Light Wave Vs Particles Electromagnetic Waves Frequency and















- Slides: 30

Light • Wave Vs. Particles • Electromagnetic Waves • Frequency and Wavelength • The Electromagnetic Spectrum • Planck’s Constant • Photo Electric Effect

Light Objectives: I can describe the dual nature of light. I can explain experimental evidence of the wave and photon nature of light.

Light: Introduction Robert Hooke In the 17 th century, Isaac Newton proposed the “corpuscular theory” stating that light is composed of particles. Other scientists, like Robert Hooke and Christian Huygens, believed light to be a wave. Christian Huygens Isaac Newton

Light: Introduction Today we know that light behaves as both a wave and as a particle. Light undergoes interference and diffraction, as all waves do, but whenever light is emitted, it is always done so in discreet packets called photons. These photons have momentum, but not mass.

Wave Vs. Particles Light is an electromagnetic wave. As light travels through space an electric field and a magnetic field oscillate perpendicular to the wave direction and perpendicular to each other. A light wave is transverse since each field oscillates in a plane perpendicular to the direction of the wave motion. Light requires no medium!

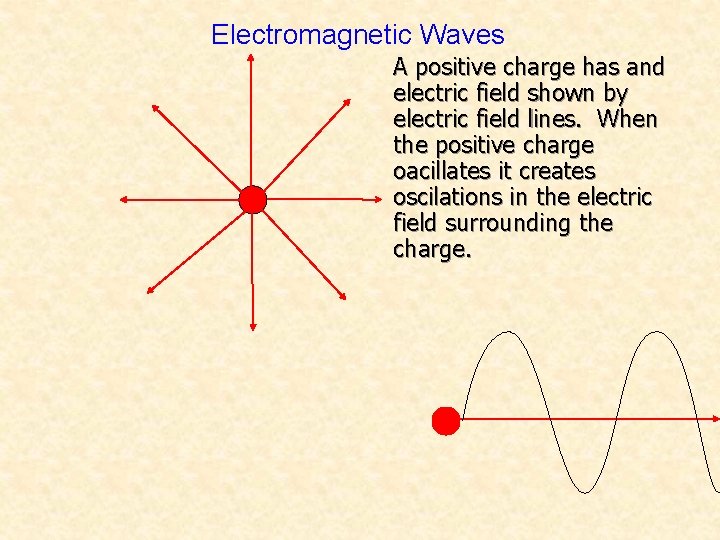
Electromagnetic Waves A positive charge has and electric field shown by electric field lines. When the positive charge oacillates it creates oscilations in the electric field surrounding the charge.

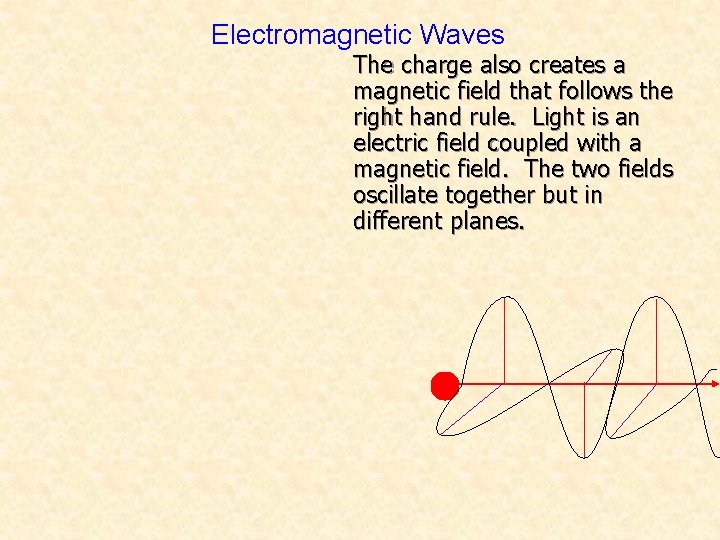
Electromagnetic Waves The charge also creates a magnetic field that follows the right hand rule. Light is an electric field coupled with a magnetic field. The two fields oscillate together but in different planes.


