Electron paramagnetic resonance EPR study of solid solutions





- Slides: 17

Electron paramagnetic resonance (EPR) study of solid solutions of Mo. O 3 in Sb. VO 5 Janusz Typek Institute of Physics West Pomeranian University of Technology Szczecin, Poland

Outline • • The aim of this work Preparation and characterisation of samples Results of the EPR study – magnetic defects Conclusions

The aim of the work Why to study these materials? • They are used widely as catalysts What are the oxidation states of ions? • Only assumed on general grounds What is the structure of the defect centres? • Not known

Components concentration triangle

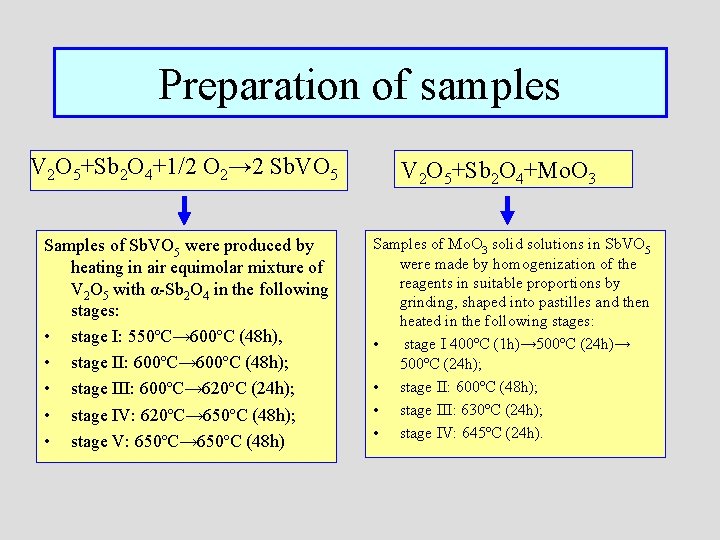
Preparation of samples V 2 O 5+Sb 2 O 4+1/2 O 2→ 2 Sb. VO 5 Samples of Sb. VO 5 were produced by heating in air equimolar mixture of V 2 O 5 with α-Sb 2 O 4 in the following stages: • stage I: 550ºC→ 600ºC (48 h), • stage II: 600ºC→ 600ºC (48 h); • stage III: 600ºC→ 620ºC (24 h); • stage IV: 620ºC→ 650ºC (48 h); • stage V: 650ºC→ 650ºC (48 h) V 2 O 5+Sb 2 O 4+Mo. O 3 Samples of Mo. O 3 solid solutions in Sb. VO 5 were made by homogenization of the reagents in suitable proportions by grinding, shaped into pastilles and then heated in the following stages: • stage I 400ºC (1 h)→ 500ºC (24 h); • stage II: 600ºC (48 h); • stage III: 630ºC (24 h); • stage IV: 645ºC (24 h).

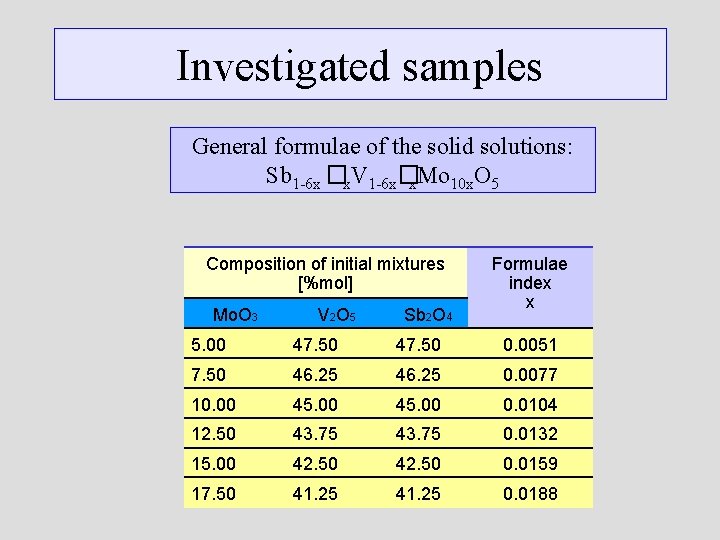
Investigated samples General formulae of the solid solutions: Sb 1 -6 x �x. V 1 -6 x�x. Mo 10 x. O 5 Composition of initial mixtures [%mol] Mo. O 3 V 2 O 5 Sb 2 O 4 Formulae index x 5. 00 47. 50 0. 0051 7. 50 46. 25 0. 0077 10. 00 45. 00 0. 0104 12. 50 43. 75 0. 0132 15. 00 42. 50 0. 0159 17. 50 41. 25 0. 0188

