Ch 13 Quantum Mechanical Model Electron Configuration Quantum






- Slides: 32

Ch. 13 Quantum Mechanical Model Electron Configuration

Quantum Mechanical Model • Quantum mechanics was developed by Erwin Schrodinger • Estimates the probability of finding an e- in a certain position • Electrons are found in an “electron cloud” or orbital

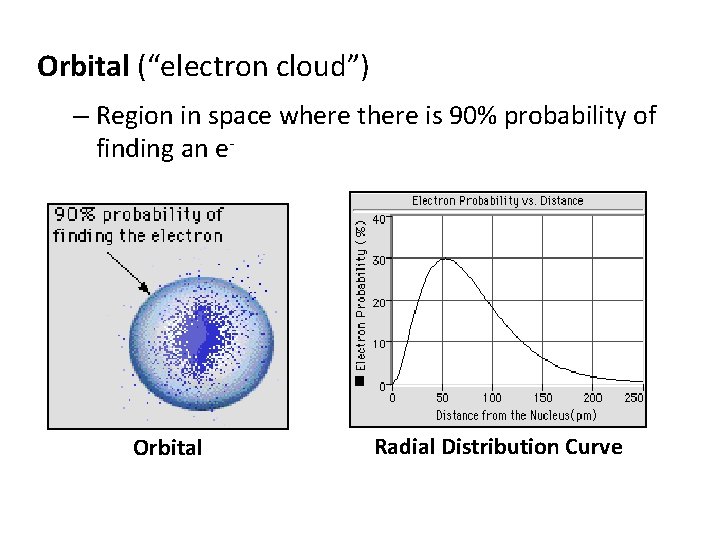
Orbital (“electron cloud”) – Region in space where there is 90% probability of finding an e- Orbital Radial Distribution Curve

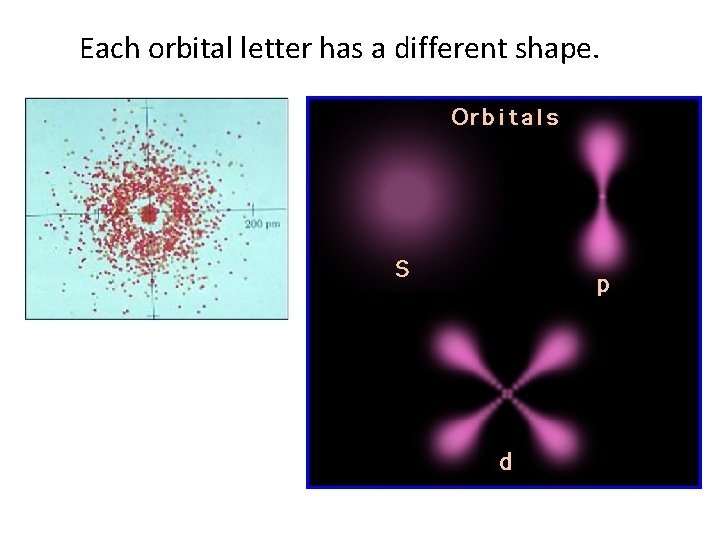
Each orbital letter has a different shape.

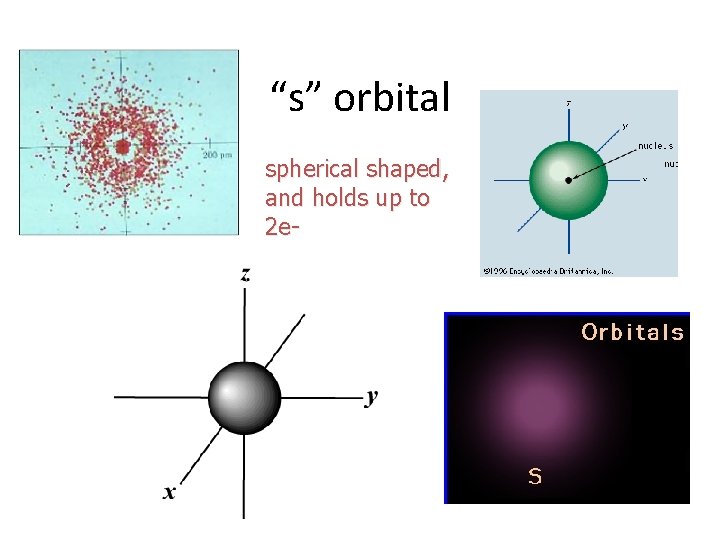
“s” orbital spherical shaped, and holds up to 2 e-