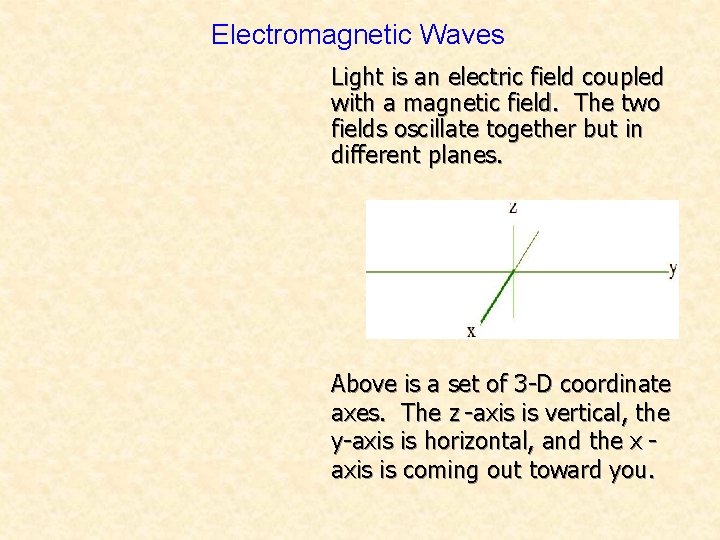
Electromagnetic Waves Light is an electric field coupled with a magnetic field. The two fields oscillate together but in different planes. Above is a set of 3 -D coordinate axes. The z -axis is vertical, the y-axis is horizontal, and the x axis is coming out toward you.

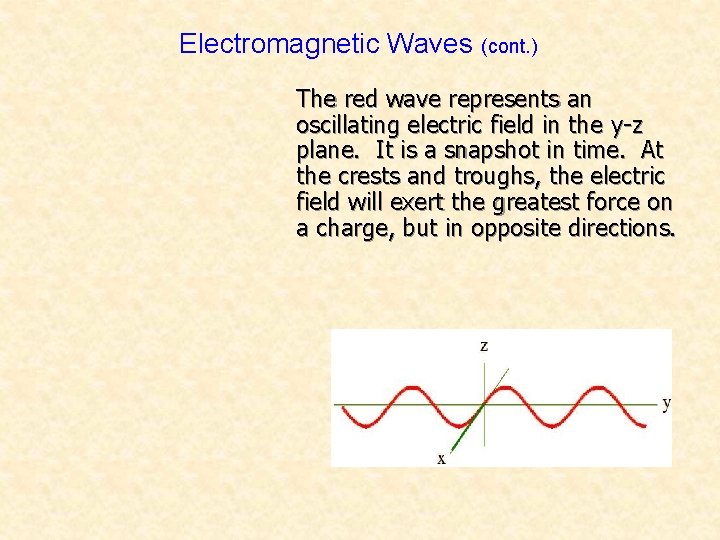
Electromagnetic Waves (cont. ) The red wave represents an oscillating electric field in the y-z plane. It is a snapshot in time. At the crests and troughs, the electric field will exert the greatest force on a charge, but in opposite directions.

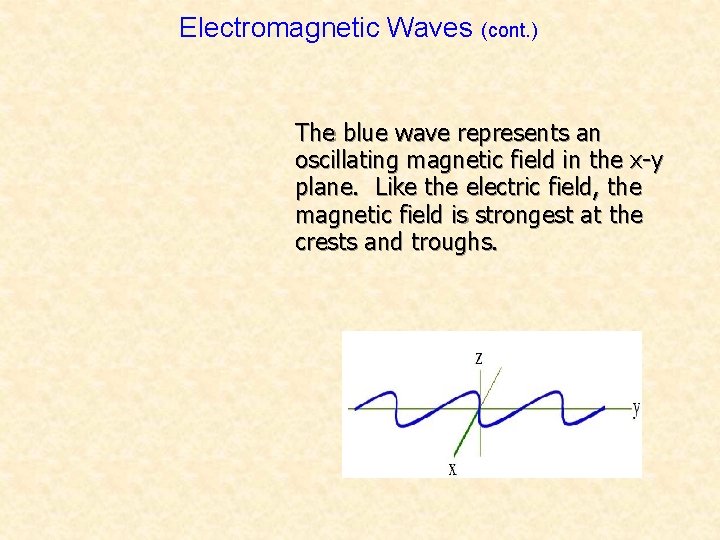
Electromagnetic Waves (cont. ) The blue wave represents an oscillating magnetic field in the x-y plane. Like the electric field, the magnetic field is strongest at the crests and troughs.

