ELECTRON SPIN RESONANCE It is also called electron
















- Slides: 33

ELECTRON SPIN RESONANCE

It is also called electron paramagnetic resonance (EPR) or electron magnetic resonance (EMR). Is a branch of absorption spectroscopy in which radiation of microwave frequency is absorbed by paramagnetic substances. It was invented by Zavoisky in 1944. Definition

ESR is concerned with magnetic behaviour of spinning electrons. ESR spectrum is obtained by transition from one spin state to other state of an electron, transition is induced by radiation of microwave frequency. ESR uses reagents called electron spin reagents, which are stable containing odd electrons have the capacity to react with aminoacids. ESR of compounds give information about structure, viscosity, polarity, phase transformation and chemical reactivity.

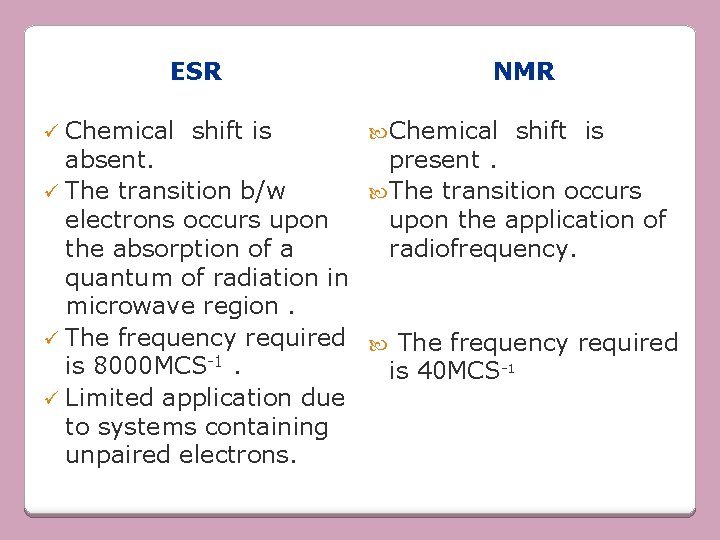
ESR ü Chemical shift is NMR Chemical shift is absent. present. ü The transition b/w The transition occurs electrons occurs upon the application of the absorption of a radiofrequency. quantum of radiation in microwave region. ü The frequency required is 8000 MCS-1. is 40 MCS-1 ü Limited application due to systems containing unpaired electrons.

The energy levels are produced by the interaction of the magnetic moment of an unpaired electron in a molecule ion with an applied magnetic field. v. ESR spectrum is due to transition b/w these energy levels by absorbing radiations of microwave frequency. v THEORY

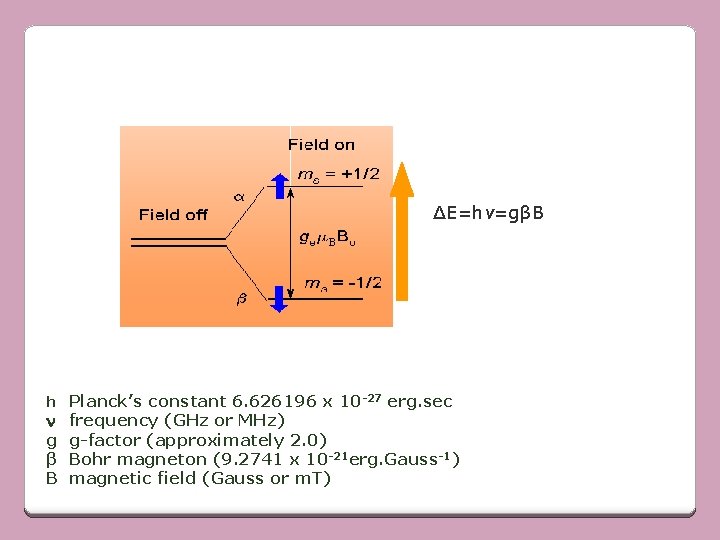
Like a proton, an electron has a spin, which gives it a magnetic property known as a magnetic moment. When an external magnetic field is supplied, the paramagnetic electrons can either orient in a direction parallel or antiparallel to the direction of the magnetic field This creates two distinct energy levels for the unpaired electrons.