The matrix: Sb. VO 5 Thickness ~0. 5 μm Length ~3÷ 10 μm Scanning Electron Microscope (SEM) picture

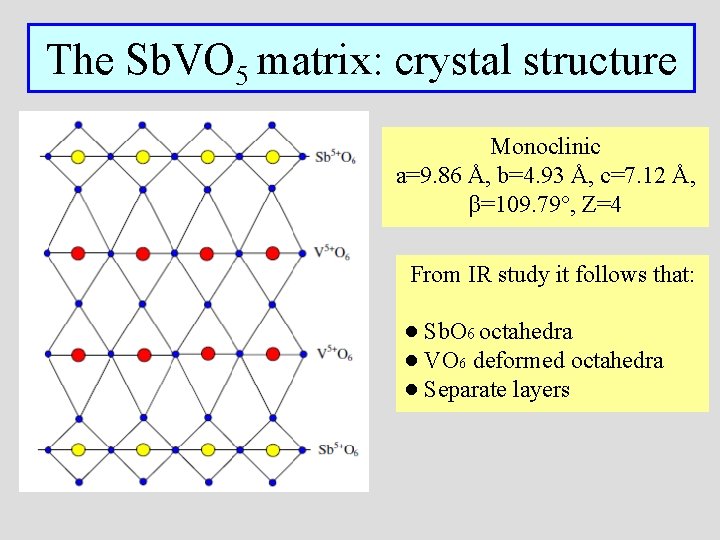
The Sb. VO 5 matrix: crystal structure Monoclinic a=9. 86 Å, b=4. 93 Å, c=7. 12 Å, β=109. 79°, Z=4 From IR study it follows that: ● Sb. O 6 octahedra ● VO 6 deformed octahedra ● Separate layers

Solid solution Sb. VO 5: Mo. O 3 More deformed, smaller sizes SEM picture of Sb. VO 5: Mo. O 3 (15 mol%)

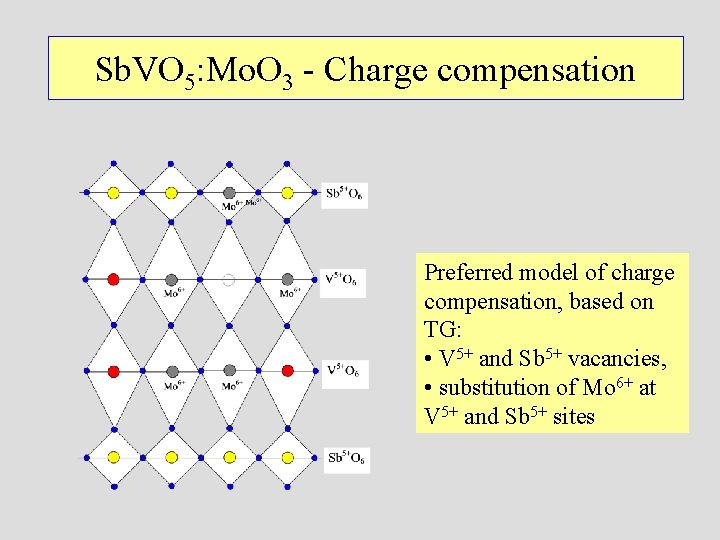
Sb. VO 5: Mo. O 3 - Charge compensation Preferred model of charge compensation, based on TG: • V 5+ and Sb 5+ vacancies, • substitution of Mo 6+ at V 5+ and Sb 5+ sites