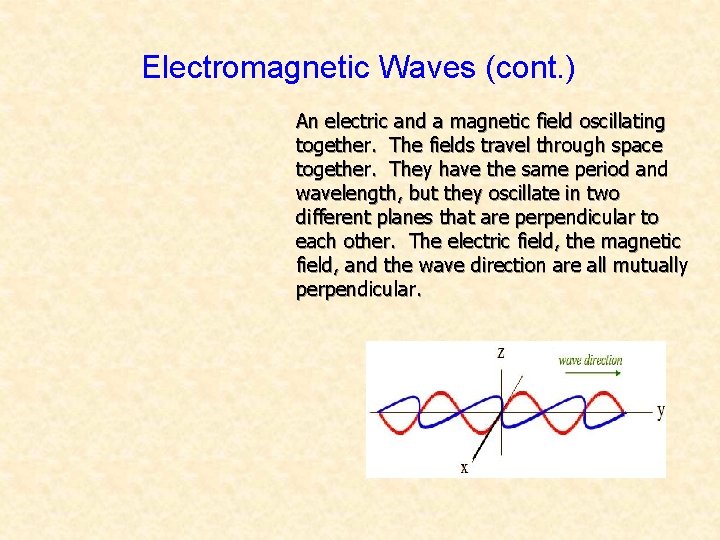
Electromagnetic Waves (cont. ) An electric and a magnetic field oscillating together. The fields travel through space together. They have the same period and wavelength, but they oscillate in two different planes that are perpendicular to each other. The electric field, the magnetic field, and the wave direction are all mutually perpendicular.

Electromagnetic Waves (cont. )

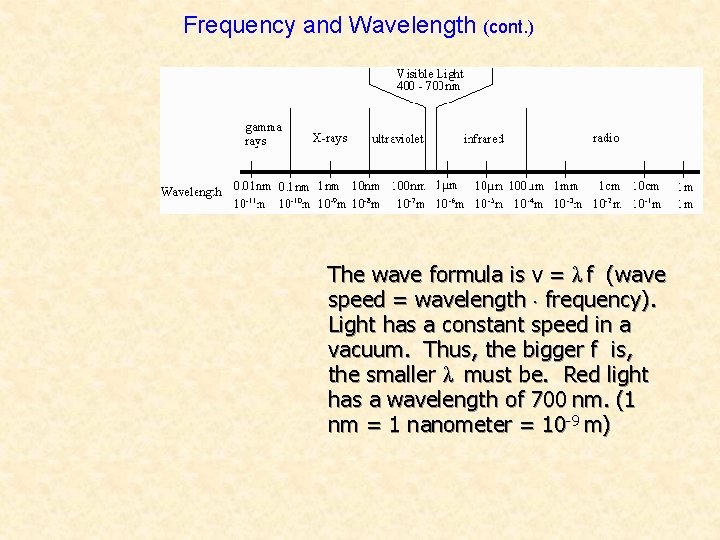
Frequency and Wavelength The frequency of a light wave corresponds to the color we see. The amplitude corresponds to brightness. Light Sound Frequency Color Pitch Amplitude Brightness Loudness Red light has a frequency of over 400 trillion Hertz. The frequency of violet light is even higher—over 750 trillion Hz. Other types of electromagnetic radiation, like X-rays, have even higher frequencies, and some have lower frequencies, like radio waves.