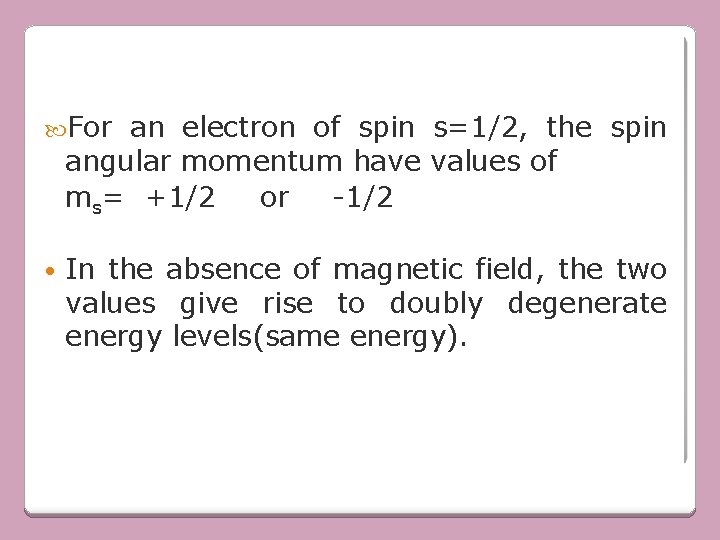
For an electron of spin s=1/2, the spin angular momentum have values of ms= +1/2 or -1/2 • In the absence of magnetic field, the two values give rise to doubly degenerate energy levels(same energy).

If a magnetic field is applied, this leads to two non-degenerate energy levels. The low energy state will have the spin magnetic moment alligned with the field (ms= -1/2), and high energy state - opposite to field (ms= +1/2).



• These two states possess energies split up from original state with no applied magnetic field by +µe H (low energy state) & -µe. H (high energy state) Where, H – magnetic field µe – magnetic moment In ESR, transition b/w different energy levels take place by absorbing quantum of radiaton of ferquency in microwave region

∆E=hv=gβB h Planck’s constant 6. 626196 x 10 -27 erg. sec n g β B frequency (GHz or MHz) g-factor (approximately 2. 0) Bohr magneton (9. 2741 x 10 -21 erg. Gauss-1) magnetic field (Gauss or m. T)

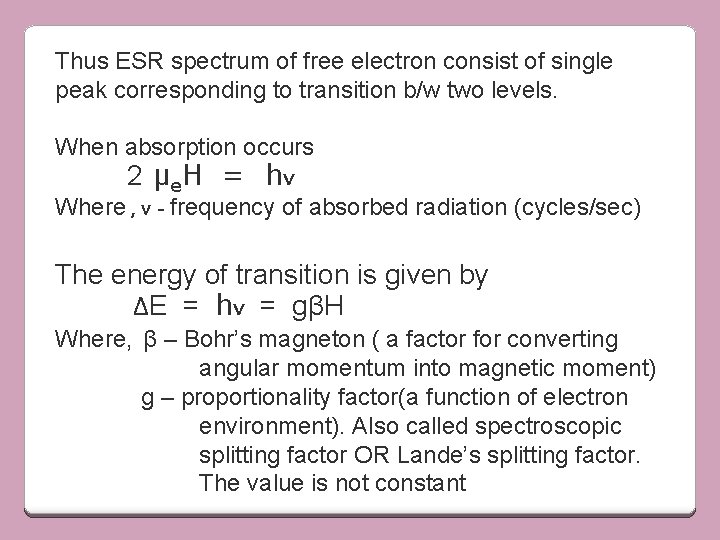
Thus ESR spectrum of free electron consist of single peak corresponding to transition b/w two levels. When absorption occurs 2 µe. H = hv Where , v - frequency of absorbed radiation (cycles/sec) The energy of transition is given by ΔE = hv = gβH Where, β – Bohr’s magneton ( a factor for converting angular momentum into magnetic moment) g – proportionality factor(a function of electron environment). Also called spectroscopic splitting factor OR Lande’s splitting factor. The value is not constant

g is proportionality factor, function of electron’s enviornment, also called as spectroscopic spliting factor or Lande’s spliting factor g is not constant. For a free electron , value is 2. 0023. In free radicals, value is same(electron character is same).

ọ In some crystals, g vary from 0. 2 to 0. 8. The reason is that unpaired electron is localised in a paricular orbital about the atom and the orbital angular momentum couples with spin angular momentum give rise to a low value of g in ionic crystals. ọ g value depend upon the orientation of the molecule having the unpaired electron w. r. t applied magnetic field. ọ In a crystal the value of g along x, y, z axes denoted as gx, gy, gz.

INSTRUMENTATION

Most EPR spectrometers are similar to the original instrumentation used for NMR before the development of pulsed Fourier Transform spectroscopy. Most also use electromagnets rather than permanent magnets or cryomagnets.