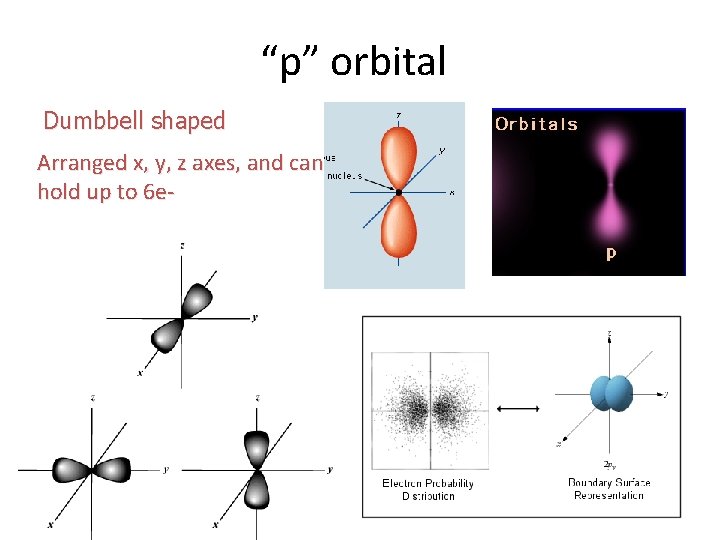
“p” orbital Dumbbell shaped Arranged x, y, z axes, and can hold up to 6 e-

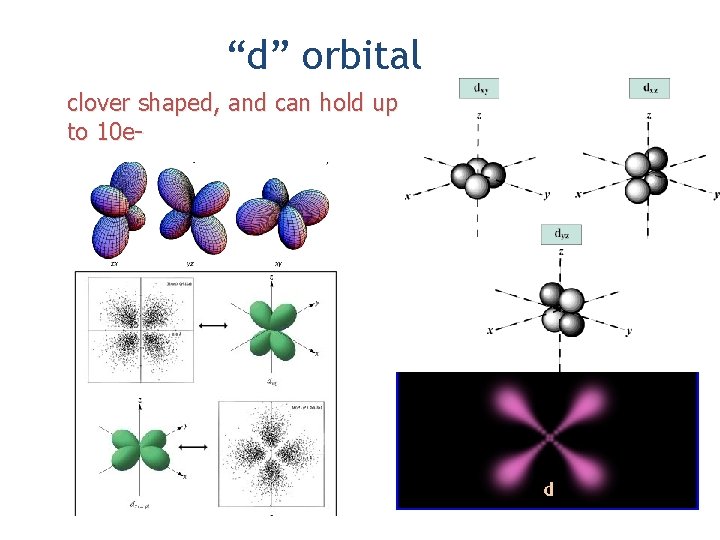
“d” orbital clover shaped, and can hold up to 10 e-

