Chemical Reactions Periodicity In the next sections periodicity

- Slides: 27

Chemical Reactions & Periodicity In the next sections periodicity will be applied to the chemical reactions of hydrogen, oxygen, and their compounds. 1

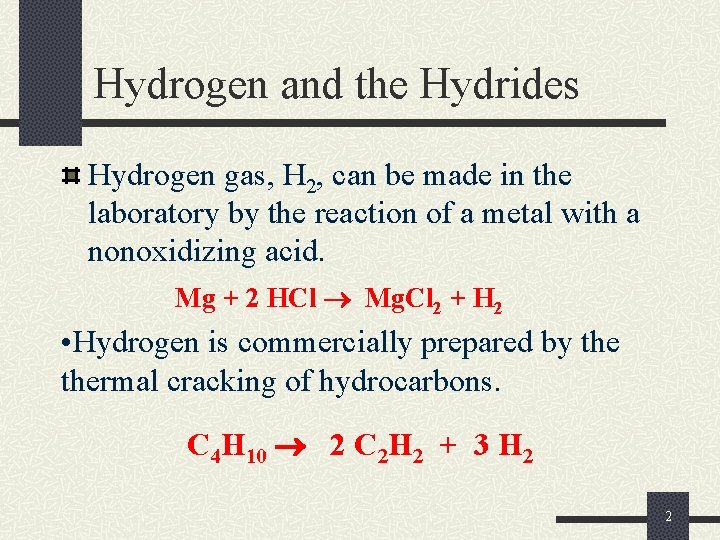
Hydrogen and the Hydrides Hydrogen gas, H 2, can be made in the laboratory by the reaction of a metal with a nonoxidizing acid. Mg + 2 HCl Mg. Cl 2 + H 2 • Hydrogen is commercially prepared by thermal cracking of hydrocarbons. C 4 H 10 2 C 2 H 2 + 3 H 2 2

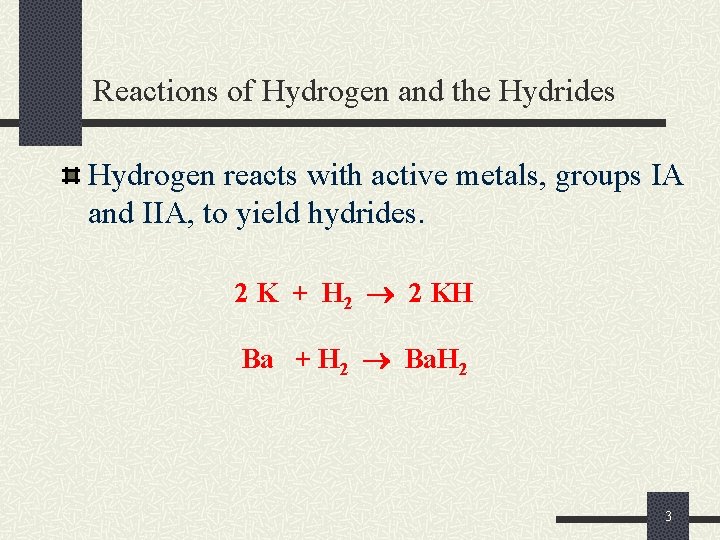
Reactions of Hydrogen and the Hydrides Hydrogen reacts with active metals, groups IA and IIA, to yield hydrides. 2 K + H 2 2 KH Ba + H 2 Ba. H 2 3

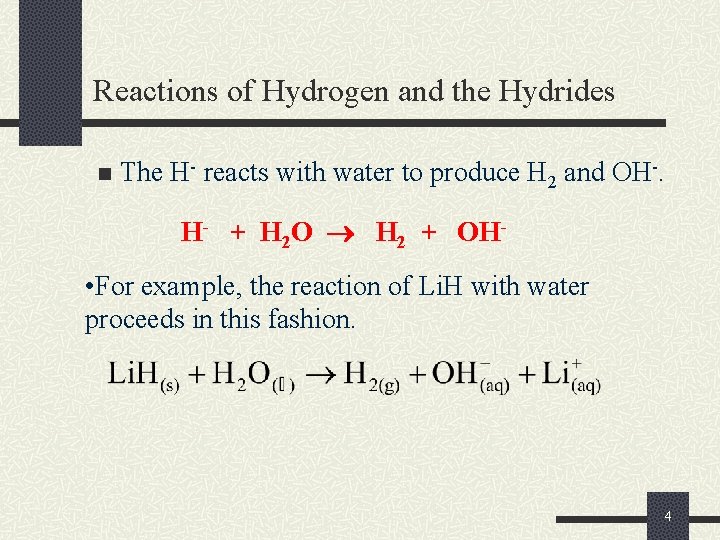
Reactions of Hydrogen and the Hydrides n The H- reacts with water to produce H 2 and OH-. H- + H 2 O H 2 + OH- • For example, the reaction of Li. H with water proceeds in this fashion. 4

