Chemical Names and Formulas Chemical Formula shows the





- Slides: 16

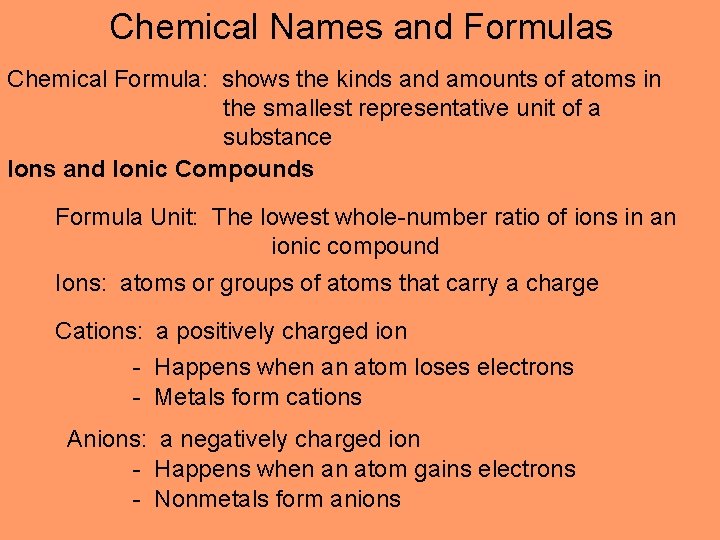
Chemical Names and Formulas Chemical Formula: shows the kinds and amounts of atoms in the smallest representative unit of a substance Ions and Ionic Compounds Formula Unit: The lowest whole-number ratio of ions in an ionic compound Ions: atoms or groups of atoms that carry a charge Cations: a positively charged ion - Happens when an atom loses electrons - Metals form cations Anions: a negatively charged ion - Happens when an atom gains electrons - Nonmetals form anions

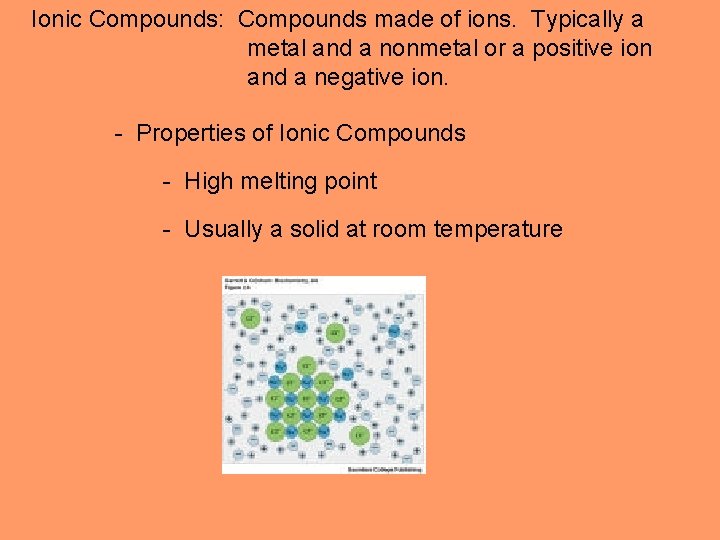
Ionic Compounds: Compounds made of ions. Typically a metal and a nonmetal or a positive ion and a negative ion. - Properties of Ionic Compounds - High melting point - Usually a solid at room temperature

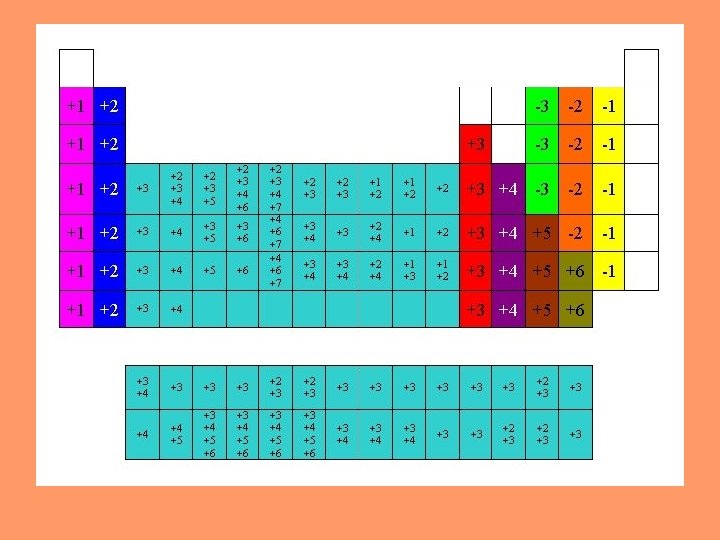
Ionic Charges Monatomic ions: How do you determine the charges from the periodic table? - Based on the element’s group’s distance from the Noble Gases Ex: Group 1 A: Always has a charge of +1 Groups 2 A: Always has a charge of +2 Groups 3 A (Al): Has a +3 charge The Nitrogen Group always has a charge of -3 The Oxygen Group always has a charge of -2 The Halogen Family always has a charge of -1 Oxidation Number: The apparent charge on an ion



