Chapter 8 Atomic Electron Configurations and Chemical Periodicity





![Atomic Electron Configurations 4 th row elements… The [Ar] 4 s 1 3 d Atomic Electron Configurations 4 th row elements… The [Ar] 4 s 1 3 d](https://slidetodoc.com/presentation_image_h2/9477a9038cd0915ee34466c73975a23a/image-23.jpg)
![Atomic Electron Configurations 4 th row elements… The [Ar] 4 s 1 3 d Atomic Electron Configurations 4 th row elements… The [Ar] 4 s 1 3 d](https://slidetodoc.com/presentation_image_h2/9477a9038cd0915ee34466c73975a23a/image-24.jpg)
![Atomic Electron Configurations Lanthanides (4 f) 2 Ba [Xe] 6 s 56 1 6 Atomic Electron Configurations Lanthanides (4 f) 2 Ba [Xe] 6 s 56 1 6](https://slidetodoc.com/presentation_image_h2/9477a9038cd0915ee34466c73975a23a/image-27.jpg)








- Slides: 71

Chapter 8 Atomic Electron Configurations and Chemical Periodicity

Chapter goals • Understanding the role magnetism plays in determining and revealing atomic structure. • Understand effective nuclear charge and its role in determining atomic properties. • Write the electron configuration of neutral atoms and monatomic ions. • Understand the fundamental physical properties of the elements and their periodic trends.

Electron Spin and the Fourth Quantum Number • The fourth quantum number is the spin quantum number which has the symbol ms. • The spin quantum number only has two possible values. ms = +1/2 or − 1/2 ms = ± 1/2 • This quantum number tells us the spin and orientation of the magnetic field of the electrons. • Wolfgang Pauli discovered the Exclusion Principle in 1925. No two electrons in an atom can have the same set of 4 quantum numbers, n, l, ml, and ms

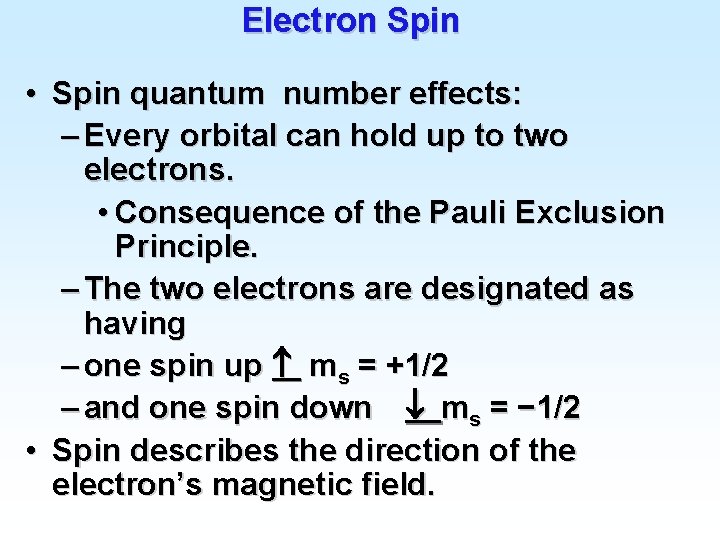
Electron Spin • Spin quantum number effects: – Every orbital can hold up to two electrons. • Consequence of the Pauli Exclusion Principle. – The two electrons are designated as having – one spin up ms = +1/2 – and one spin down ms = − 1/2 • Spin describes the direction of the electron’s magnetic field.

Paramagnetism and Diamagnetism • Unpaired electrons have their spins aligned or (in diff. orbitals) – This increases the magnetic field of the atom. Total spin 0, because they add up. • Atoms with unpaired electrons are called paramagnetic. – Paramagnetic atoms are attracted to a magnet.

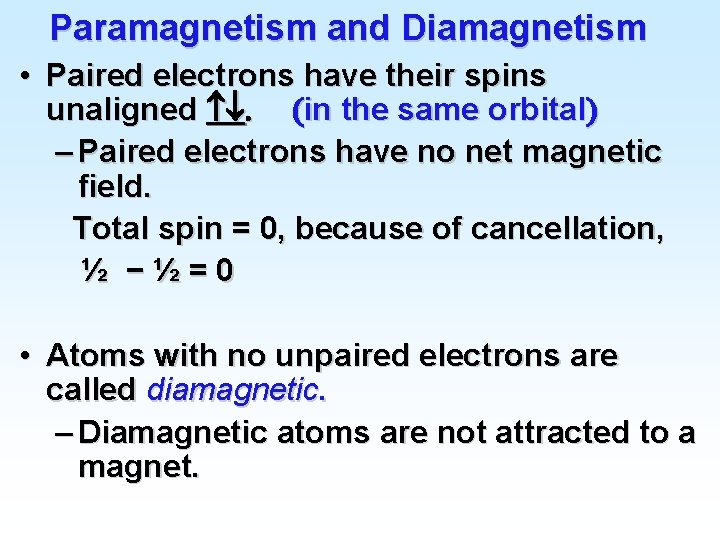
Paramagnetism and Diamagnetism • Paired electrons have their spins unaligned . (in the same orbital) – Paired electrons have no net magnetic field. Total spin = 0, because of cancellation, ½ −½=0 • Atoms with no unpaired electrons are called diamagnetic. – Diamagnetic atoms are not attracted to a magnet.

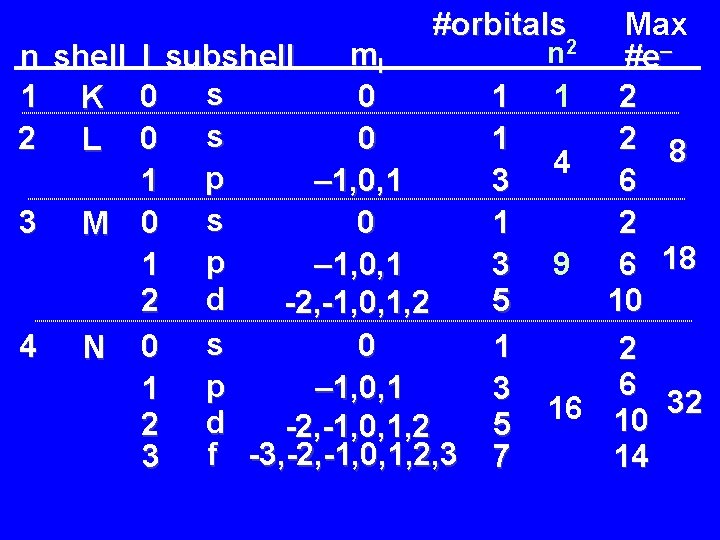
Atomic Orbitals, Spin, and # of Electrons • Because two electrons in the same orbital must be paired (due to Pauli’s Exclusion Principle), it is possible to calculate the number of orbitals and the number of electrons in each n shell. • The number of orbitals per n level is given by n 2 (see table at end of chapter 7. ) • The maximum number of electrons per n level is 2 n 2 (two electrons per orbital. ) – The value is 2 n 2 because of the two paired electrons per orbital.

#orbitals ml n shell l subshell s 0 1 K 0 1 s 0 2 L 0 1 – 1, 0, 1 1 p 3 0 3 M 0 s 1 – 1, 0, 1 1 p 3 2 d 5 -2, -1, 0, 1, 2 0 4 N 0 s 1 – 1, 0, 1 1 p 3 2 d 5 -2, -1, 0, 1, 2 3 f -3, -2, -1, 0, 1, 2, 3 7 Max n 2 #e– 1 2 2 8 4 6 2 6 18 9 10 2 6 16 10 32 14

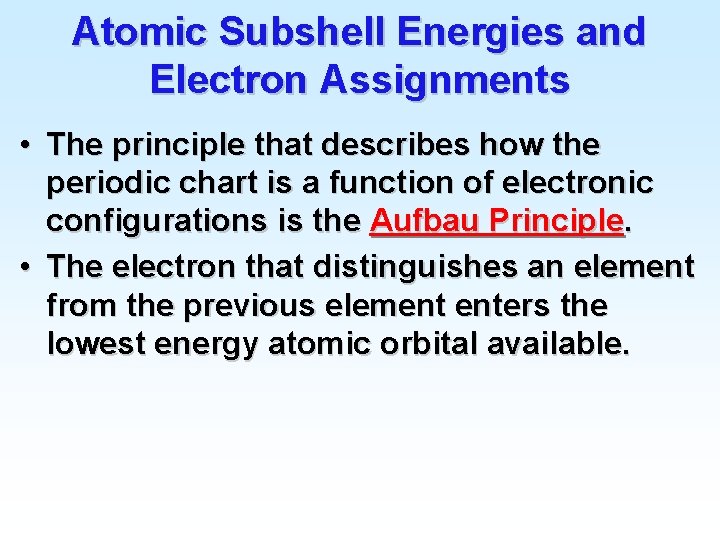
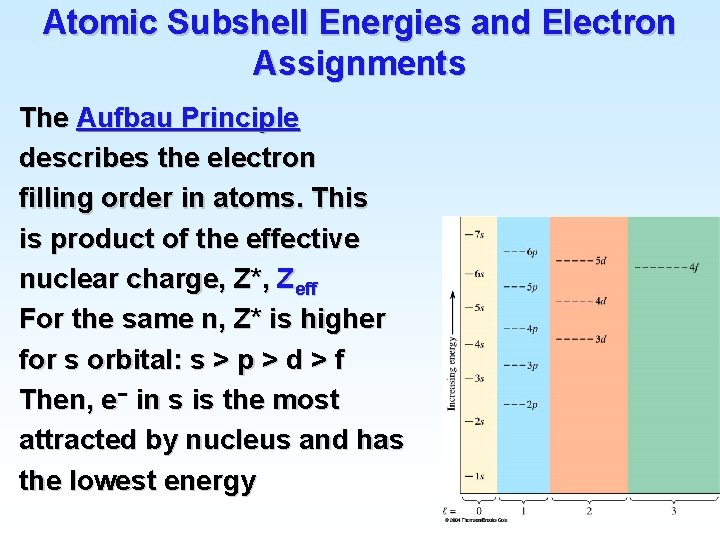
Atomic Subshell Energies and Electron Assignments • The principle that describes how the periodic chart is a function of electronic configurations is the Aufbau Principle. • The electron that distinguishes an element from the previous element enters the lowest energy atomic orbital available.