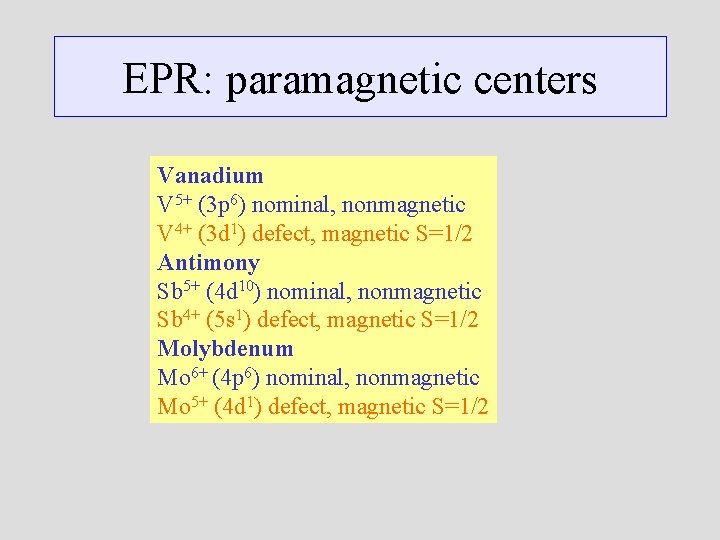
EPR: paramagnetic centers Vanadium V 5+ (3 p 6) nominal, nonmagnetic V 4+ (3 d 1) defect, magnetic S=1/2 Antimony Sb 5+ (4 d 10) nominal, nonmagnetic Sb 4+ (5 s 1) defect, magnetic S=1/2 Molybdenum Mo 6+ (4 p 6) nominal, nonmagnetic Mo 5+ (4 d 1) defect, magnetic S=1/2

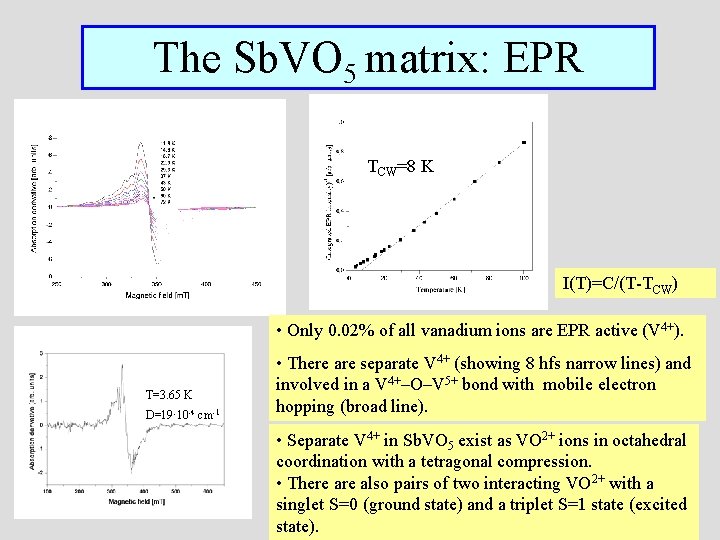
The Sb. VO 5 matrix: EPR TCW=8 K I(T)=C/(T-TCW) • Only 0. 02% of all vanadium ions are EPR active (V 4+). T=3. 65 K D=19·10 -4 cm-1 • There are separate V 4+ (showing 8 hfs narrow lines) and involved in a V 4+–O–V 5+ bond with mobile electron hopping (broad line). • Separate V 4+ in Sb. VO 5 exist as VO 2+ ions in octahedral coordination with a tetragonal compression. • There also pairs of two interacting VO 2+ with a singlet S=0 (ground state) and a triplet S=1 state (excited state).

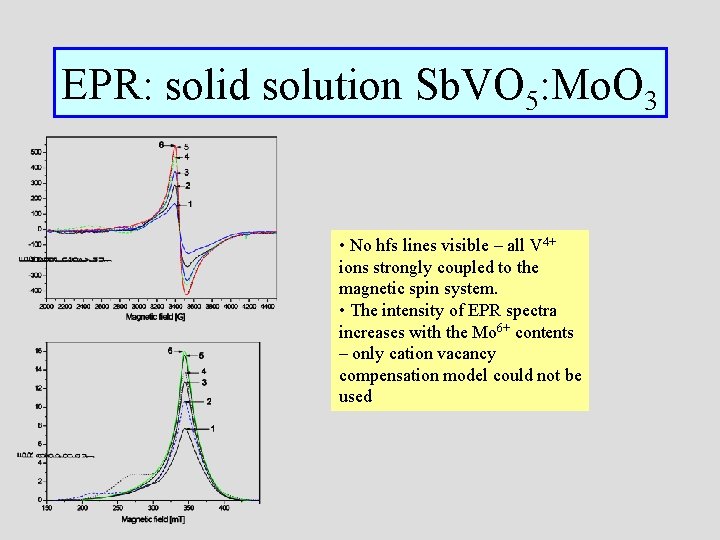
EPR: solid solution Sb. VO 5: Mo. O 3 • No hfs lines visible – all V 4+ ions strongly coupled to the magnetic spin system. • The intensity of EPR spectra increases with the Mo 6+ contents – only cation vacancy compensation model could not be used