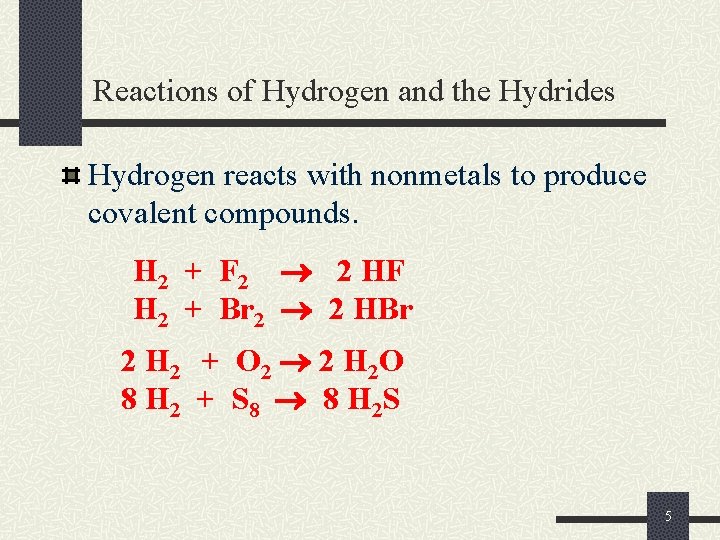
Reactions of Hydrogen and the Hydrides Hydrogen reacts with nonmetals to produce covalent compounds. H 2 + F 2 2 HF H 2 + Br 2 2 HBr 2 H 2 + O 2 2 H 2 O 8 H 2 + S 8 8 H 2 S 5

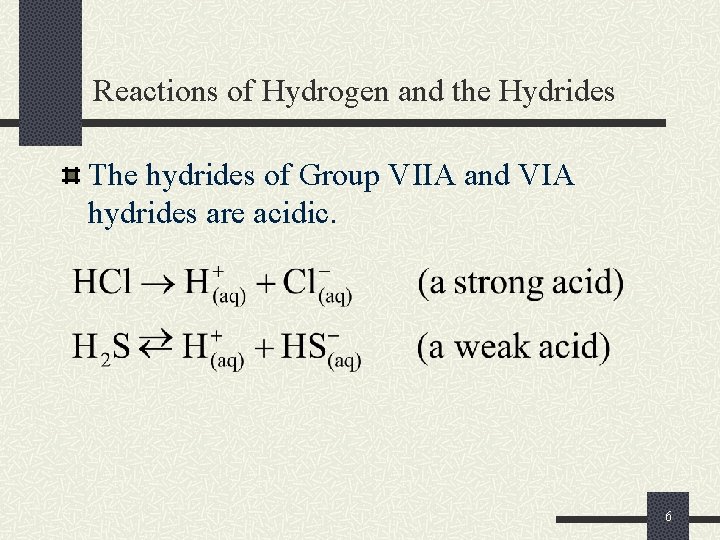
Reactions of Hydrogen and the Hydrides The hydrides of Group VIIA and VIA hydrides are acidic. 6

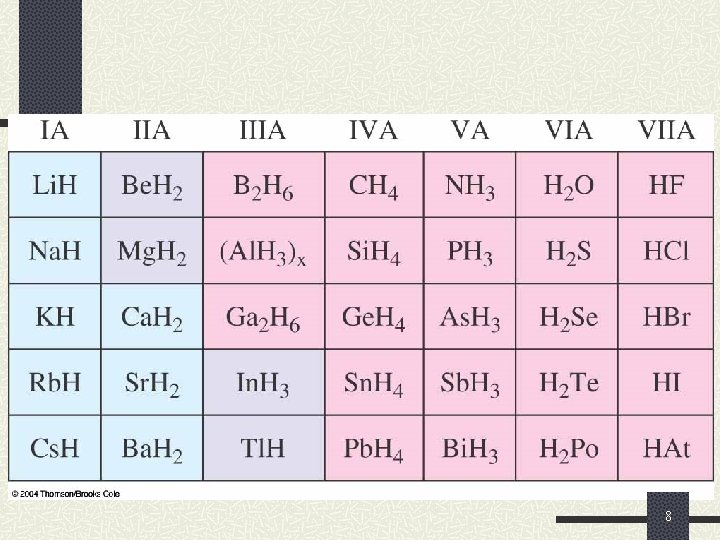
Reactions of Hydrogen and the Hydrides There is an important periodic trend evident in the ionic or covalent character of hydrides. 1. Metal hydrides are ionic compounds and form basic aqueous solutions. 2. Nonmetal hydrides are covalent compounds and form acidic aqueous solutions. 7

