Noble Gas Core Electron Configurations • Recall, the electron configuration for Na is: Na: 1 s 2 2 p 6 3 s 1 • We can abbreviate the electron configuration by indicating the innermost electrons with the symbol of the preceding noble gas. • The preceding noble gas with an atomic number less than sodium is neon, Ne. We rewrite the electron configuration: Na: [Ne] 3 s 1

Let’s Look at it backwards! ID the elements with the following configurations… • 1 s 22 p 63 s 23 p 4 Sulfur • 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 Rubidium • [Kr] 5 s 24 d 105 p 3 Antimony • [Xe] 6 s 24 f 145 d 6 Osmium • [Rn] 7 s 25 f 11 Einsteinium