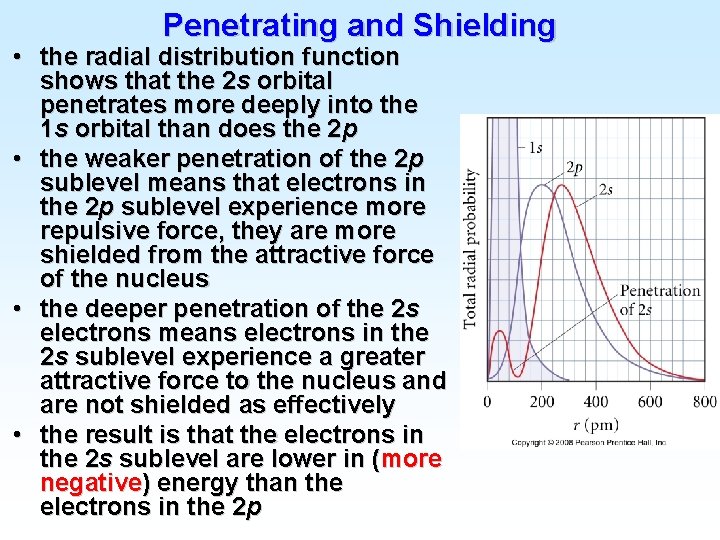
Penetrating and Shielding • the radial distribution function shows that the 2 s orbital penetrates more deeply into the 1 s orbital than does the 2 p • the weaker penetration of the 2 p sublevel means that electrons in the 2 p sublevel experience more repulsive force, they are more shielded from the attractive force of the nucleus • the deeper penetration of the 2 s electrons means electrons in the 2 s sublevel experience a greater attractive force to the nucleus and are not shielded as effectively • the result is that the electrons in the 2 s sublevel are lower in (more negative) energy than the electrons in the 2 p

Atomic Subshell Energies and Electron Assignments The Aufbau Principle describes the electron filling order in atoms. This is product of the effective nuclear charge, Z*, Zeff For the same n, Z* is higher for s orbital: s > p > d > f Then, e− in s is the most attracted by nucleus and has the lowest energy

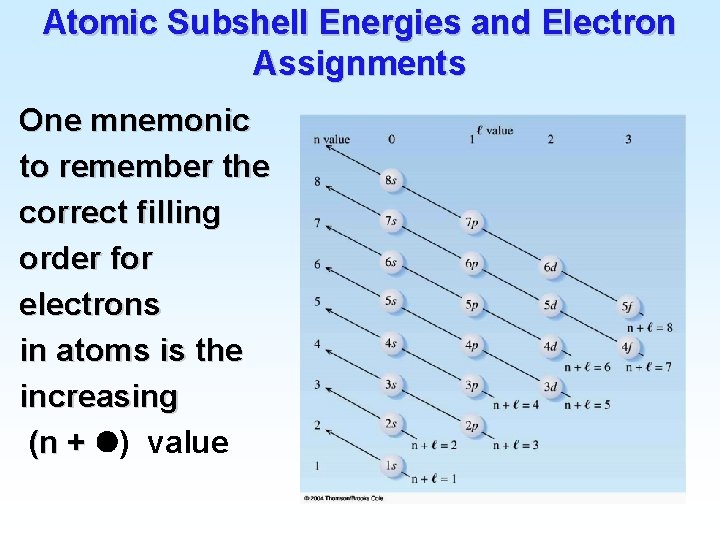
Atomic Subshell Energies and Electron Assignments One mnemonic to remember the correct filling order for electrons in atoms is the increasing (n + ) value

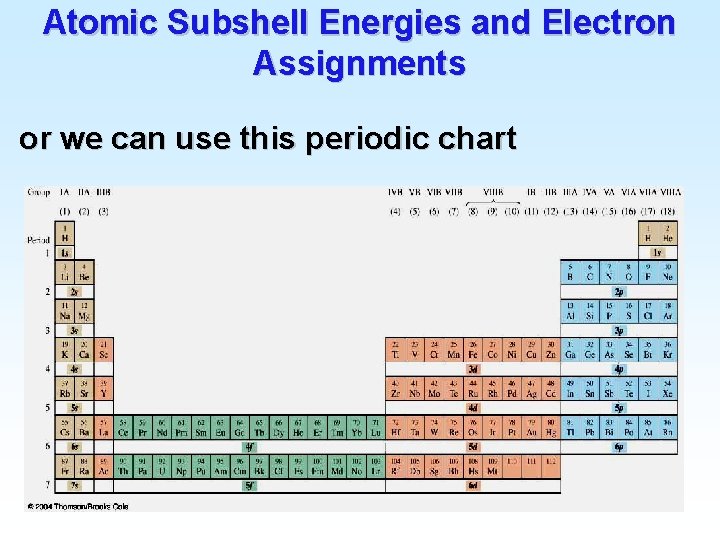
Atomic Subshell Energies and Electron Assignments or we can use this periodic chart

Atomic Electron Configurations • Now we will use the Aufbau Principle to determine the electronic configurations of the elements on the periodic chart. • 1 st row elements

Atomic Electron Configurations Hund’s rule tells us that the electrons will fill the p and d orbitals by placing electrons in each orbital singly and with same spin until half-filled. That is the rule of maximum spin. Then the electrons will pair to finish the p orbitals. Electrons in orbitals of or same kind, such as p or d orbitals, in the same shell (n), have the same energy; the are said to be degenerate.

Atomic Electron Configurations 3 rd row elements…

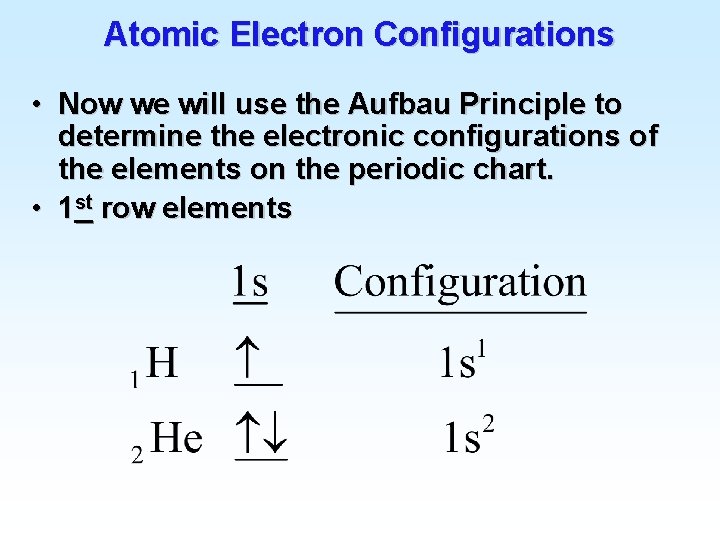
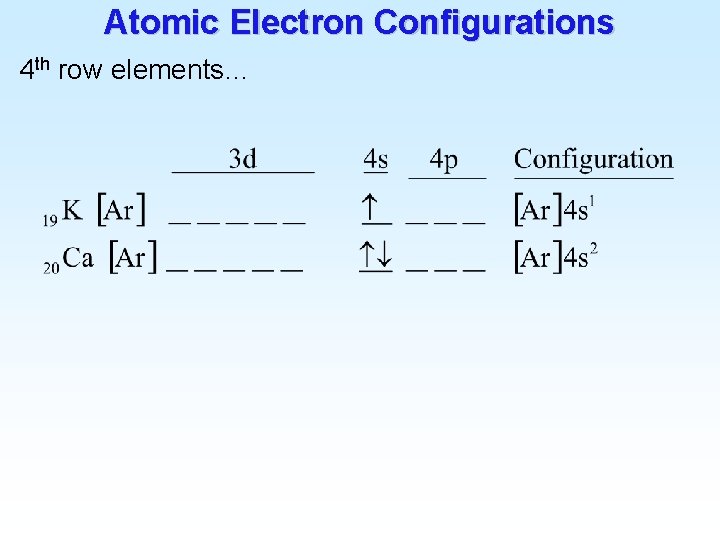
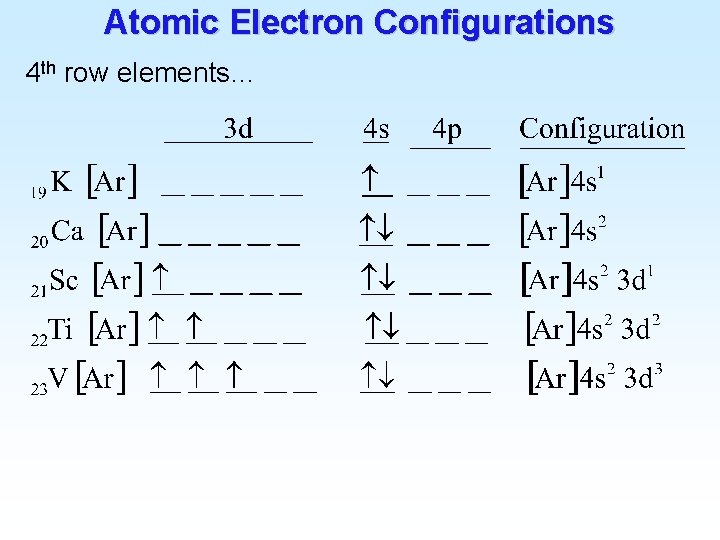
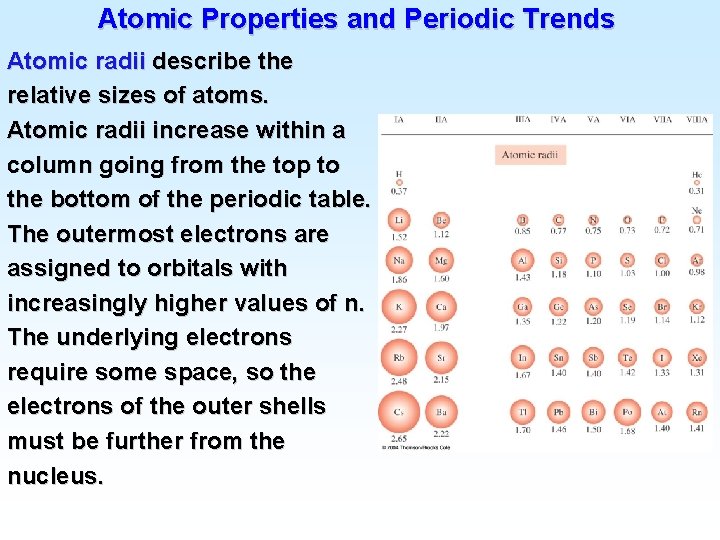
Atomic Electron Configurations 4 th row elements…

Atomic Electron Configurations 4 th row elements…

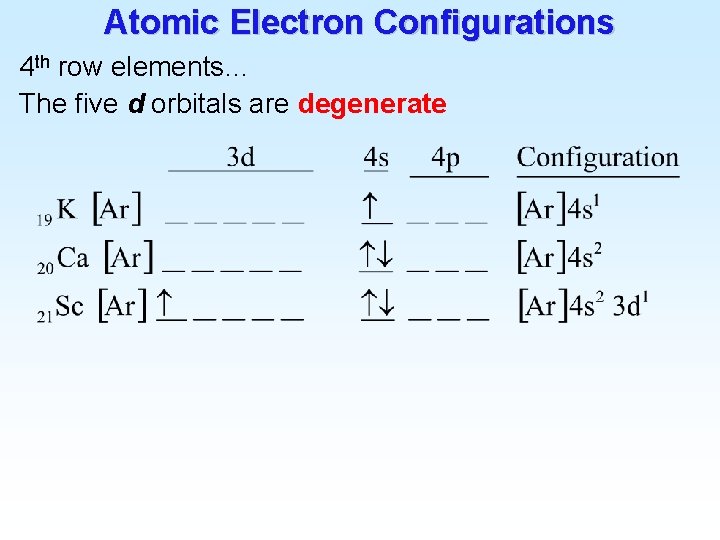
Atomic Electron Configurations 4 th row elements… The five d orbitals are degenerate