Frequency and Wavelength (cont. ) The wave formula is v = λ f (wave speed = wavelength × frequency). Light has a constant speed in a vacuum. Thus, the bigger f is, the smaller λ must be. Red light has a wavelength of 700 nm. (1 nm = 1 nanometer = 10 -9 m)

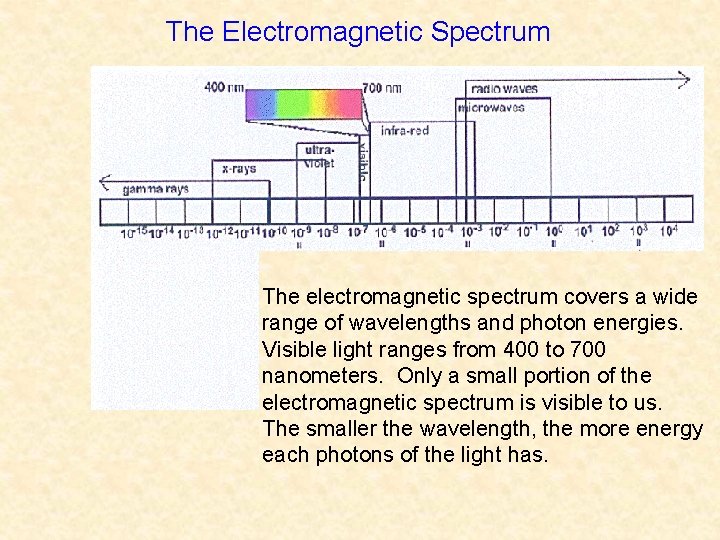
The Electromagnetic Spectrum The electromagnetic spectrum covers a wide range of wavelengths and photon energies. Visible light ranges from 400 to 700 nanometers. Only a small portion of the electromagnetic spectrum is visible to us. The smaller the wavelength, the more energy each photons of the light has.

Electromagnetic Spectrum (cont. ) Wavelengths other that visible light serve useful purposes: Radio waves are very long (a few centimeters to 6 football fields) and can be used to send signals. These signals are transmitted by radio stations. They transmit information and music via amplitude modulation (AM) and frequency modulation (FM). Microwaves (a few millimeters long) are also used in communications. Microwave ovens are great for heating food since food is primarily water, and microwaves have just the right frequency to get water molecules vibrating.

Electromagnetic Spectrum (cont. ) Infrared (micrometers in length) are used in remote controls to change the channel, and they are also radiated by objects that are warmer than their surrounding (like your body). They make night vision equipment possible. Ultraviolet light is harmful to our bodies because its wavelength is so small. Short wavelength mean high energy for photons. UV causes our skin to tan and burn. Fortunately, the ozone layer blocks most UV radiation, but prolonged exposure to the sun should be avoided, since UV rays can cause skin cancer. On the positive side UV radiation helps people to produce their own vitamin D.

Electromagnetic Spectrum (cont. ) X-rays are even more energetic, and hence more dangerous, than UV rays, but luckily they cannot penetrate our ozone layer. They are produced in space and of course are used by doctors to get pictures of your bones. Gamma rays are the most energetic of the light waves and little is known about them other than they are very harmful to living cells and are used by doctors to kill certain cells and for other operations. They are produced in nuclear explosions. Like other high energy rays, our atmosphere protects us from gamma rays.

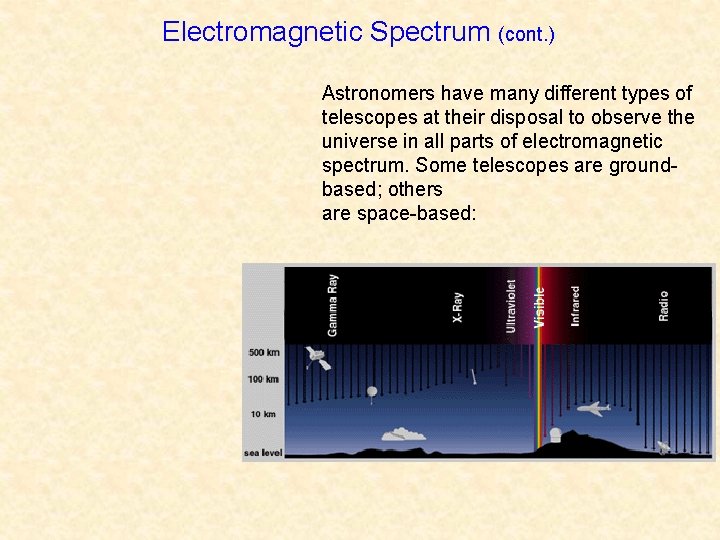
Electromagnetic Spectrum (cont. ) Astronomers have many different types of telescopes at their disposal to observe the universe in all parts of electromagnetic spectrum. Some telescopes are groundbased; others are space-based:

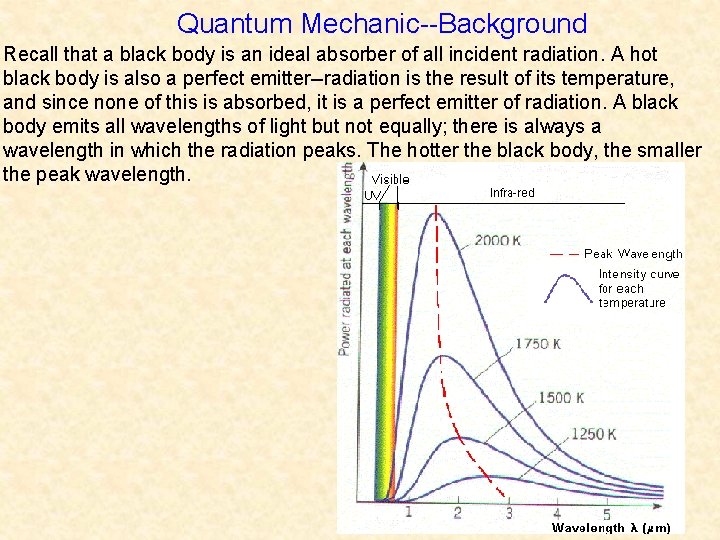
Quantum Mechanic--Background Recall that a black body is an ideal absorber of all incident radiation. A hot black body is also a perfect emitter--radiation is the result of its temperature, and since none of this is absorbed, it is a perfect emitter of radiation. A black body emits all wavelengths of light but not equally; there is always a wavelength in which the radiation peaks. The hotter the black body, the smaller the peak wavelength.

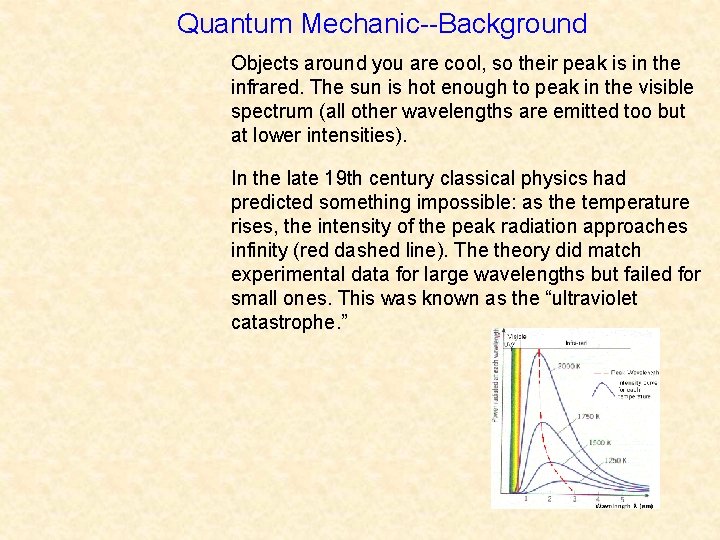
Quantum Mechanic--Background Objects around you are cool, so their peak is in the infrared. The sun is hot enough to peak in the visible spectrum (all other wavelengths are emitted too but at lower intensities). In the late 19 th century classical physics had predicted something impossible: as the temperature rises, the intensity of the peak radiation approaches infinity (red dashed line). The theory did match experimental data for large wavelengths but failed for small ones. This was known as the “ultraviolet catastrophe. ”

