Electron Paramagnetic Resonance at Hunter College Makia Hughes



- Slides: 7

Electron Paramagnetic Resonance at Hunter College Makia Hughes John F. Kennedy High School Physics Department, Hunter College of Solid State NMR/EPR Lab EPR measurements of Lithiated Silver Vanadium Oxide Dr. John Flowers, Professor of Chemistry at Medgar Evers College of CUNY

Our Goal l To use EPR to examine and confirm the mechanisms of discharge in a Sulfur Vanadium Oxide (SVO) battery in order to develop higher quality batteries.

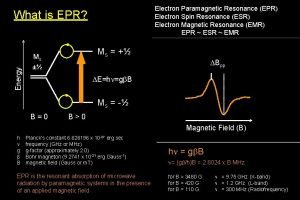
What is EPR? Electron Paramagnetic Resonance also known as Electron Spin Resonance is a spectroscopic technique which detect species that have unpaired electrons. l A surprisingly large number of materials have unpaired electrons. (examples: free radicals and many transition metal ions) l EPR alone yields inconvertible evidence of free radicals and has the unique power of identifying the paramagnetic species. l Emblem of the Society of Free Radical Research

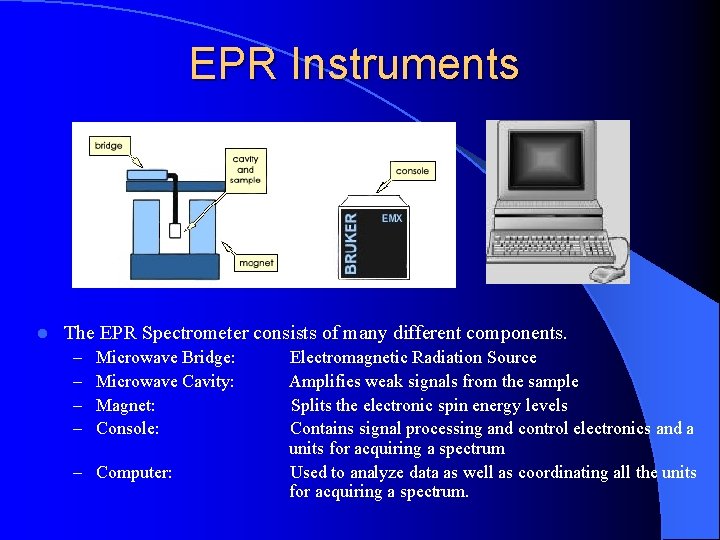
EPR Instruments l The EPR Spectrometer consists of many different components. – – Microwave Bridge: Microwave Cavity: Magnet: Console: – Computer: Electromagnetic Radiation Source Amplifies weak signals from the sample Splits the electronic spin energy levels Contains signal processing and control electronics and a units for acquiring a spectrum Used to analyze data as well as coordinating all the units for acquiring a spectrum.

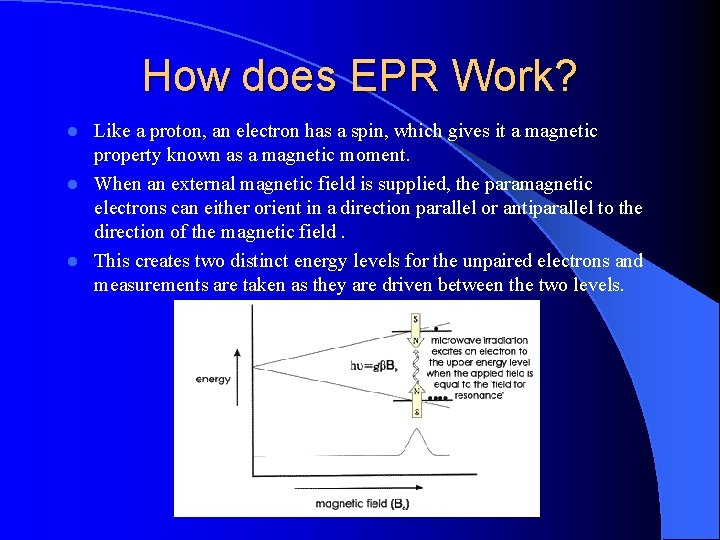
How does EPR Work? Like a proton, an electron has a spin, which gives it a magnetic property known as a magnetic moment. l When an external magnetic field is supplied, the paramagnetic electrons can either orient in a direction parallel or antiparallel to the direction of the magnetic field. l This creates two distinct energy levels for the unpaired electrons and measurements are taken as they are driven between the two levels. l

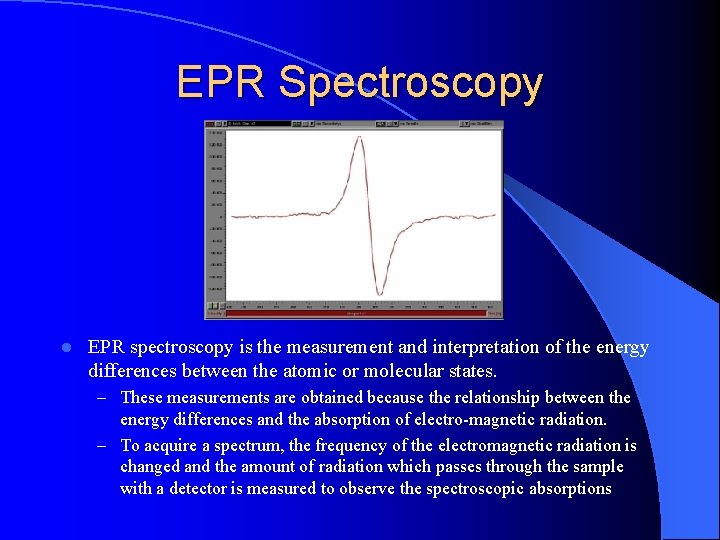
EPR Spectroscopy l EPR spectroscopy is the measurement and interpretation of the energy differences between the atomic or molecular states. – These measurements are obtained because the relationship between the energy differences and the absorption of electro-magnetic radiation. – To acquire a spectrum, the frequency of the electromagnetic radiation is changed and the amount of radiation which passes through the sample with a detector is measured to observe the spectroscopic absorptions

EPR Results l 4 Samples Tested – 1) Pure SVO- – 2) Li 0. 72 Ag 2 V 4 O 11 – 3) Li 2. 13 Ag 2 V 4 O 11 – 4) Li 5. 59 Ag 2 V 4 O 11 l Gives EPR signal due to the paramagnetism of SVO; High Q value Weak or No EPR signal; Lower Q value Definite EPR Signal; Lower Q value Supports our Hypothesis