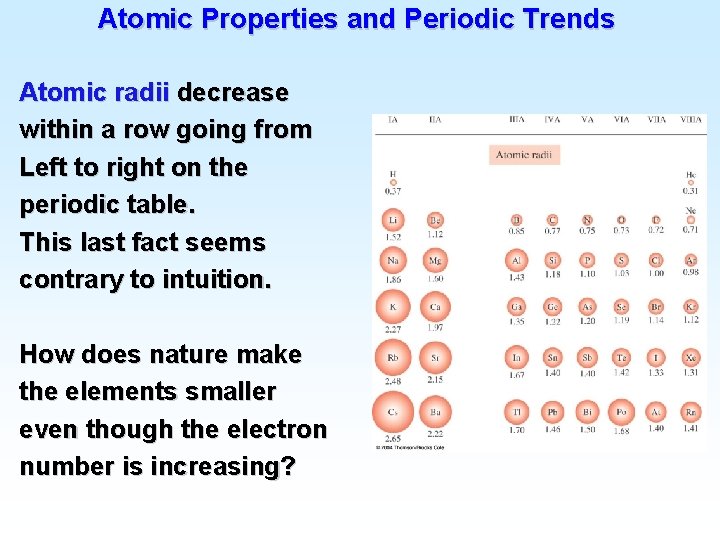
Atomic Electron Configurations 4 th row elements…

Atomic Electron Configurations 4 th row elements… The five d orbitals are degenerate

Atomic Electron Configurations 4 th row elements…
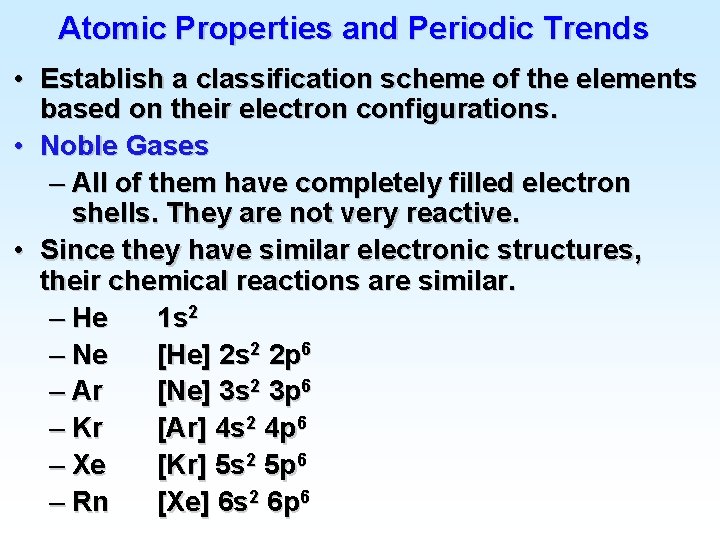
![Atomic Electron Configurations 4 th row elements The Ar 4 s 1 3 d Atomic Electron Configurations 4 th row elements… The [Ar] 4 s 1 3 d](https://slidetodoc.com/presentation_image_h2/9477a9038cd0915ee34466c73975a23a/image-23.jpg)
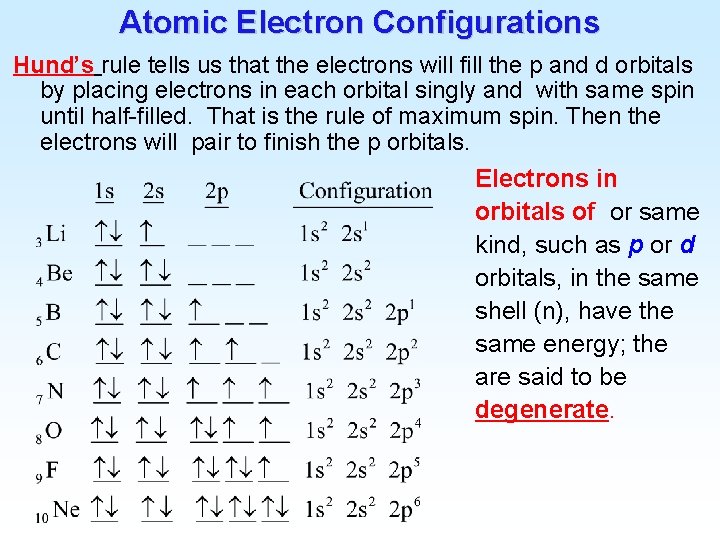
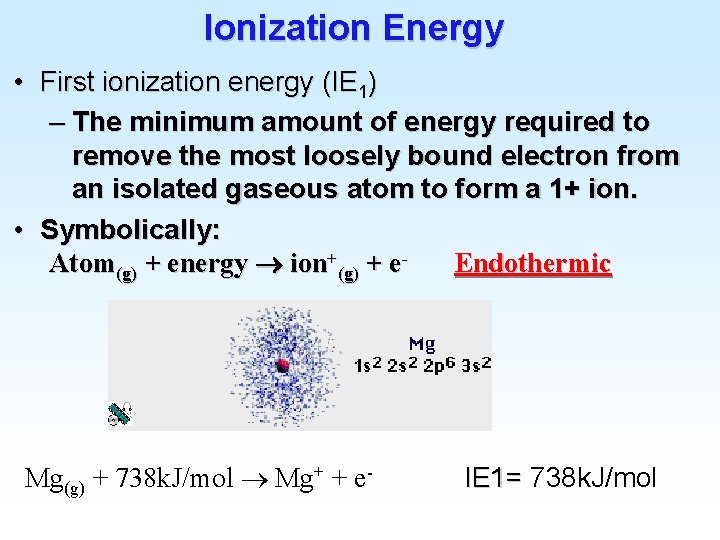
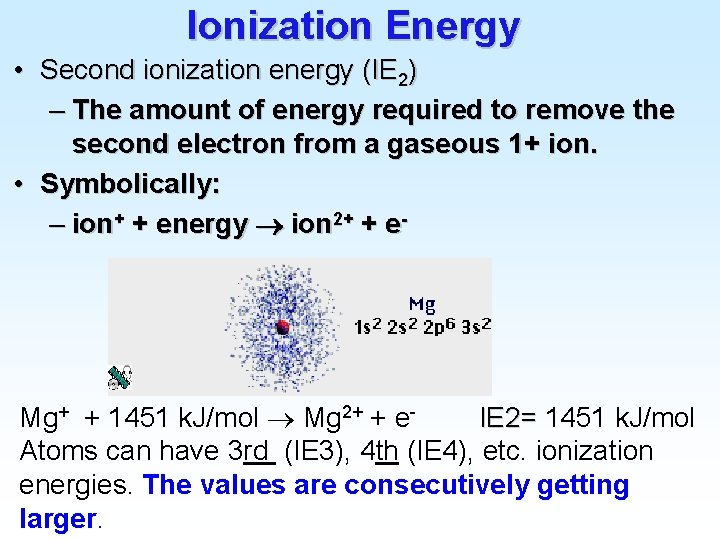
Atomic Electron Configurations 4 th row elements… The [Ar] 4 s 1 3 d 5 configuration of Cr is more stable than [Ar] 4 s 2 3 d 4 (expected)
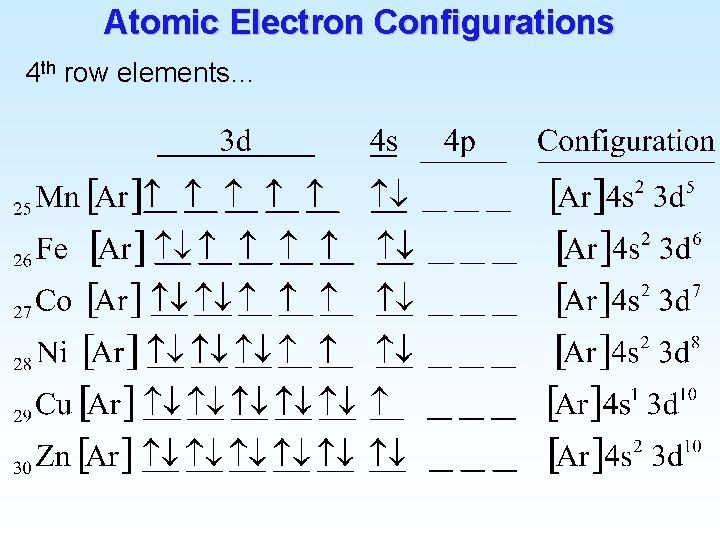
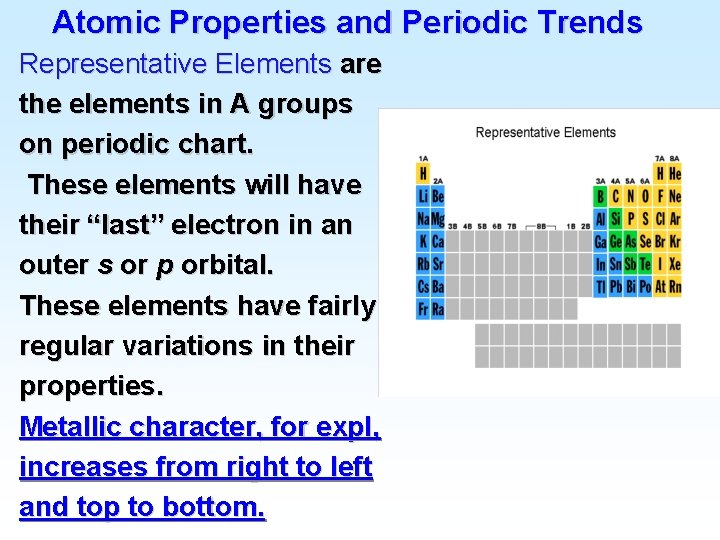
![Atomic Electron Configurations 4 th row elements The Ar 4 s 1 3 d Atomic Electron Configurations 4 th row elements… The [Ar] 4 s 1 3 d](https://slidetodoc.com/presentation_image_h2/9477a9038cd0915ee34466c73975a23a/image-24.jpg)
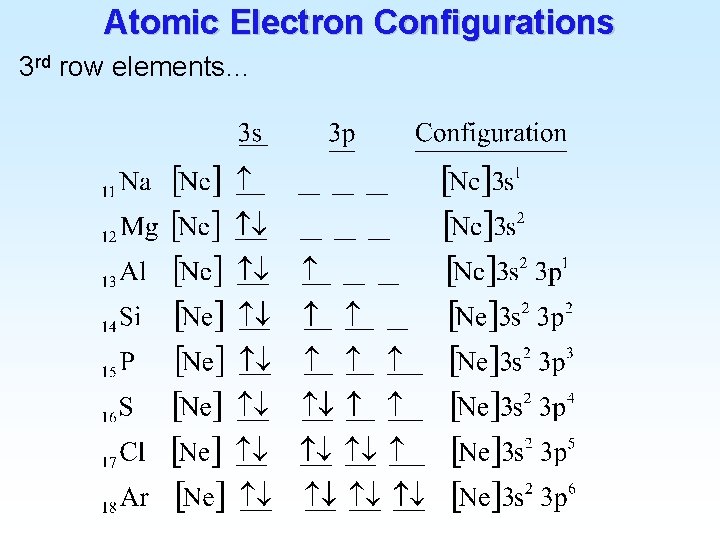
Atomic Electron Configurations 4 th row elements… The [Ar] 4 s 1 3 d 10 full d configuration of Cu is more stable than [Ar] 4 s 2 3 d 9 (expected)