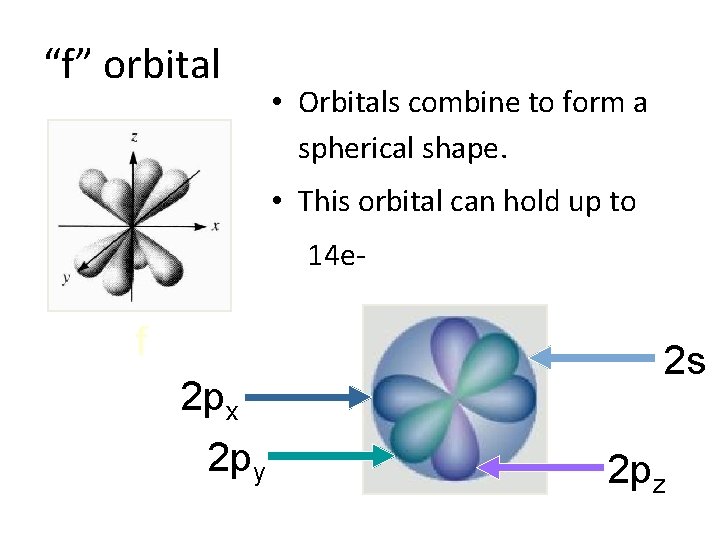
“f” orbital • Orbitals combine to form a spherical shape. • This orbital can hold up to 14 e- f 2 px 2 py 2 s 2 pz

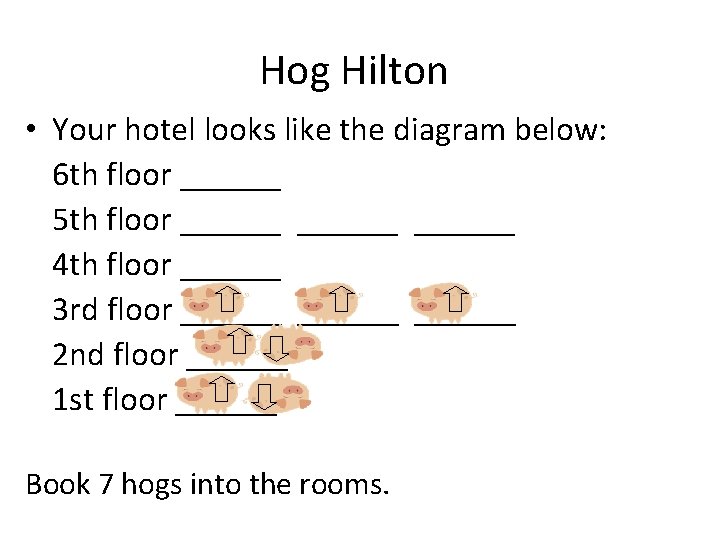
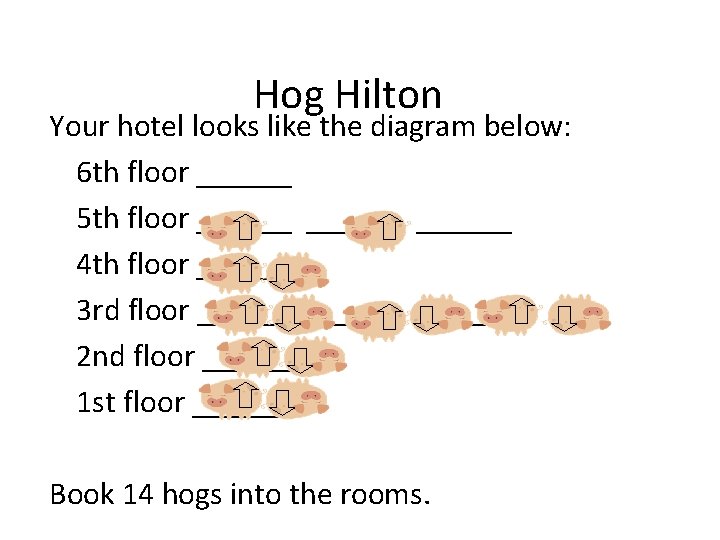
Hog Hilton You are the manager of a prestigious new hotel in downtown Midland—the “Hog Hilton”. It’s just the “snort of the town” and you want to keep its reputation a cut above all the other hotels. Your problem is your clientele. They are hogs in the truest sense. Your major task is to fill rooms in your hotel. The Hog Hilton only has stairs. You must fill up your hotel keeping the following rules in mind: 1) Hogs are lazy, they don’t want to walk up stairs! 2) Hogs want to room by themselves, but they would rather room with another hog than walk up more stairs. 3) If hogs are in the same room they will face in opposite directions. 4) They stink, so you can’t put more than two hogs in each room.