8

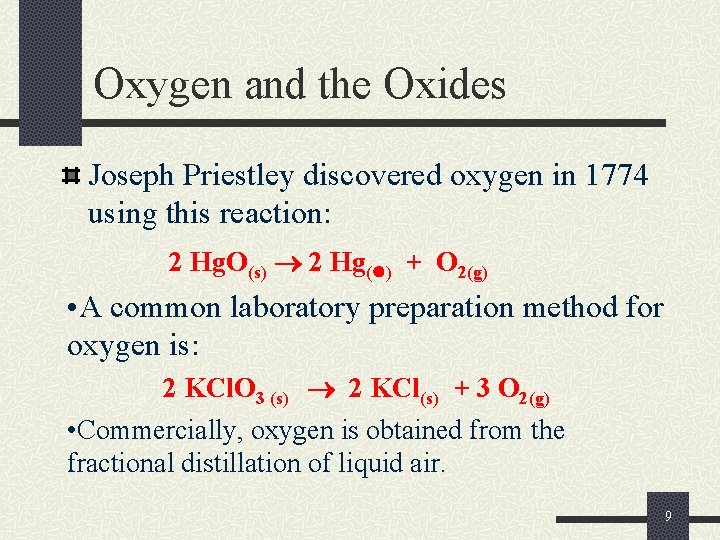
Oxygen and the Oxides Joseph Priestley discovered oxygen in 1774 using this reaction: 2 Hg. O(s) 2 Hg( ) + O 2(g) • A common laboratory preparation method for oxygen is: 2 KCl. O 3 (s) 2 KCl(s) + 3 O 2(g) • Commercially, oxygen is obtained from the fractional distillation of liquid air. 9

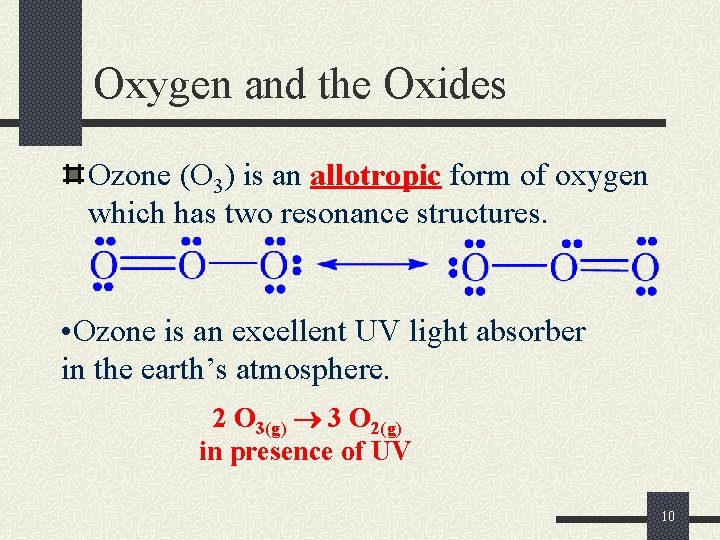
Oxygen and the Oxides Ozone (O 3) is an allotropic form of oxygen which has two resonance structures. • Ozone is an excellent UV light absorber in the earth’s atmosphere. 2 O 3(g) 3 O 2(g) in presence of UV 10

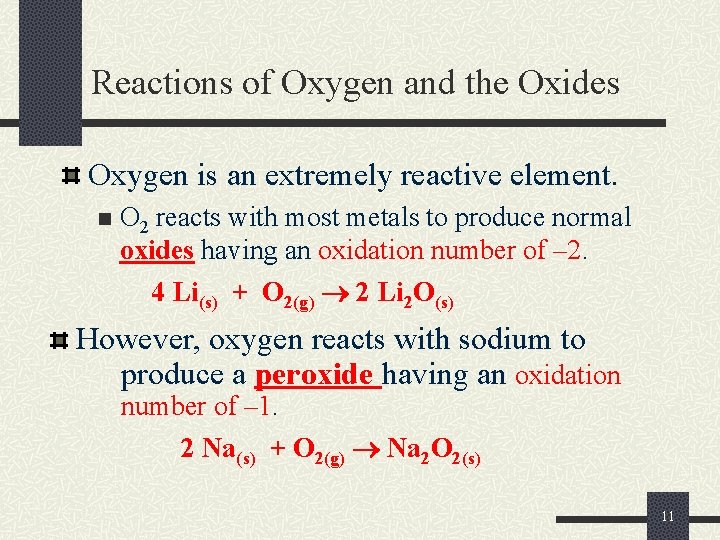
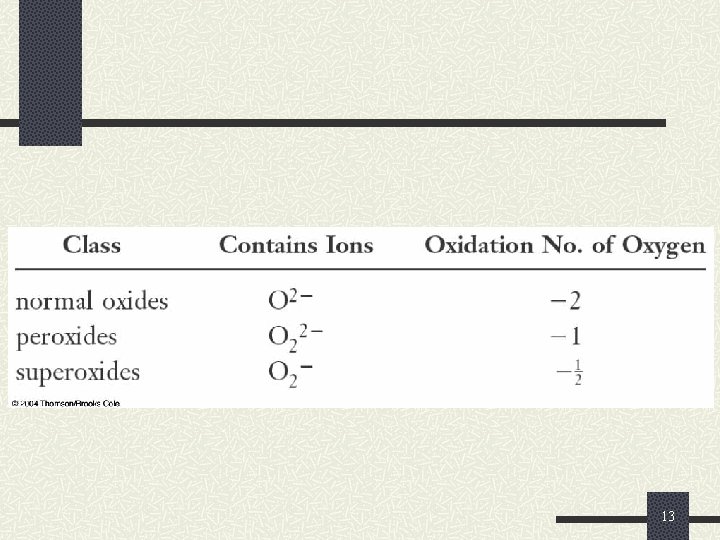
Reactions of Oxygen and the Oxides Oxygen is an extremely reactive element. n O 2 reacts with most metals to produce normal oxides having an oxidation number of – 2. 4 Li(s) + O 2(g) 2 Li 2 O(s) However, oxygen reacts with sodium to produce a peroxide having an oxidation number of – 1. 2 Na(s) + O 2(g) Na 2 O 2(s) 11