Atomic Electron Configurations 4 th row elements…

Atomic Electron Configurations 4 th row elements… (remember Hund’s rule): __ is better (lower energy) than __ __ 4 p 4 p
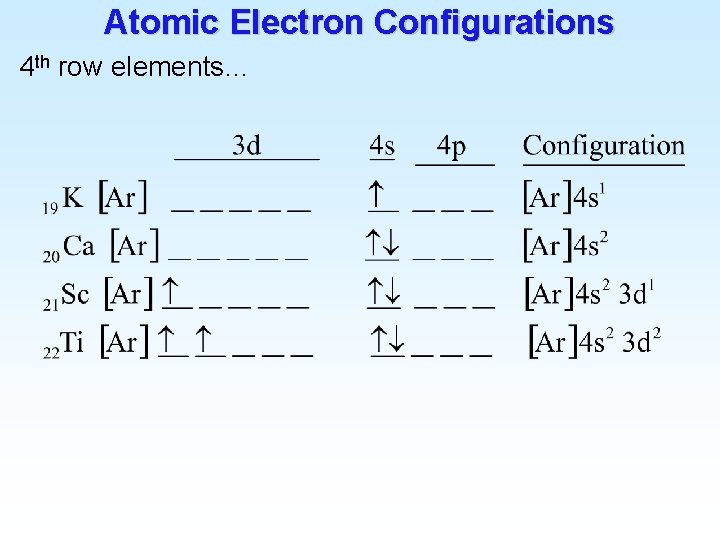
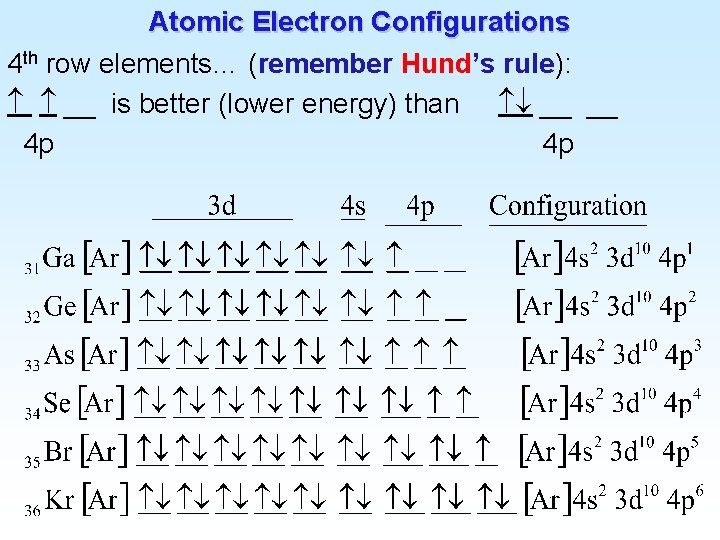
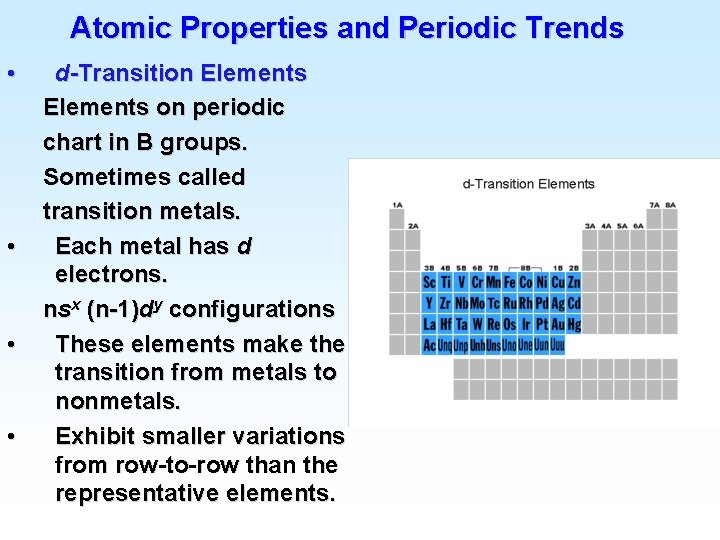
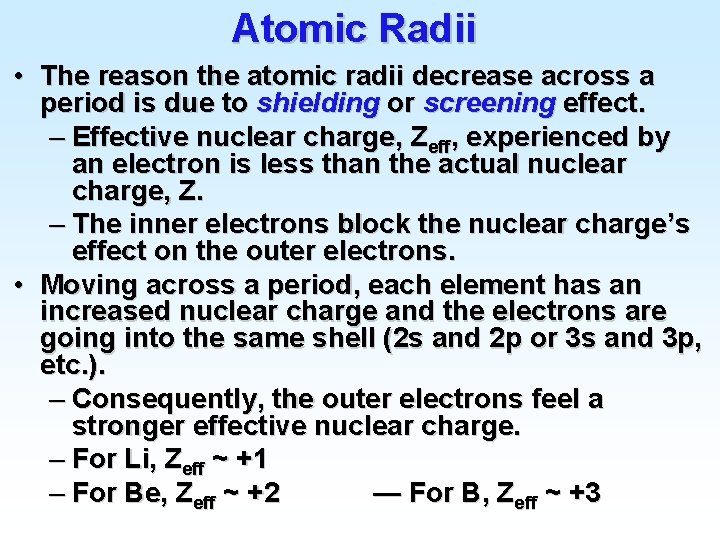
![Atomic Electron Configurations Lanthanides 4 f 2 Ba Xe 6 s 56 1 6 Atomic Electron Configurations Lanthanides (4 f) 2 Ba [Xe] 6 s 56 1 6](https://slidetodoc.com/presentation_image_h2/9477a9038cd0915ee34466c73975a23a/image-27.jpg)
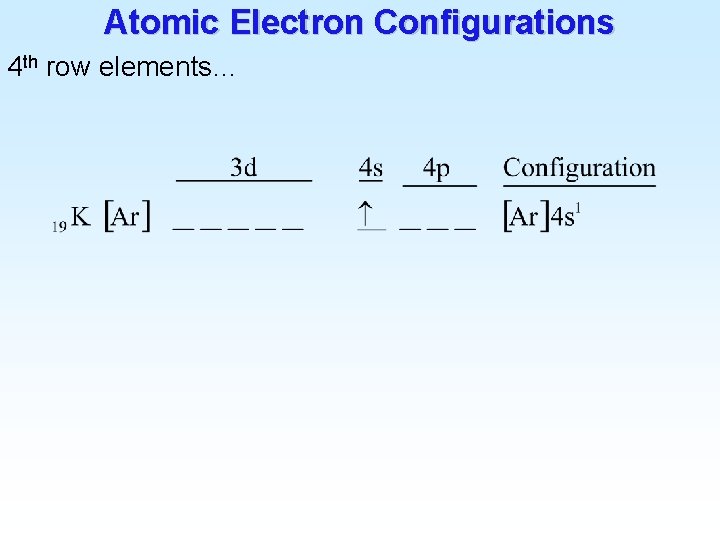
Atomic Electron Configurations Lanthanides (4 f) 2 Ba [Xe] 6 s 56 1 6 s 2 La [Xe] 5 d 57 1 5 d 1 6 s 2 Ce [Xe] 4 f 58 3 6 s 2 Pr [Xe] 4 f 59 Praseodymium 14 6 s 2 Yb [Xe] 4 f 70 Ytterbium 14 5 d 1 6 s 2 Lu [Xe] 4 f 71 Lutetium

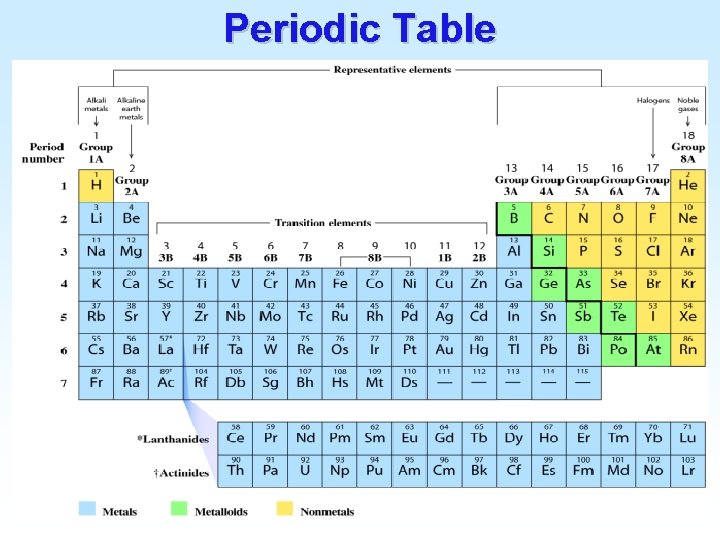
Periodic Table

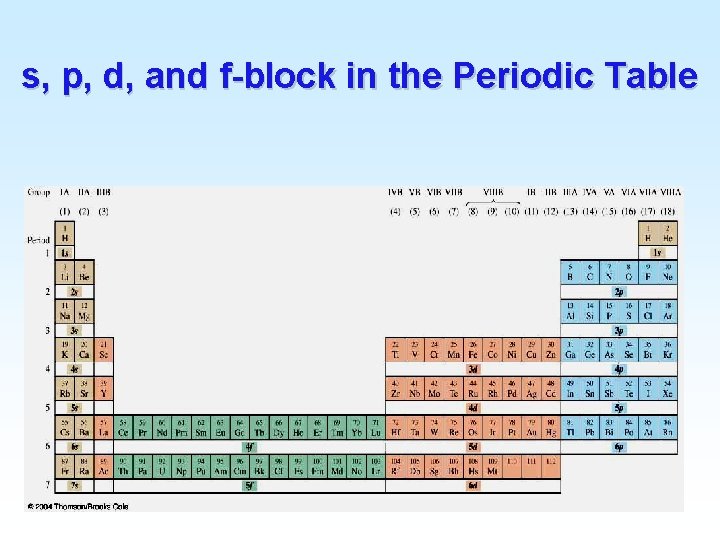
s, p, d, and f-block in the Periodic Table