Hog Hilton • Your hotel looks like the diagram below: 6 th floor ______ 5 th floor ______ 4 th floor ______ 3 rd floor ______ 2 nd floor ______ 1 st floor ______ Book 7 hogs into the rooms.

Hog Hilton Your hotel looks like the diagram below: 6 th floor ______ 5 th floor ______ 4 th floor ______ 3 rd floor ______ 2 nd floor ______ 1 st floor ______ Book 14 hogs into the rooms.

Let’s play Hog Hilton!!

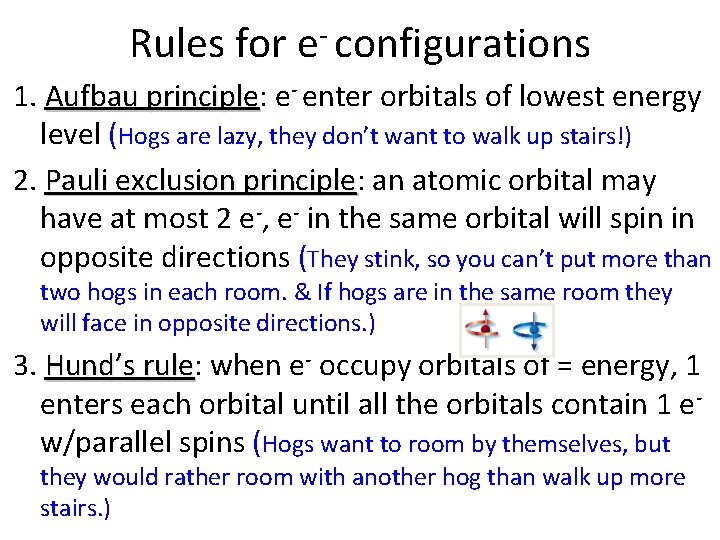
Rules for e configurations 1. Aufbau principle: principle e- enter orbitals of lowest energy level (Hogs are lazy, they don’t want to walk up stairs!) 2. Pauli exclusion principle: principle an atomic orbital may have at most 2 e-, e- in the same orbital will spin in opposite directions (They stink, so you can’t put more than two hogs in each room. & If hogs are in the same room they will face in opposite directions. ) 3. Hund’s rule: rule when e- occupy orbitals of = energy, 1 enters each orbital until all the orbitals contain 1 ew/parallel spins (Hogs want to room by themselves, but they would rather room with another hog than walk up more stairs. )

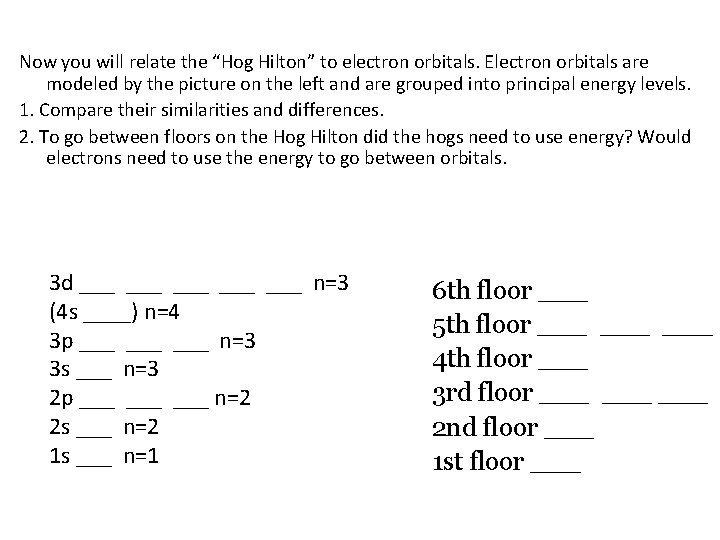
Now you will relate the “Hog Hilton” to electron orbitals. Electron orbitals are modeled by the picture on the left and are grouped into principal energy levels. 1. Compare their similarities and differences. 2. To go between floors on the Hog Hilton did the hogs need to use energy? Would electrons need to use the energy to go between orbitals. 3 d ___ ___ ___ n=3 (4 s ____) n=4 3 p ___ ___ n=3 3 s ___ n=3 2 p ___ ___ n=2 2 s ___ n=2 1 s ___ n=1 6 th floor ___ 5 th floor ___ ___ 4 th floor ___ 3 rd floor ___ ___ 2 nd floor ___ 1 st floor ___