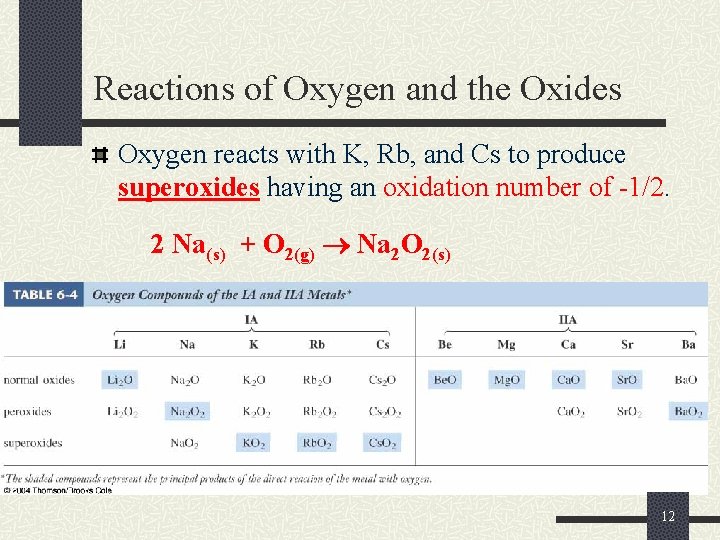
Reactions of Oxygen and the Oxides Oxygen reacts with K, Rb, and Cs to produce superoxides having an oxidation number of -1/2. 2 Na(s) + O 2(g) Na 2 O 2(s) 12

13

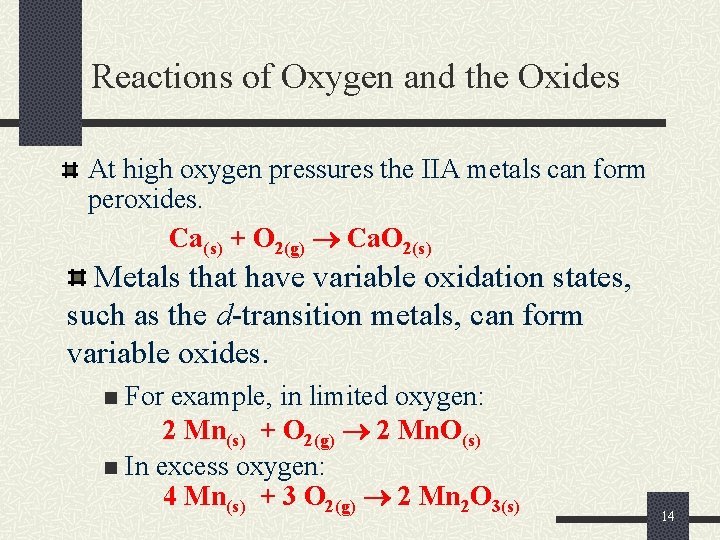
Reactions of Oxygen and the Oxides At high oxygen pressures the IIA metals can form peroxides. Ca(s) + O 2(g) Ca. O 2(s) Metals that have variable oxidation states, such as the d-transition metals, can form variable oxides. For example, in limited oxygen: 2 Mn(s) + O 2(g) 2 Mn. O(s) n In excess oxygen: 4 Mn(s) + 3 O 2(g) 2 Mn 2 O 3(s) n 14

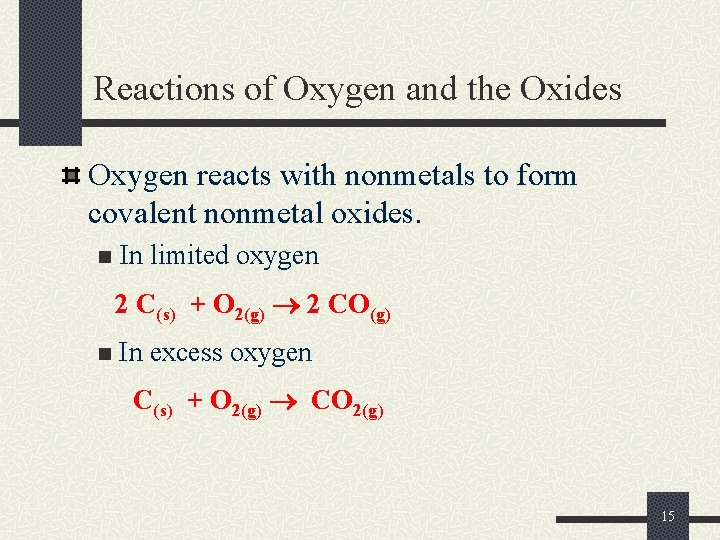
Reactions of Oxygen and the Oxides Oxygen reacts with nonmetals to form covalent nonmetal oxides. n In limited oxygen 2 C(s) + O 2(g) 2 CO(g) n In excess oxygen C(s) + O 2(g) CO 2(g) 15