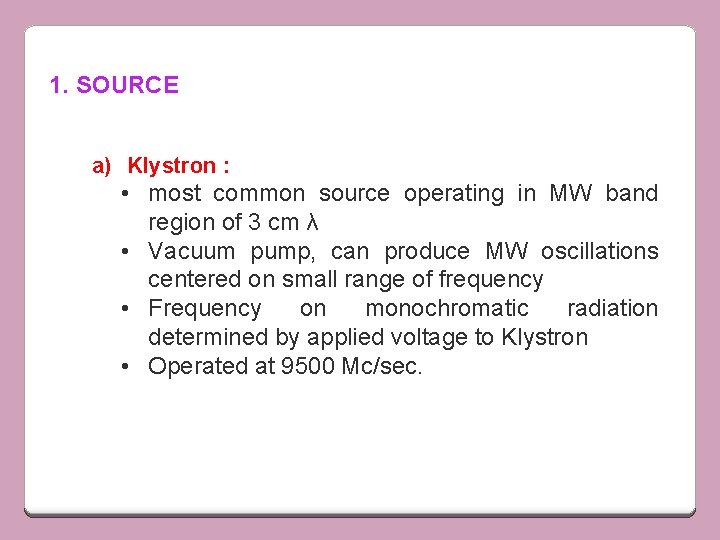
1. SOURCE a) Klystron : • most common source operating in MW band region of 3 cm λ • Vacuum pump, can produce MW oscillations centered on small range of frequency • Frequency on monochromatic radiation determined by applied voltage to Klystron • Operated at 9500 Mc/sec.

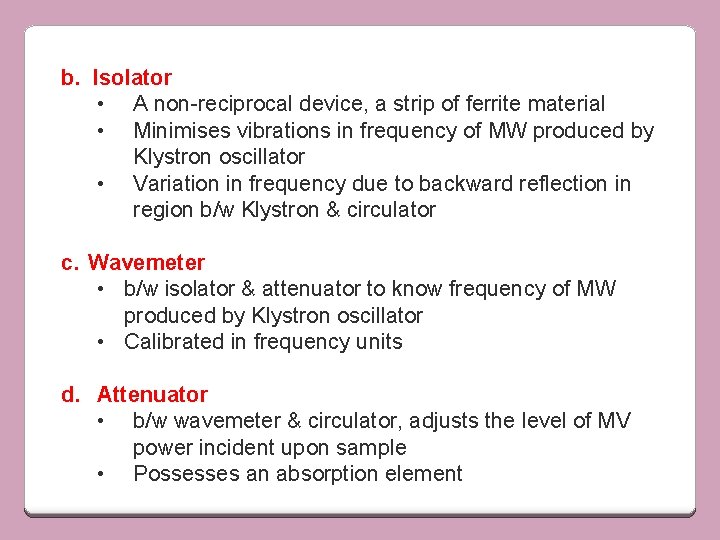
b. Isolator • A non-reciprocal device, a strip of ferrite material • Minimises vibrations in frequency of MW produced by Klystron oscillator • Variation in frequency due to backward reflection in region b/w Klystron & circulator c. Wavemeter • b/w isolator & attenuator to know frequency of MW produced by Klystron oscillator • Calibrated in frequency units d. Attenuator • b/w wavemeter & circulator, adjusts the level of MV power incident upon sample • Possesses an absorption element

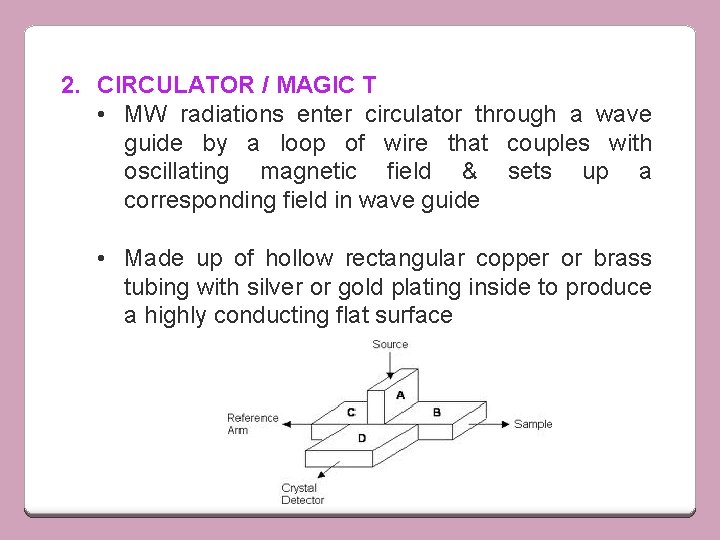
2. CIRCULATOR / MAGIC T • MW radiations enter circulator through a wave guide by a loop of wire that couples with oscillating magnetic field & sets up a corresponding field in wave guide • Made up of hollow rectangular copper or brass tubing with silver or gold plating inside to produce a highly conducting flat surface