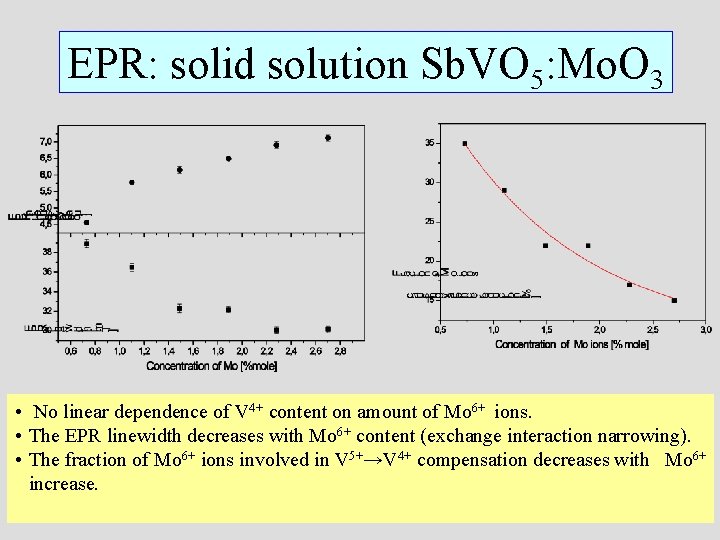
EPR: solid solution Sb. VO 5: Mo. O 3 • No linear dependence of V 4+ content on amount of Mo 6+ ions. • The EPR linewidth decreases with Mo 6+ content (exchange interaction narrowing). • The fraction of Mo 6+ ions involved in V 5+→V 4+ compensation decreases with Mo 6+ increase.

Solid solution: possible centres Possible paramagnetic Mo 6+-V 4+ centres involving one V 4+ ion

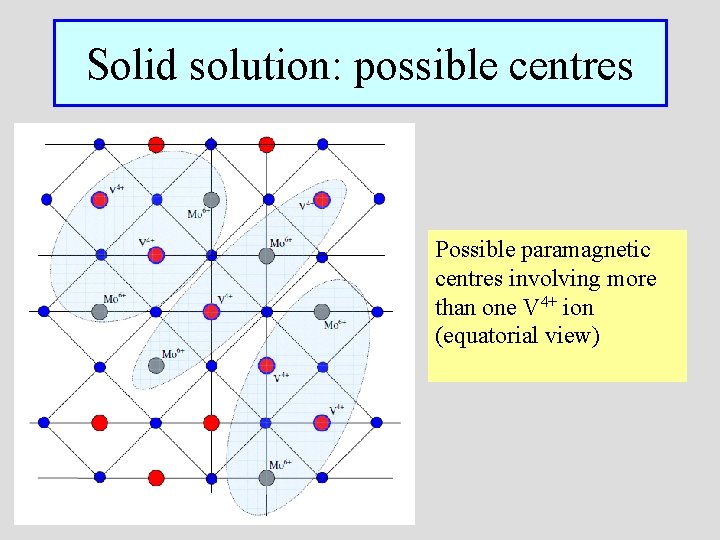
Solid solution: possible centres Possible paramagnetic centres involving more than one V 4+ ion (equatorial view)

Conclusions • At least one third of Mo 6+ ions are involved in charge compensation through changing the oxidation state of cations • Compensation mechanism through cation vacancy is more efficient for larger concentrations of Mo 6+ ions • V 4+ ions are strongly coupled to the rest of spin system – no distant charge compensation