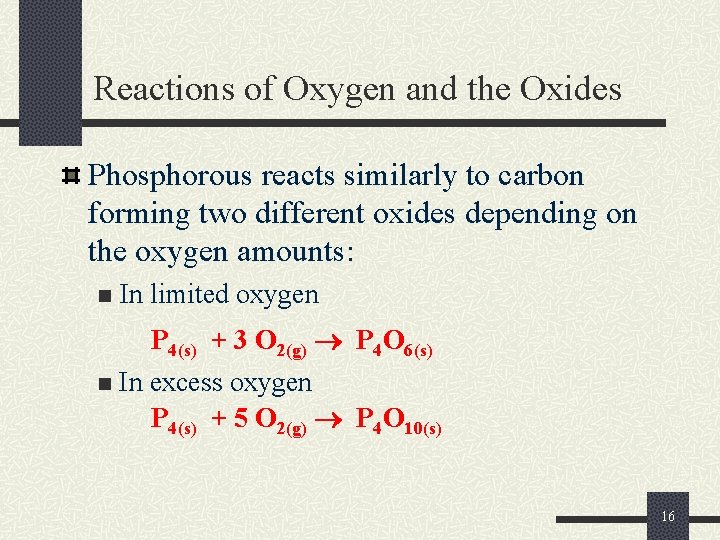
Reactions of Oxygen and the Oxides Phosphorous reacts similarly to carbon forming two different oxides depending on the oxygen amounts: n In limited oxygen P 4(s) + 3 O 2(g) P 4 O 6(s) n In excess oxygen P 4(s) + 5 O 2(g) P 4 O 10(s) 16

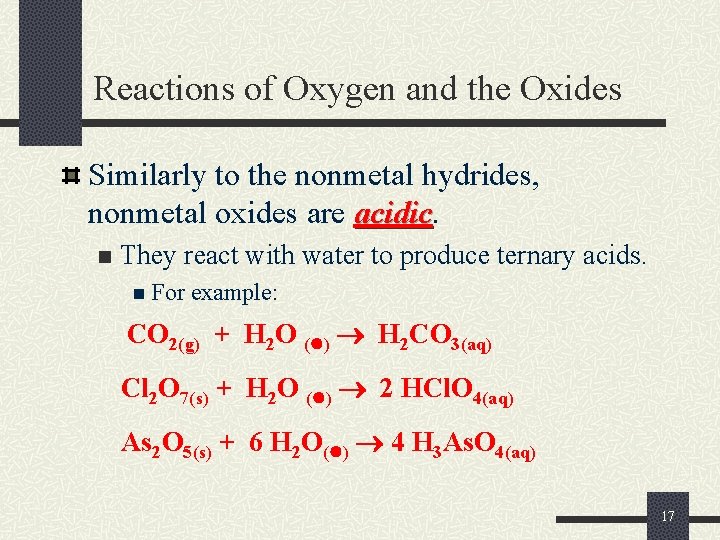
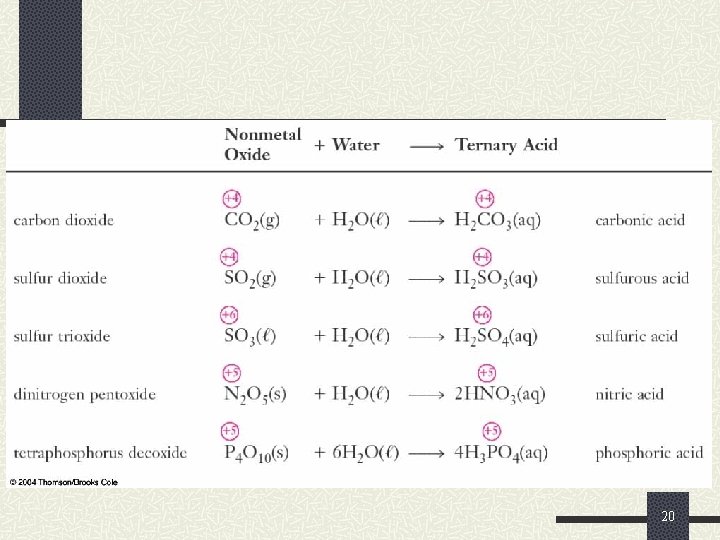
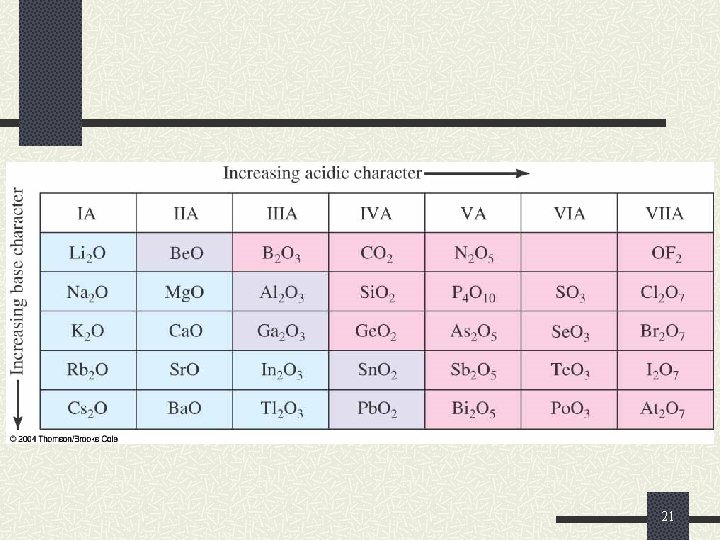
Reactions of Oxygen and the Oxides Similarly to the nonmetal hydrides, nonmetal oxides are acidic n They react with water to produce ternary acids. n For example: CO 2(g) + H 2 O ( ) H 2 CO 3(aq) Cl 2 O 7(s) + H 2 O ( ) 2 HCl. O 4(aq) As 2 O 5(s) + 6 H 2 O( ) 4 H 3 As. O 4(aq) 17