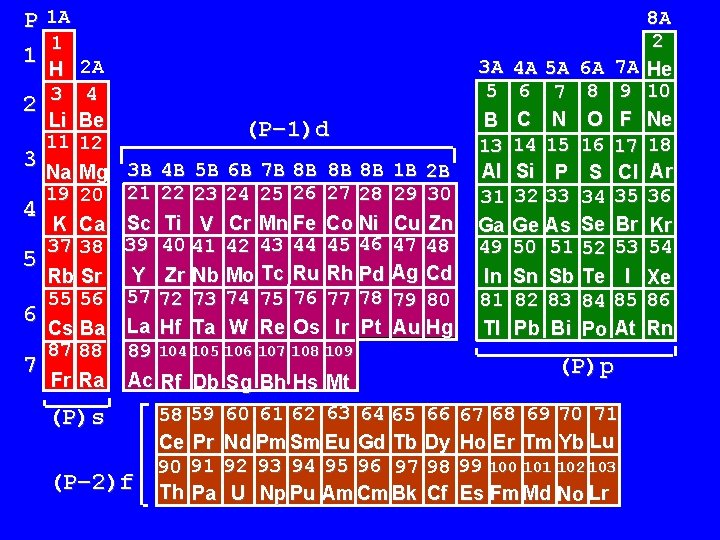
P 1 A 1 1 H 2 A 2 3 4 5 6 7 Li Be 11 12 Na Mg 19 20 K Ca 37 38 Rb Sr 55 56 Cs Ba 87 88 Fr Ra (P– 1)d 3 B 4 B 5 B 6 B 7 B 8 B 8 B 8 B 21 22 23 24 25 26 27 28 Sc Ti V Cr Mn Fe Co Ni 39 40 41 42 43 44 45 46 Y Zr Nb Mo Tc Ru Rh Pd 57 72 73 74 75 76 77 78 La Hf Ta W Re Os Ir Pt 89 104 105 106 107 108 109 Ac Rf Db Sg Bh Hs Mt (P)s (P– 2)f 58 59 Ce Pr 90 91 Th Pa 1 B 2 B 29 30 Cu Zn 47 48 Ag Cd 79 80 Au Hg 60 61 62 63 64 65 66 Nd Pm Sm Eu Gd Tb Dy 92 93 94 95 96 97 98 U Np Pu Am Cm Bk Cf 3 A 5 B 13 Al 31 Ga 49 In 81 Tl 4 A 5 A 6 A 7 A 6 7 8 9 C N O F 14 15 16 17 Si P S Cl 32 33 34 35 Ge As Se Br 50 51 52 53 Sn Sb Te I 82 83 84 85 Pb Bi Po At (P)p 67 68 69 70 71 Ho Er Tm Yb Lu 99 100 101 102 103 Es Fm Md No Lr 8 A 2 He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn

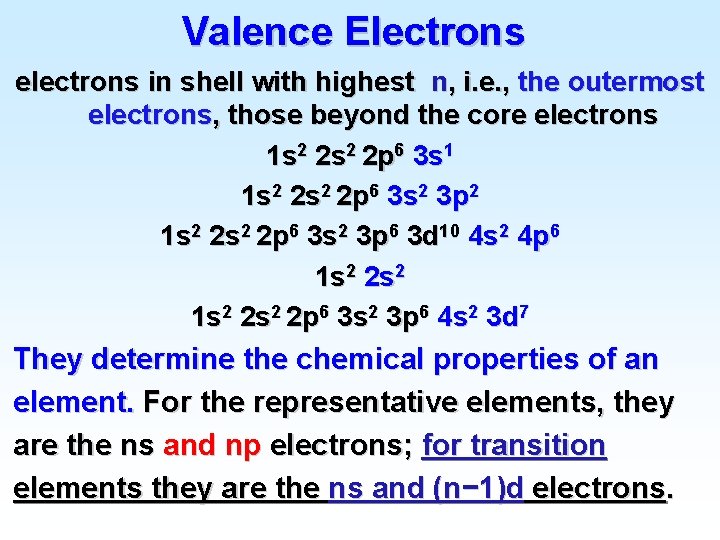
Valence Electrons electrons in shell with highest n, i. e. , the outermost electrons, those beyond the core electrons 1 s 2 2 p 6 3 s 1 1 s 2 2 p 6 3 s 2 3 p 2 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 7 They determine the chemical properties of an element. For the representative elements, they are the ns and np electrons; for transition elements they are the ns and (n− 1)d electrons.

P 1 A 1 1 1 s 1 2 2 s 1 3 4 5 6 7 H 3 Li 11 Na 19 K 37 Rb 55 Cs 87 Fr 3 s 1 4 s 1 5 s 1 6 s 1 7 s 1 # of valence electrons = 1

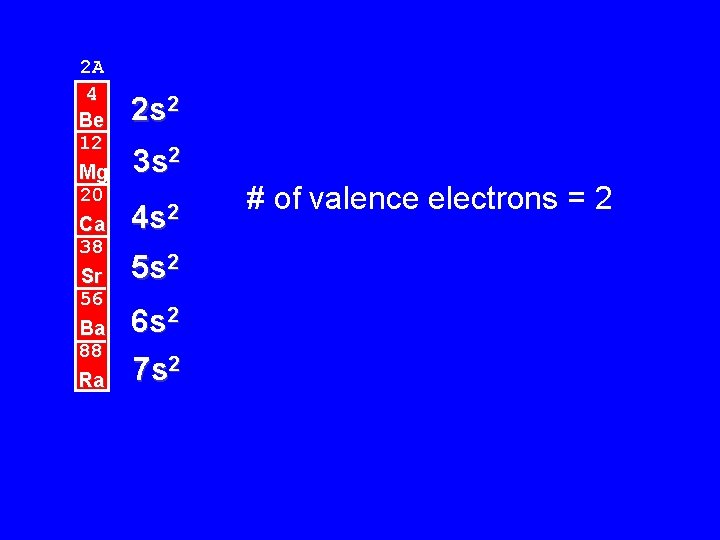
2 A 4 Be 12 Mg 20 Ca 38 Sr 56 Ba 88 Ra 2 s 2 3 s 2 4 s 2 5 s 2 6 s 2 7 s 2 # of valence electrons = 2

3 A 5 B 13 Al 31 Ga 49 In 81 Tl 2 s 2 2 p 1 3 s 2 3 p 1 4 s 2 4 p 1 5 s 2 5 p 1 6 s 2 6 p 1 # of valence electrons = 3

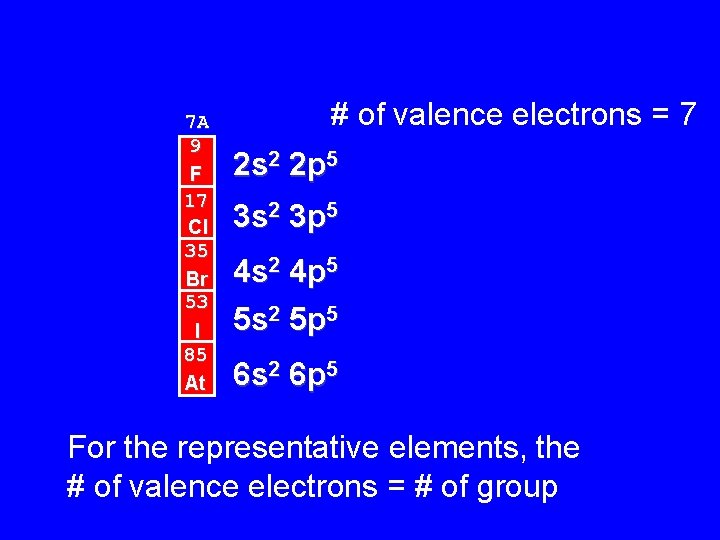
7 A 9 F 17 Cl 35 Br 53 I 85 At # of valence electrons = 7 2 s 2 2 p 5 3 s 2 3 p 5 4 s 2 4 p 5 5 s 2 5 p 5 6 s 2 6 p 5 For the representative elements, the # of valence electrons = # of group

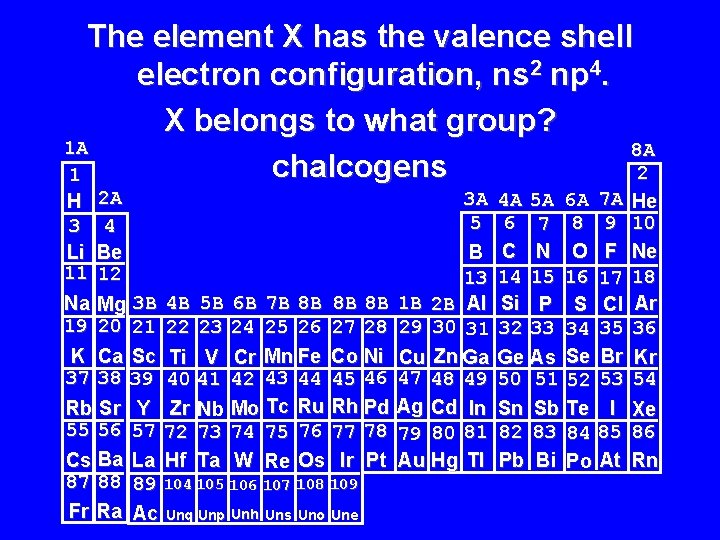
The element X has the valence shell electron configuration, ns 2 np 4. X belongs to what group? 1 A 8 A chalcogens 2 1 H 3 Li 11 2 A 4 Be 12 Na Mg 3 B 4 B 5 B 6 B 7 B 8 B 8 B 8 B 19 20 21 22 23 24 25 26 27 28 K Ca Sc Ti V Cr Mn Fe Co Ni 37 38 39 40 41 42 43 44 45 46 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd 55 56 57 72 73 74 75 76 77 78 Cs Ba La Hf Ta W Re Os Ir Pt 87 88 89 104 105 106 107 108 109 Fr Ra Ac Unq Unp Unh Uns Uno Une 3 A 5 B 13 1 B 2 B Al 29 30 31 Cu Zn Ga 47 48 49 Ag Cd In 79 80 81 Au Hg Tl 4 A 5 A 6 A 7 A 6 7 8 9 C N O F 14 15 16 17 Si P S Cl 32 33 34 35 Ge As Se Br 50 51 52 53 Sn Sb Te I 82 83 84 85 Pb Bi Po At He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn

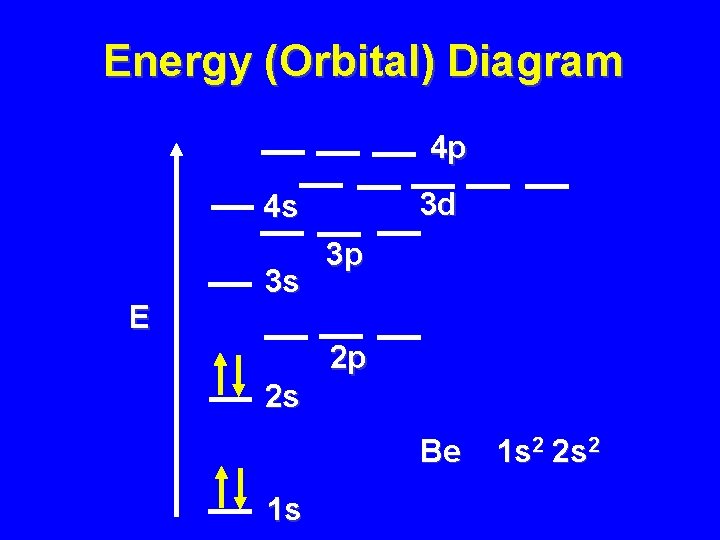
Energy (Orbital) Diagram 4 p 3 d 4 s E 3 s 3 p 2 p 2 s Be 1 s 1 s 2 2 s 2

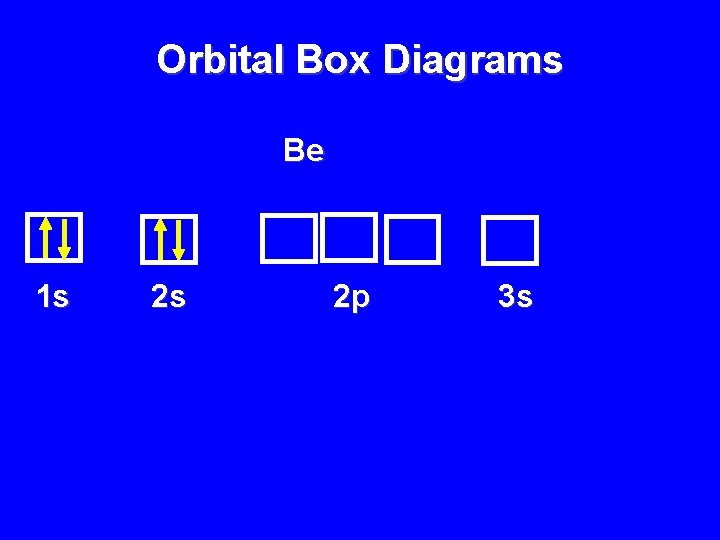
Orbital Box Diagrams Be 1 s 2 s 2 p 3 s

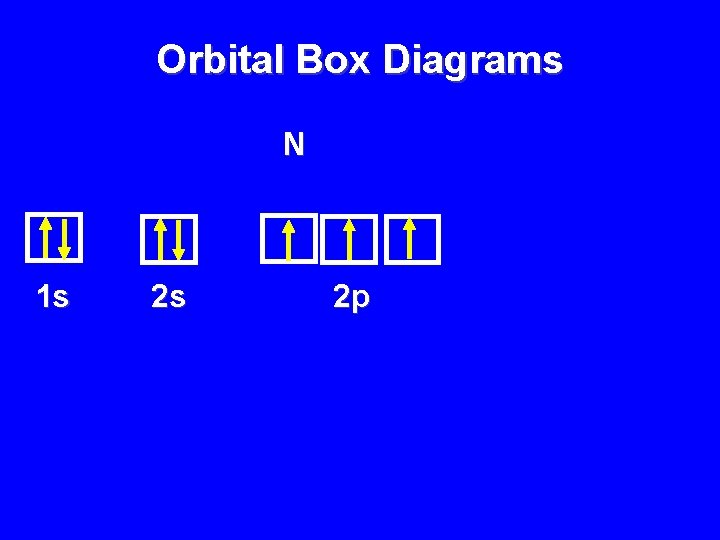
Orbital Box Diagrams N 1 s 2 s 2 p

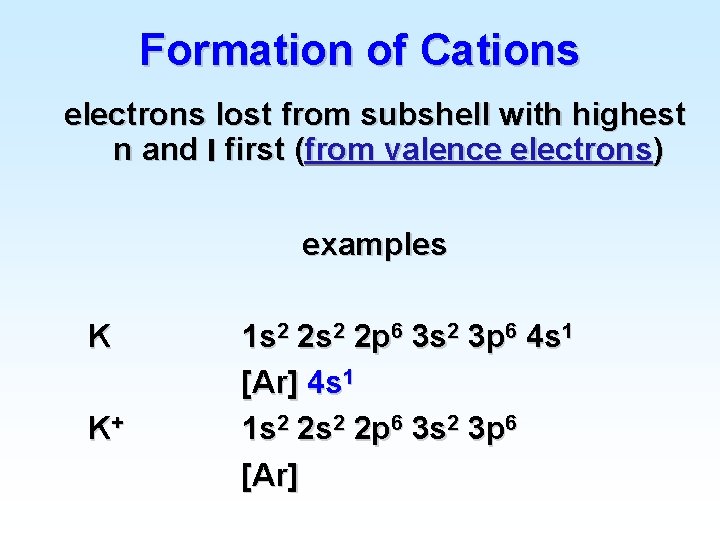
Formation of Cations electrons lost from subshell with highest n and l first (from valence electrons) examples K K+ 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 [Ar] 4 s 1 1 s 2 2 p 6 3 s 2 3 p 6 [Ar]

Ca Ca 2+ Al Al 3+ In In 3+ 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 [Ar] 4 s 2 1 s 2 2 p 6 3 s 2 3 p 6 [Ar] 1 s 2 2 p 6 3 s 2 3 p 1 [Ne] [Kr] 4 d 10 5 s 2 5 p 1 [Kr] 4 d 10

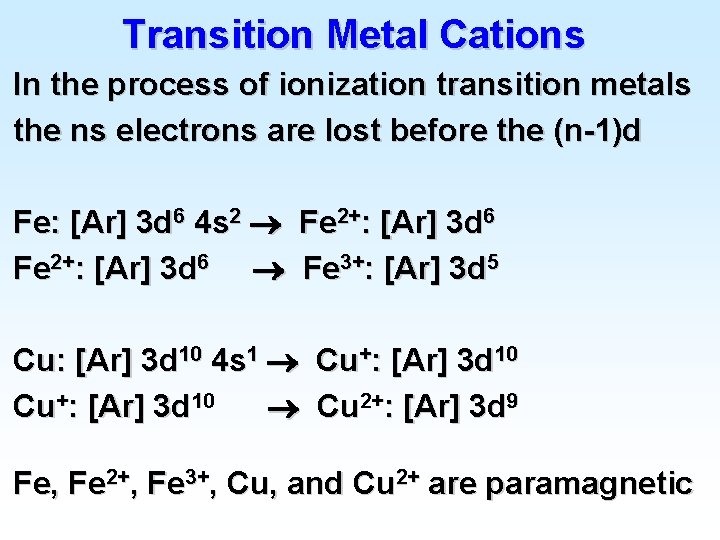
Transition Metal Cations In the process of ionization transition metals the ns electrons are lost before the (n-1)d Fe: [Ar] 3 d 6 4 s 2 Fe 2+: [Ar] 3 d 6 Fe 3+: [Ar] 3 d 5 Cu: [Ar] 3 d 10 4 s 1 Cu+: [Ar] 3 d 10 Cu 2+: [Ar] 3 d 9 Fe, Fe 2+, Fe 3+, Cu, and Cu 2+ are paramagnetic

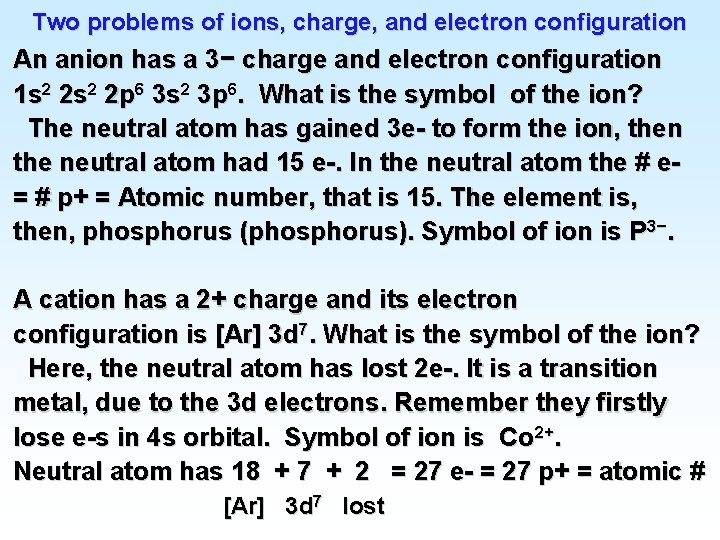
Two problems of ions, charge, and electron configuration An anion has a 3− charge and electron configuration 1 s 2 2 p 6 3 s 2 3 p 6. What is the symbol of the ion? The neutral atom has gained 3 e- to form the ion, then the neutral atom had 15 e-. In the neutral atom the # e= # p+ = Atomic number, that is 15. The element is, then, phosphorus (phosphorus). Symbol of ion is P 3−. A cation has a 2+ charge and its electron configuration is [Ar] 3 d 7. What is the symbol of the ion? Here, the neutral atom has lost 2 e-. It is a transition metal, due to the 3 d electrons. Remember they firstly lose e-s in 4 s orbital. Symbol of ion is Co 2+. Neutral atom has 18 + 7 + 2 = 27 e- = 27 p+ = atomic # [Ar] 3 d 7 lost

Atomic Properties and Periodic Trends Periodic Properties of the Elements 1. Atomic Radii 2. Ionization Energy 3. Electron Affinity 4. Ionic Radii

Atomic Properties and Periodic Trends • Establish a classification scheme of the elements based on their electron configurations. • Noble Gases – All of them have completely filled electron shells. They are not very reactive. • Since they have similar electronic structures, their chemical reactions are similar. – He 1 s 2 – Ne [He] 2 s 2 2 p 6 – Ar [Ne] 3 s 2 3 p 6 – Kr [Ar] 4 s 2 4 p 6 – Xe [Kr] 5 s 2 5 p 6 – Rn [Xe] 6 s 2 6 p 6

Atomic Properties and Periodic Trends Representative Elements are the elements in A groups on periodic chart. These elements will have their “last” electron in an outer s or p orbital. These elements have fairly regular variations in their properties. Metallic character, for expl, increases from right to left and top to bottom.

Atomic Properties and Periodic Trends • • d-Transition Elements on periodic chart in B groups. Sometimes called transition metals. Each metal has d electrons. nsx (n-1)dy configurations These elements make the transition from metals to nonmetals. Exhibit smaller variations from row-to-row than the representative elements.