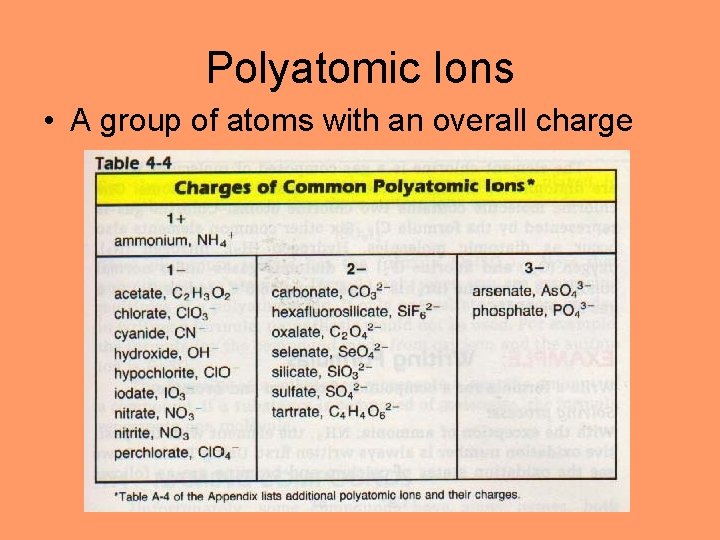
Polyatomic Ions • A group of atoms with an overall charge

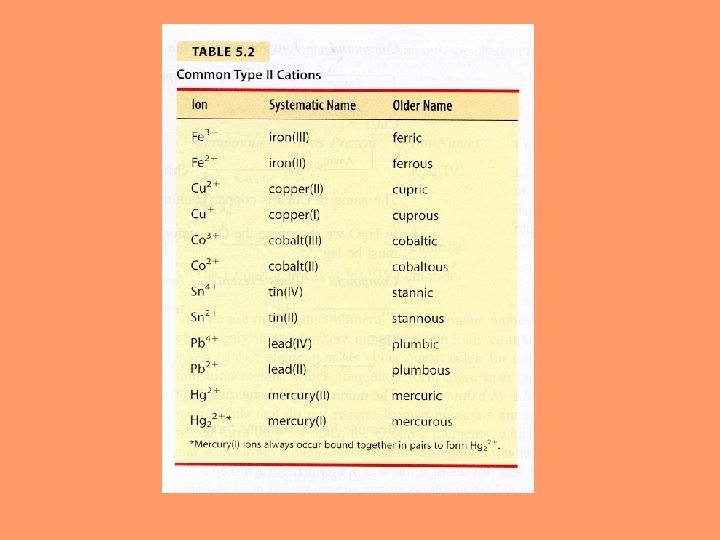
Naming Ionic Compounds Rules: 1. The positive ion (cation) is given first in the formula 2. The suffix –ide is added to the nonmetal stem 3. The subscripts in the formula produce an electrically neutral formula unit (adjust the subscripts as needed) 4. The subscripts should be reduced to the smallest set of whole numbers possible 5. For transition metals with more than one ion charge possible write the name of the element and without a space write a charge of the metal with a Roman Numeral in parenthesis.

Practice – Cs. F – Hg 2 O – Ca(CN)2 – Hg 3 P 2


Rules: 1. The positive ion (cation) is given first in the formula 2. The suffix –ide is added to the nonmetal stem 3. The subscripts in the formula produce an electrically neutral formula unit (adjust the subscripts as needed) 4. The subscripts should be reduced to the smallest set of whole numbers possible 5. For transition metals with more than one ion charge possible write the name of the element and without a space write a charge of the metal with a Roman Numeral in parenthesis. Fe. I 2 Iron (II) Iodide Potassium Sulfide K 2 S