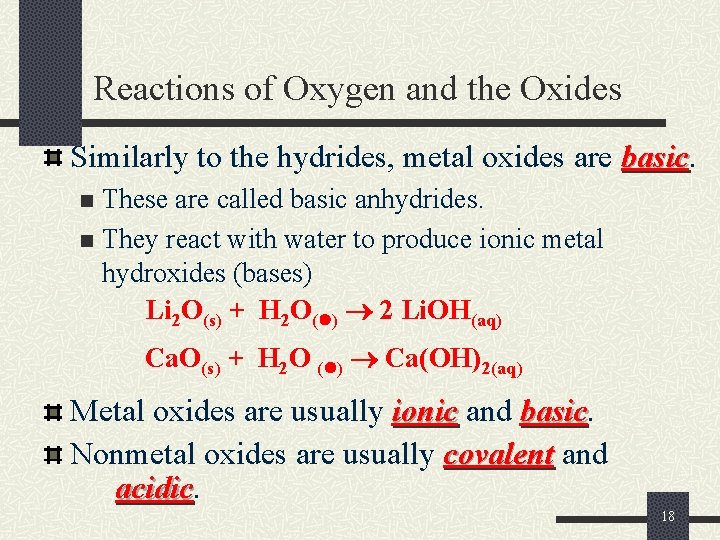
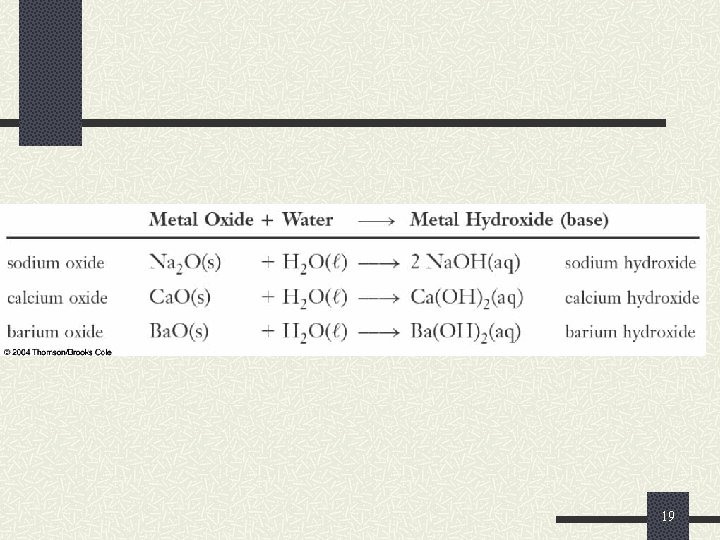
Reactions of Oxygen and the Oxides Similarly to the hydrides, metal oxides are basic These are called basic anhydrides. n They react with water to produce ionic metal hydroxides (bases) Li 2 O(s) + H 2 O( ) 2 Li. OH(aq) n Ca. O(s) + H 2 O ( ) Ca(OH)2(aq) Metal oxides are usually ionic and basic Nonmetal oxides are usually covalent and acidic 18