Atomic Properties and Periodic Trends • f - transition metals Sometimes called inner transition metals. • Electrons are being added to f orbitals. • Electrons are being added two shells below the valence shell! • Consequently, very slight variations of properties from one element to another.

Atomic Properties and Periodic Trends Outermost electrons (valence electrons) have the greatest Influence on the chemical properties of elements.

Atomic Properties and Periodic Trends Atomic radii describe the relative sizes of atoms. Atomic radii increase within a column going from the top to the bottom of the periodic table. The outermost electrons are assigned to orbitals with increasingly higher values of n. The underlying electrons require some space, so the electrons of the outer shells must be further from the nucleus.

Atomic Properties and Periodic Trends Atomic radii decrease within a row going from Left to right on the periodic table. This last fact seems contrary to intuition. How does nature make the elements smaller even though the electron number is increasing?


Atomic Radii • The reason the atomic radii decrease across a period is due to shielding or screening effect. – Effective nuclear charge, Zeff, experienced by an electron is less than the actual nuclear charge, Z. – The inner electrons block the nuclear charge’s effect on the outer electrons. • Moving across a period, each element has an increased nuclear charge and the electrons are going into the same shell (2 s and 2 p or 3 s and 3 p, etc. ). – Consequently, the outer electrons feel a stronger effective nuclear charge. – For Li, Zeff ~ +1 – For Be, Zeff ~ +2 — For B, Zeff ~ +3

Atomic Radii • Example: Arrange these elements based on their increasing atomic radii. – Se, S, O, Te O < Se < Te In the same group atomic size increases as n (and Z) increases ─ Br, Ca, Ge, F F < Br < Ge < Ca same group same period

Ionization Energy • First ionization energy (IE 1) – The minimum amount of energy required to remove the most loosely bound electron from an isolated gaseous atom to form a 1+ ion. • Symbolically: Atom(g) + energy ion+(g) + e. Endothermic Mg(g) + 738 k. J/mol Mg+ + e- IE 1= 738 k. J/mol

Ionization Energy • Second ionization energy (IE 2) – The amount of energy required to remove the second electron from a gaseous 1+ ion. • Symbolically: – ion+ + energy ion 2+ + e- Mg+ + 1451 k. J/mol Mg 2+ + e. IE 2= 1451 k. J/mol Atoms can have 3 rd (IE 3), 4 th (IE 4), etc. ionization energies. The values are consecutively getting larger.

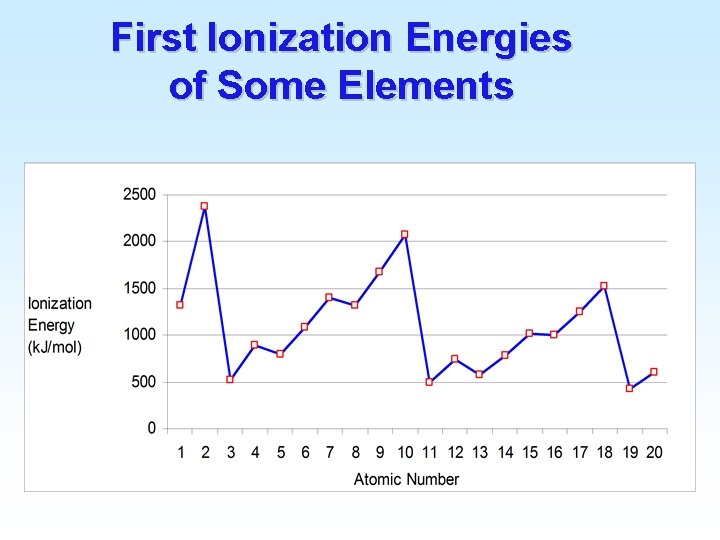
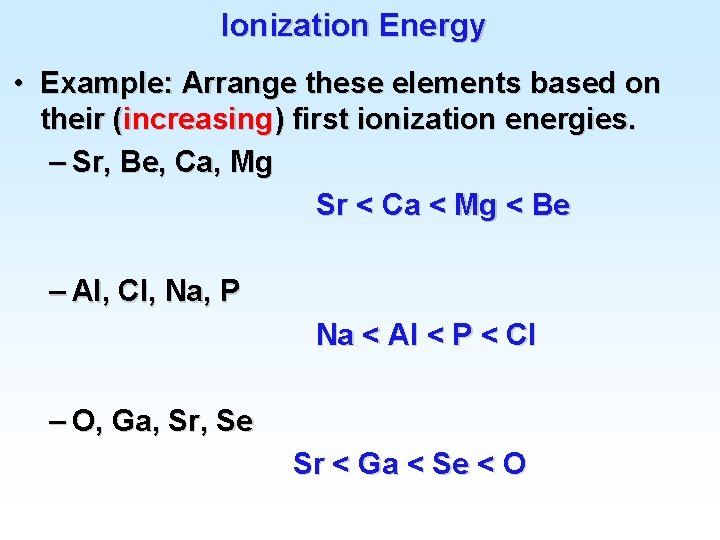
Ionization Energy Periodic trends for Ionization Energy: 1) IE 2 > IE 1 It always takes more energy to remove a second electron from an ion than from a neutral atom. 2) IE 1 generally increases moving from IA elements to VIIIA elements. Important exceptions at Be & B, N & O, etc. due to s and p and half-filled subshells. 3) IE 1 generally decreases moving down a family. IE 1 for Li > IE 1 for Na, etc

First Ionization Energies of Some Elements

Ionization Energy • Example: Arrange these elements based on their (increasing) first ionization energies. – Sr, Be, Ca, Mg Sr < Ca < Mg < Be – Al, Cl, Na, P Na < Al < P < Cl – O, Ga, Sr, Se Sr < Ga < Se < O

Ionization Energy • The reason Na forms Na+ and not Na 2+ is that the energy difference between IE 1 and IE 2 is so large. – Requires more than 9 times more energy to remove the second electron than the first one. • The same trend is persistent throughout the series. – Thus Mg forms Mg 2+ and not Mg 3+. – Al forms Al 3+ and not Al 4+.

H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K 1312 2371 520 900 800 1086 1402 1314 1681 2080 496 738 577 786 1012 1000 1255 1520 419 Ionization Energies (k. J/mole) 5247 7297 1757 2430 2352 2857 3391 3375 3963 4565 1450 1816 1577 1896 2260 2297 2665 3069 11810 14840 3659 4619 4577 5301 6045 6276 6912 7732 2744 3229 2910 3380 3850 3947 4600 21000 25020 6221 7473 7468 8418 9376 9540 10550 11580 4356 4954 4565 5146 5770 5879 32810 37800 9443 10980 11020 12190 13360 13620 15030 16080 6272 6996 6544 7240 7971 47300 53250 13320 15160 15230 16610 18000 18370 19790 21270 8490 9330 8810 9619 64340 71300 84850 17860 92000 20110 21700 23290 23780 25410 28080 11020 11970 11380 25490 25660 27460 29250 29840 31720 33600 13840 14950

Electron Affinity (EA) • Electron affinity is the amount of energy absorbed or emitted when an electron is added to an isolated gaseous atom to form an ion with a 1 - charge. • Sign conventions for electron affinity. – If EA > 0 energy is absorbed (difficult) – If EA < 0 energy is released (easy) • Electron affinity is a measure of an atom’s ability to form negative ions. • Symbolically: atom(g) + e- ion-(g) EA (k. J/mol)

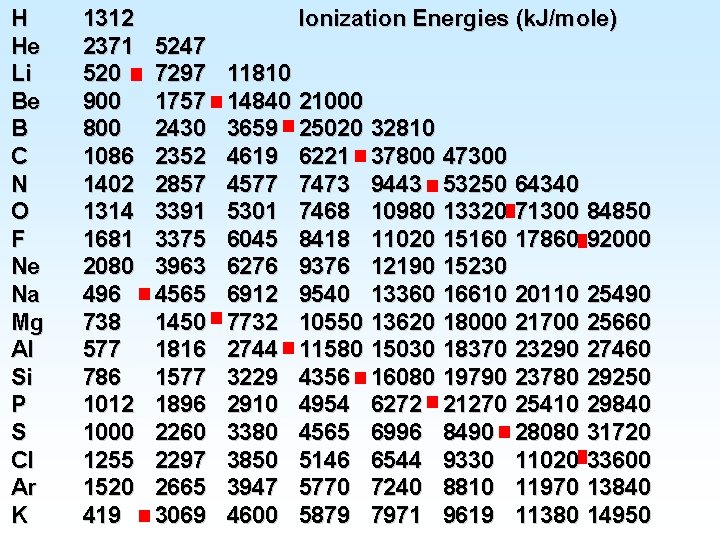
Electron Affinity • General periodic trend for electron affinity is – the values become more negative from left to right across a period on the periodic chart (affinity for electron increases). – the values become more negative from bottom to top at a group on the periodic chart. −Noble gases have EA > 0 (full electron confg) • An element with a high ionization energy generally has a high affinity for an electron, i. e. , EA is largely negative. That is the case for halogens (F, Cl, Br, I), O, and S.

Electron Affinity F (Z= 9) and Cl (Z = 17) have the most negative EA Noble gases, He (2), Ne (10), and Ar (18), EA > 0; also Be, Mg, N They are all first Electron Affinity. A(g)- + e- A 2 -(g) EA 2(k. J/mol) is the 2 nd

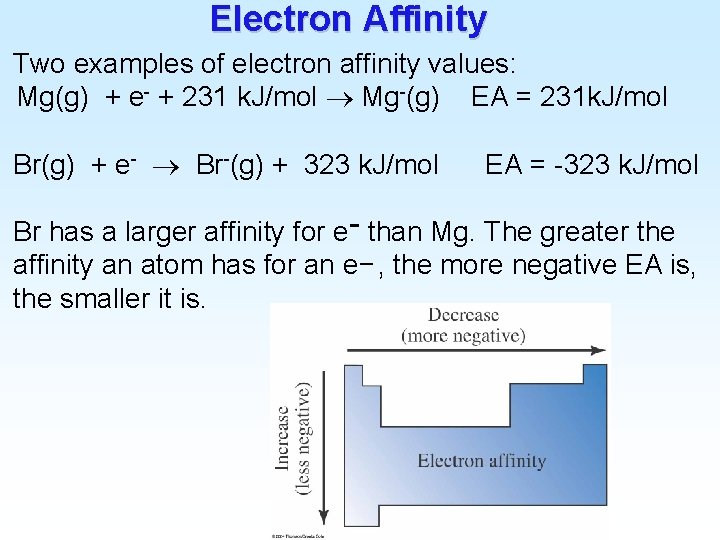
Electron Affinity Two examples of electron affinity values: Mg(g) + e- + 231 k. J/mol Mg-(g) EA = 231 k. J/mol Br(g) + e- Br-(g) + 323 k. J/mol EA = -323 k. J/mol Br has a larger affinity for e− than Mg. The greater the affinity an atom has for an e− , the more negative EA is, the smaller it is.