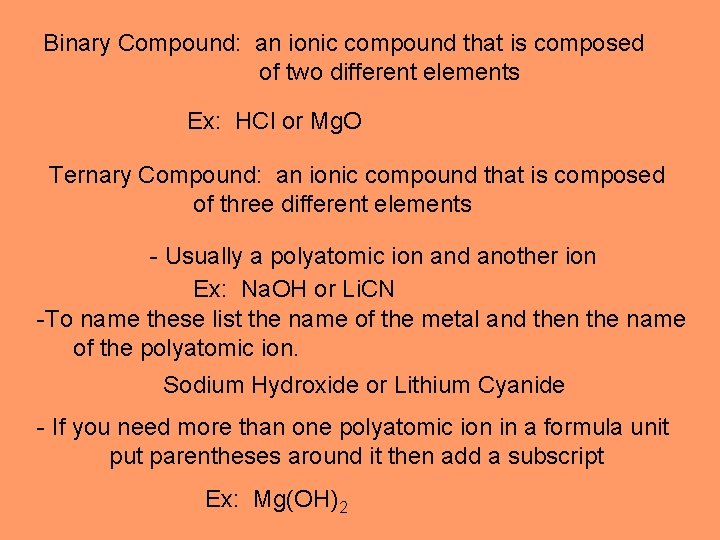
Binary Compound: an ionic compound that is composed of two different elements Ex: HCl or Mg. O Ternary Compound: an ionic compound that is composed of three different elements - Usually a polyatomic ion and another ion Ex: Na. OH or Li. CN -To name these list the name of the metal and then the name of the polyatomic ion. Sodium Hydroxide or Lithium Cyanide - If you need more than one polyatomic ion in a formula unit put parentheses around it then add a subscript Ex: Mg(OH)2

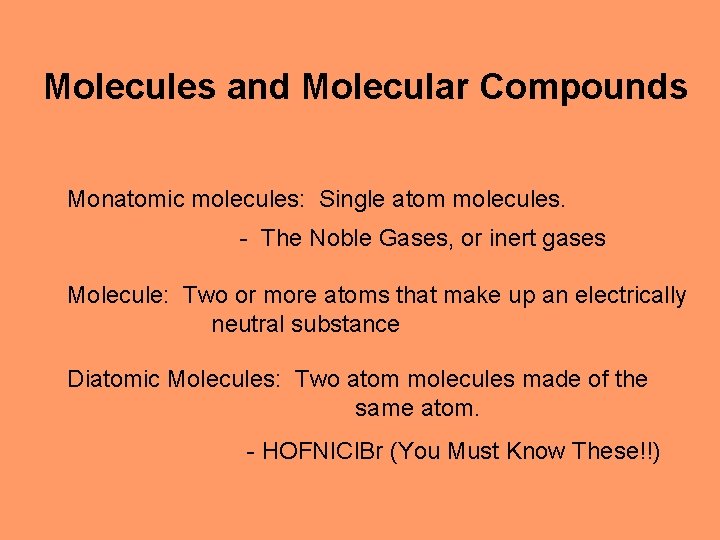
Molecules and Molecular Compounds Monatomic molecules: Single atom molecules. - The Noble Gases, or inert gases Molecule: Two or more atoms that make up an electrically neutral substance Diatomic Molecules: Two atom molecules made of the same atom. - HOFNICl. Br (You Must Know These!!)

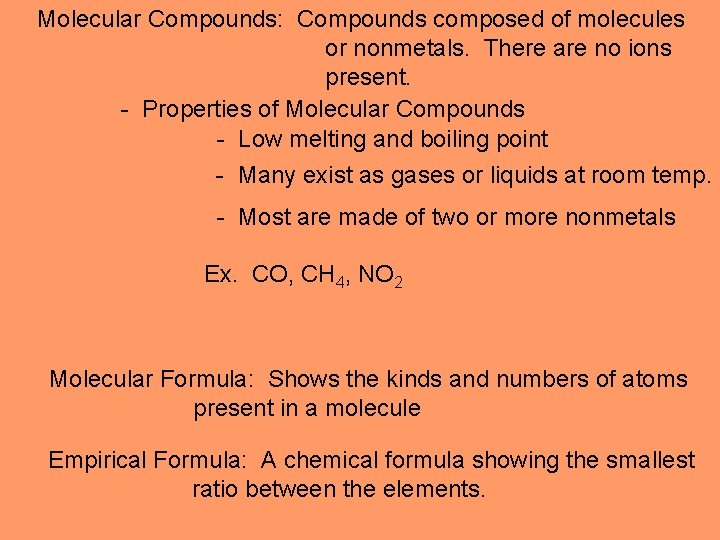
Molecular Compounds: Compounds composed of molecules or nonmetals. There are no ions present. - Properties of Molecular Compounds - Low melting and boiling point - Many exist as gases or liquids at room temp. - Most are made of two or more nonmetals Ex. CO, CH 4, NO 2 Molecular Formula: Shows the kinds and numbers of atoms present in a molecule Empirical Formula: A chemical formula showing the smallest ratio between the elements.