19

20

21

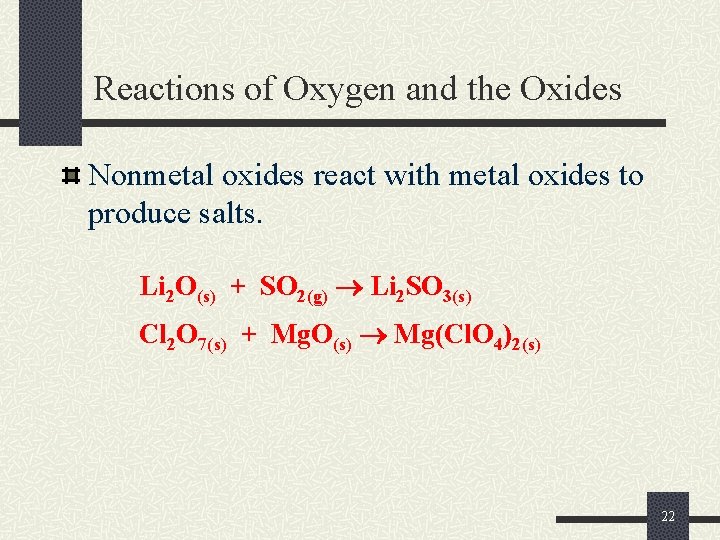
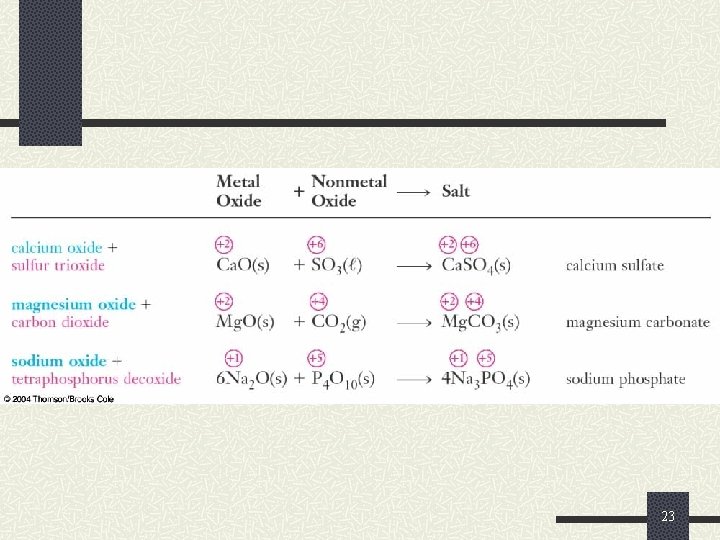
Reactions of Oxygen and the Oxides Nonmetal oxides react with metal oxides to produce salts. Li 2 O(s) + SO 2(g) Li 2 SO 3(s) Cl 2 O 7(s) + Mg. O(s) Mg(Cl. O 4)2(s) 22

23

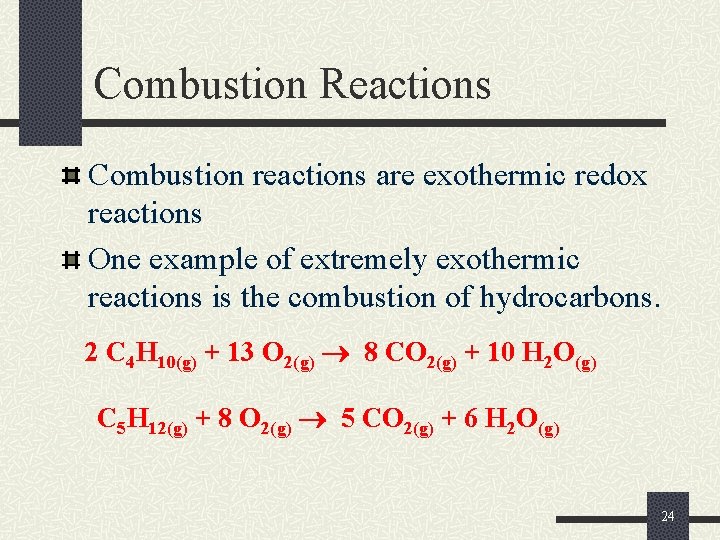
Combustion Reactions Combustion reactions are exothermic redox reactions One example of extremely exothermic reactions is the combustion of hydrocarbons. 2 C 4 H 10(g) + 13 O 2(g) 8 CO 2(g) + 10 H 2 O(g) C 5 H 12(g) + 8 O 2(g) 5 CO 2(g) + 6 H 2 O(g) 24

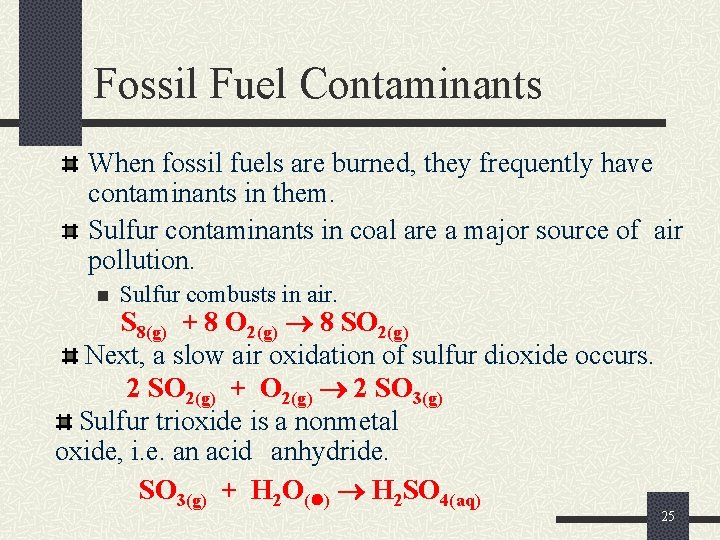
Fossil Fuel Contaminants When fossil fuels are burned, they frequently have contaminants in them. Sulfur contaminants in coal are a major source of air pollution. n Sulfur combusts in air. S 8(g) + 8 O 2(g) 8 SO 2(g) Next, a slow air oxidation of sulfur dioxide occurs. 2 SO 2(g) + O 2(g) 2 SO 3(g) Sulfur trioxide is a nonmetal oxide, i. e. an acid anhydride. SO 3(g) + H 2 O( ) H 2 SO 4(aq) 25