Black Body Radiation • Phet: Black Body Radiation Simulation

Planck’s Constant In 1900 Max Planck came up with a revolutionary way to resolve the problem by assuming that energy came in discrete amounts (quanta). This was the beginning of quantum mechanics. Each quantum of light is called a photon, and its energy is given by E = h f, where f is the frequency of the radiation and h is the constant of proportionality called Plank’s constant. The formula states that higher frequency light has proportionally more energy per photon. Einstein lent credence to Plank’s ideas by explaining the photoelectric effect in a similar manner. Robert Millikan did a series of experiments involving the photoelectric effect and calculated the constant: h = 6. 626 × 10 -34 J s. Max Plank

Planck’s Constant Before Planck light was considered to be a wave. Today we know it can be interpreted as either a particle or a wave. As a wave, bright light can be explained as a large amplitude in the electric and magnetic fields. As a particle, bright light would be explained by a large number of photons.

Wave Particle Duality Mechanical Universe: Wave Particle Duality Phet: Fourier Transformation Making Waves

A great follow up on this presentation would be to watch the Mechanical Universe: Wave Particle Duality that is part of this Bundle.

28

Credits Numerous Images as well as information were obtained from the following sources: http: //archive. ncsa. uiuc. edu/Cyberia/Bima/spectrum. html http: //www. christiananswers. net/q-eden/star-distance. html http: //abalone. cwru. edu/tutorial/enhanced/files/lc/light. htm http: //www. netzmedien. de/software/download/java/polarisation/ http: //www. howstuffworks. com/sunglass 4. htm http: //www. colorado. edu/physics/2000/polarization. I. html http: //www. cs. brown. edu/exploratory/research/applets/catalog. html http: //www. intl-light. com/handbook/flux. html http: //www. schorsch. com/kbase/glossary/luminous_flux. html http: //www. natmus. min. dk/cons/tp/lightcd/lumen. htm http: //www. bipm. fr/enus/5_Scientific/e_rad_phot/photometry/luminous_flux. html http: //webdesign. about. com/library/weekly/aa 111201 a. htm http: //www. glenbrook. k 12. il. us/gbssci/phys/Class/light/u 12 l 2 a. html http: //cowan. bendnet. com/darksky/Illuminance. htm http: //www. chemie. de/tools/units. php 3? language=e&property=cd*sr%2 Fm%5 E 2

Credits (cont. ) http: //www. worldlights. com/world/candela. html http: //www. westsidesystems. com/rays. html http: //www. electro-optical. com/bb_rad/emspect. htm http: //violet. pha. jhu. edu/~wpb/spectroscopy/em_spec. html http: //www. electro-optical. com/bb_rad/emspect. htmacadem/physics/physlets/resources-1/dav_optics/EMWave. html http: //littleshop. physics. colostate. edu/Color_Mixing. html http: //www. phy. ntnu. edu. tw/java/image/rgb. Color. html http: //www. nobel. se/physics/educational/tools/relativity/experiment-1. html http: //www. encyclopedia. com/ http: //www. colorado. edu/physics/2000/quantumzone/photoelectric 2. html http: //www. askjeeves. com/main/followup. asp? qcat=ref_&ask=what+is+parallax&qsrc=0&o=0&snp=jeeves&qid=9 FD 4952 E 186 C E 248994 B 552 F 9 E 7 DDF 63&dt=020415095320&back=ask%3 Dwhat%2 Bis%2 Bparallax%26 o%3 D 0%26 fmt%3 D&qcatid=62&scor e=0. 76&aj_ques=snapshot%3 DJeeves%26 kbid%3 D 1967190%26 item 1%3 D 19900982247110&aj_logid=9 FD 4952 E 186 CE 248994 B 552 F 9 E 7 DDF 63&aj_rank=1&aj_score=0. 76&aj_list 1=19900982247110&x=26&y=13 http: //www. holonorth. com/anatom. htm http: //www. phys. ufl. edu/~avery/course/3400/f 2001/lectures/lecture_lumens. pdf http: //www. schorsch. com/kbase/glossary/solid_angle. html http: //www. egglescliffe. org. uk/physics/astronomy/blackbody/bbody. html