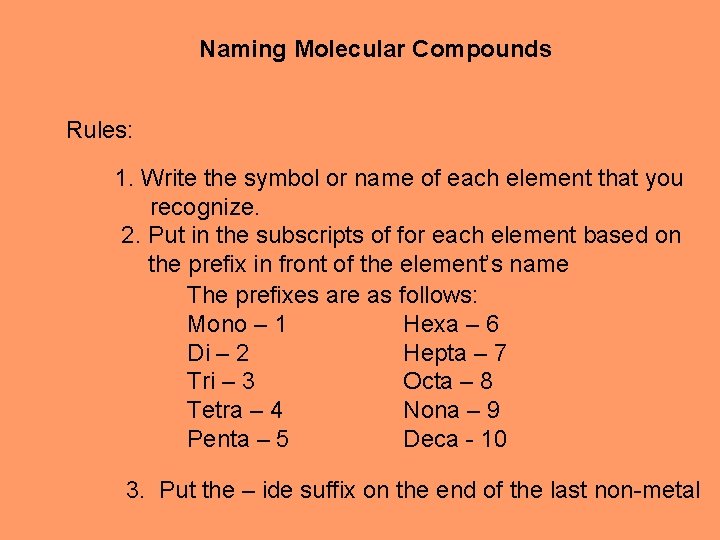
Naming Molecular Compounds Rules: 1. Write the symbol or name of each element that you recognize. 2. Put in the subscripts of for each element based on the prefix in front of the element’s name The prefixes are as follows: Mono – 1 Hexa – 6 Di – 2 Hepta – 7 Tri – 3 Octa – 8 Tetra – 4 Nona – 9 Penta – 5 Deca - 10 3. Put the – ide suffix on the end of the last non-metal

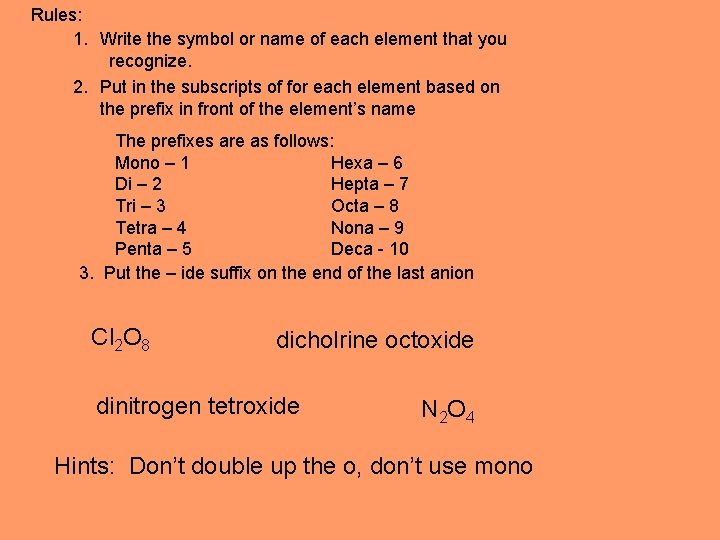
Rules: 1. Write the symbol or name of each element that you recognize. 2. Put in the subscripts of for each element based on the prefix in front of the element’s name The prefixes are as follows: Mono – 1 Hexa – 6 Di – 2 Hepta – 7 Tri – 3 Octa – 8 Tetra – 4 Nona – 9 Penta – 5 Deca - 10 3. Put the – ide suffix on the end of the last anion Cl 2 O 8 dicholrine octoxide dinitrogen tetroxide N 2 O 4 Hints: Don’t double up the o, don’t use mono

Naming Common Acids: compounds that produce hydrogen when dissolved in water. Know the names and formulas of the following acids. Hydrochloric Acid Nitric Acid Sulfuric Acid Phosphoric Acid Acetic Acid HCl HNO 3 H 2 SO 4 H 3 PO 4 HC 2 H 3 O 2