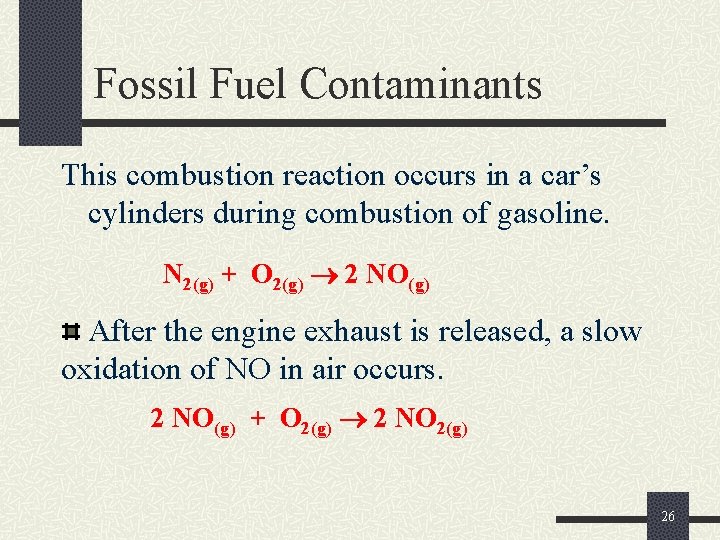
Fossil Fuel Contaminants This combustion reaction occurs in a car’s cylinders during combustion of gasoline. N 2(g) + O 2(g) 2 NO(g) After the engine exhaust is released, a slow oxidation of NO in air occurs. 2 NO(g) + O 2(g) 2 NO 2(g) 26

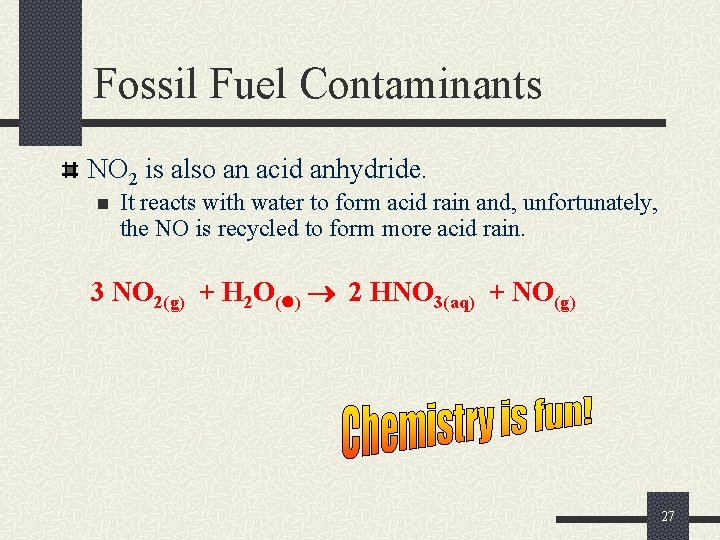
Fossil Fuel Contaminants NO 2 is also an acid anhydride. n It reacts with water to form acid rain and, unfortunately, the NO is recycled to form more acid rain. 3 NO 2(g) + H 2 O( ) 2 HNO 3(aq) + NO(g) 27
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions X.next = x.next.next
X.next = x.next.next Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Electronic configuration of mo (z=42)
Electronic configuration of mo (z=42) Are kc and kp equal
Are kc and kp equal Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Balancing redox reactions
Balancing redox reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Wuchereria bancrofti
Wuchereria bancrofti Texas health steps quick reference guide
Texas health steps quick reference guide Aap bright futures periodicity schedule
Aap bright futures periodicity schedule Ap chemistry atomic structure and periodicity
Ap chemistry atomic structure and periodicity Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity Ikan euryphagic
Ikan euryphagic Oxygen periodic trends
Oxygen periodic trends Chemsheets periodicity
Chemsheets periodicity Ionization energy on periodic table
Ionization energy on periodic table What is periodicity?
What is periodicity? Electron configuration and periodicity
Electron configuration and periodicity Mass relationships in chemical reactions
Mass relationships in chemical reactions Types of reaction
Types of reaction Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Solvent in chemical reactions
Solvent in chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Non organic solvents
Non organic solvents Chemical reactions in soil
Chemical reactions in soil Describing chemical reactions
Describing chemical reactions