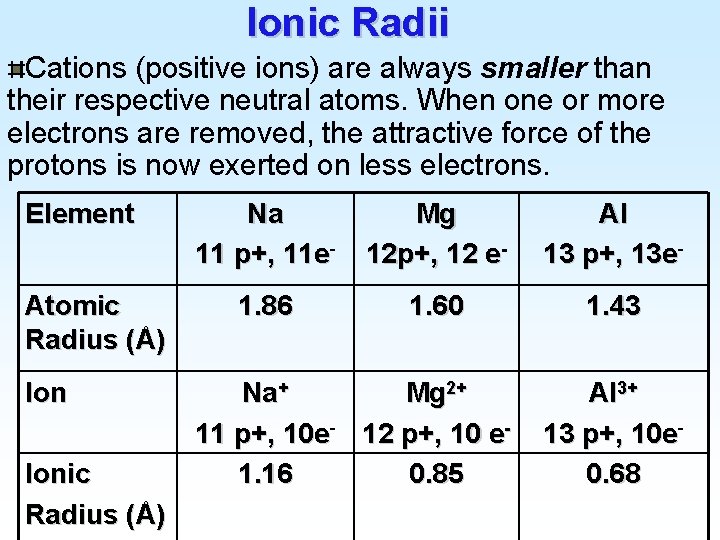
Ionic Radii Cations (positive ions) are always smaller than their respective neutral atoms. When one or more electrons are removed, the attractive force of the protons is now exerted on less electrons. Element Atomic Radius (Å) Ionic Radius (Å) Na 11 p+, 11 e- Mg 12 p+, 12 e- Al 13 p+, 13 e- 1. 86 1. 60 1. 43 Na+ Mg 2+ 11 p+, 10 e- 12 p+, 10 e 1. 16 0. 85 Al 3+ 13 p+, 10 e 0. 68

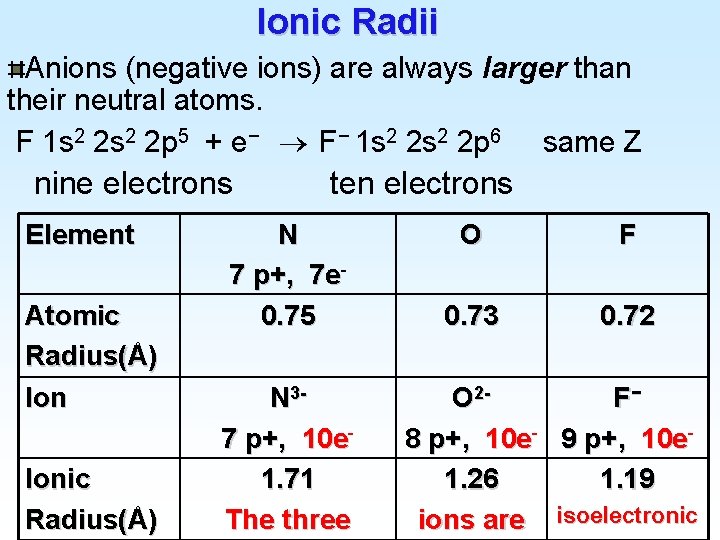
Ionic Radii Anions (negative ions) are always larger than their neutral atoms. F 1 s 2 2 p 5 + e− F− 1 s 2 2 p 6 same Z nine electrons Element Atomic Radius(Å) Ionic Radius(Å) ten electrons N 7 p+, 7 e 0. 75 N 37 p+, 10 e 1. 71 The three O F 0. 73 0. 72 O 2 F− 8 p+, 10 e- 9 p+, 10 e 1. 26 1. 19 ions are isoelectronic

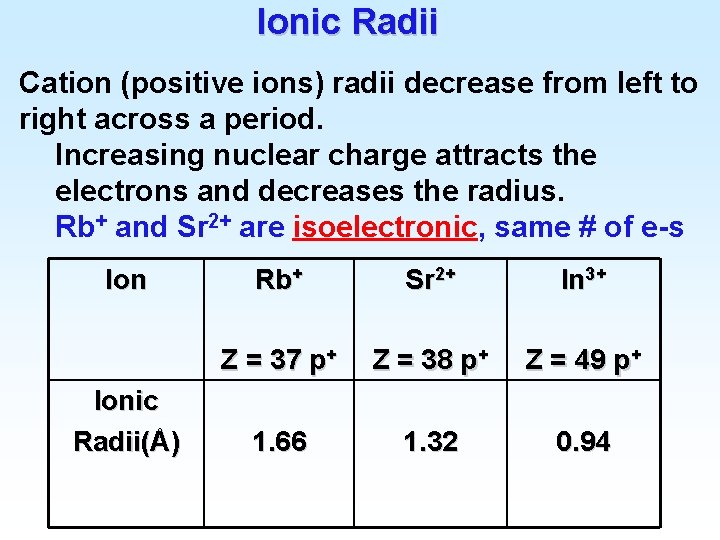
Ionic Radii Cation (positive ions) radii decrease from left to right across a period. Increasing nuclear charge attracts the electrons and decreases the radius. Rb+ and Sr 2+ are isoelectronic, same # of e-s Ionic Radii(Å) Rb+ Sr 2+ In 3+ Z = 37 p+ Z = 38 p+ Z = 49 p+ 1. 66 1. 32 0. 94

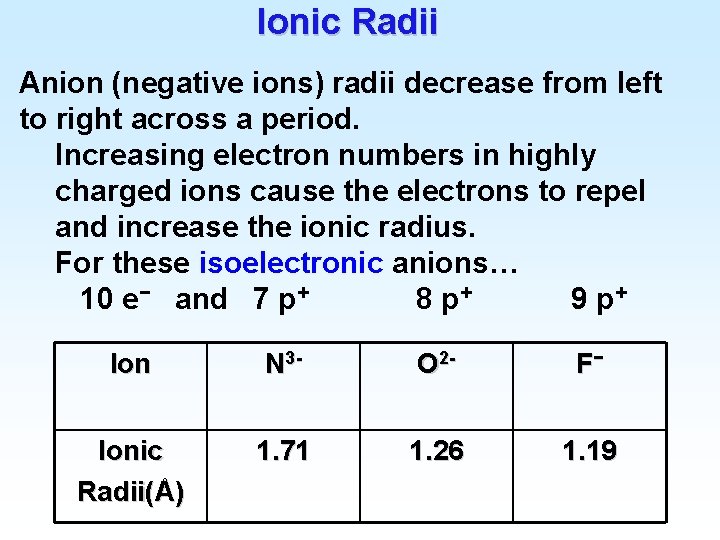
Ionic Radii Anion (negative ions) radii decrease from left to right across a period. Increasing electron numbers in highly charged ions cause the electrons to repel and increase the ionic radius. For these isoelectronic anions… 10 e− and 7 p+ 8 p+ 9 p+ Ion N 3 - O 2 - F− Ionic Radii(Å) 1. 71 1. 26 1. 19

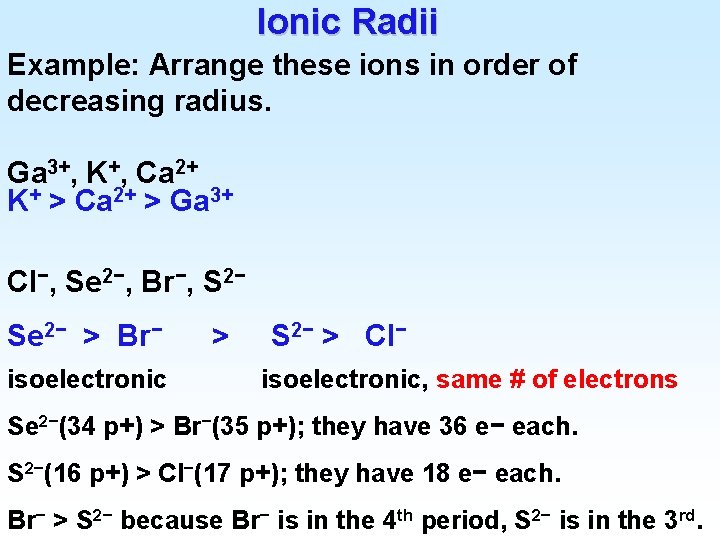
Ionic Radii Example: Arrange these ions in order of decreasing radius. Ga 3+, K+, Ca 2+ K+ > Ca 2+ > Ga 3+ Cl−, Se 2−, Br−, S 2− Se 2− > Br− isoelectronic > S 2− > Cl− isoelectronic, same # of electrons Se 2−(34 p+) > Br−(35 p+); they have 36 e− each. S 2−(16 p+) > Cl−(17 p+); they have 18 e− each. Br− > S 2− because Br− is in the 4 th period, S 2− is in the 3 rd.

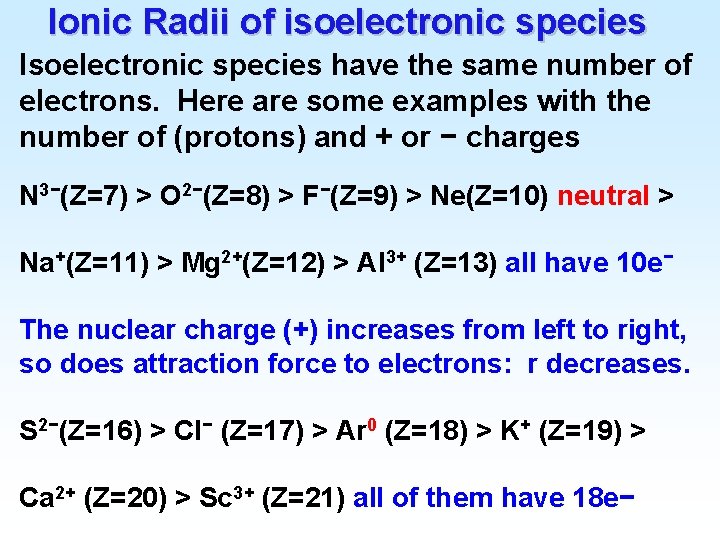
Ionic Radii of isoelectronic species Isoelectronic species have the same number of electrons. Here are some examples with the number of (protons) and + or − charges N 3−(Z=7) > O 2−(Z=8) > F−(Z=9) > Ne(Z=10) neutral > Na+(Z=11) > Mg 2+(Z=12) > Al 3+ (Z=13) all have 10 e− The nuclear charge (+) increases from left to right, so does attraction force to electrons: r decreases. S 2−(Z=16) > Cl− (Z=17) > Ar 0 (Z=18) > K+ (Z=19) > Ca 2+ (Z=20) > Sc 3+ (Z=21) all of them have 18 e−