3. SAMPLE CAVITY • Cavity system is constructed in such a way as to maximise applied magnetic field along the sample dimension • 0. 15 – 0. 5 ml sample can be used (less DEC) • Flat cells (0. 25 mm thickness) used for samples (high DEC) – 0. 05 ml sample • Most ESR spectrometers use dual sample cavities for sample and reference

4. MAGNET SYSTEM • A static magnetic field is provided by an electromagnet with a current- regulated power supply. • A homogeneous and stable field is required for best results. • The resonant cavity is placed b/w pole pieces of electromagnet • A Hall probe, driven from a stable constant-current power system, with a digital multimeter (DMM) reading the Hall voltage, is used to measure the value of the magnetic field between the poles of the magnet

5. CRYSTAL DETECTORS Silicon crystal is most commonly used detector Act as a microwave rectifier Convert MW power into direct current output 6. AMPLIFIER AND PHASE SENSITIVE DETECTOR The signal from crystal detector undergo narrow-band amplification by auto amplifier Noise present in the amplified signal is removed by phase sensitive detector 7. OSCILLOSCOPE AND PEN RECORDER

• • • WORKING Sample is kept in resonant cavity The cavity is a long path-length cell in which wavas are reflected to & fro thousand of times Klystron oscillator produce MW that pass through isolator, wave meter and attenuator to reach circulator through arm 1 MW power divide b/w arms 2 & 3 (sample cavity) Arm 2 have balancing resistance • When total resistance on 2 & 3 are same, MW power is absorbed completely • When R on arm 3 changes due to some ESR resonance absorption by the sample, then some MW power enter into detector through arm 4

• detector convert the MW power into DC • If the magnetic field around the sample is changed to the value required for resonance, absorption takes place by the sample • The current is recorded by the recorder and show an absorbance peak • If main magnetic field is swept slowly for several minutes, recorder shows the derivative of the absorption spectrum • generally absorption spectra are recorded as first derivative spectra


PRESENTATION OF ESR SPECTRUM • ESR spectrum is obtained by plotting intensity against the strength of magnetic field • ESR spectrum is better represented by derivative curve • First derivative (slope) of absorption curve is plotted against strength of magnetic field • Each negative slope in derv. curve represents a peak or shoulder in absorption spectrum • Every crossing of the derv. axis with a negative slope indicates a true maximum and crossing with positive slope indicates minimum • Thus no. of peaks/shoulders in absorption curve can be determined from no. of minima or maxima in the derivative curve.

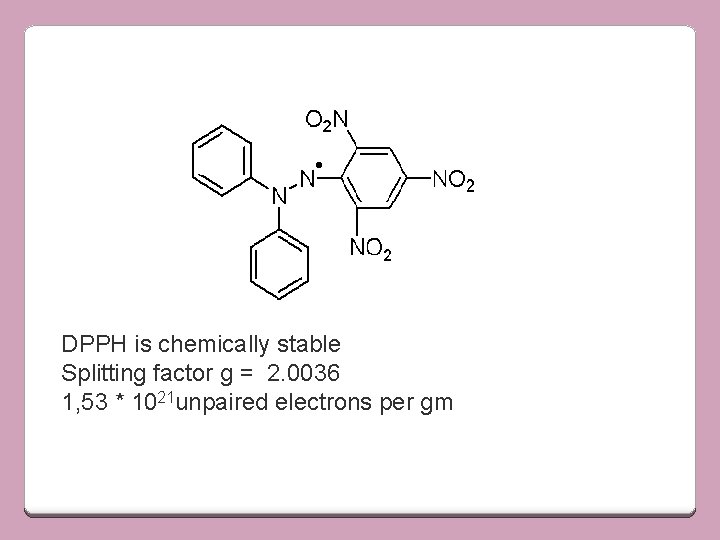
• Area covered by either the absorption or derivative curve is proportional to no of unpaired electrons in the sample • To calculate no of electrons in the sample, comparison is made with standard sample having known no of unpaired electrons and possessing the same line shape • Most widely used std is 1, 1 -diphenyl-picrylhydrazyl (DPPH) free radical

DPPH is chemically stable Splitting factor g = 2. 0036 1, 53 * 1021 unpaired electrons per gm