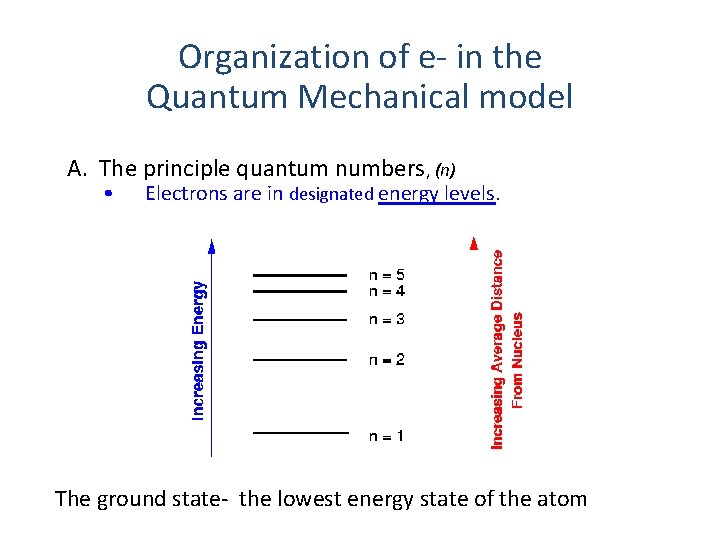
Organization of e- in the Quantum Mechanical model A. The principle quantum numbers, (n) • Electrons are in designated energy levels. The ground state- the lowest energy state of the atom

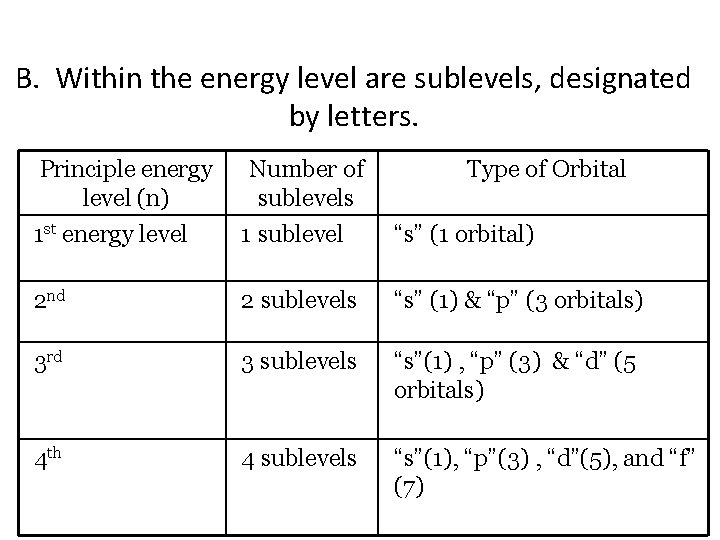
B. Within the energy level are sublevels, designated by letters. Principle energy level (n) Number of sublevels Type of Orbital 1 st energy level 1 sublevel “s” (1 orbital) 2 nd 2 sublevels “s” (1) & “p” (3 orbitals) 3 rd 3 sublevels “s”(1) , “p” (3) & “d” (5 orbitals) 4 th 4 sublevels “s”(1), “p”(3) , “d”(5), and “f” (7)

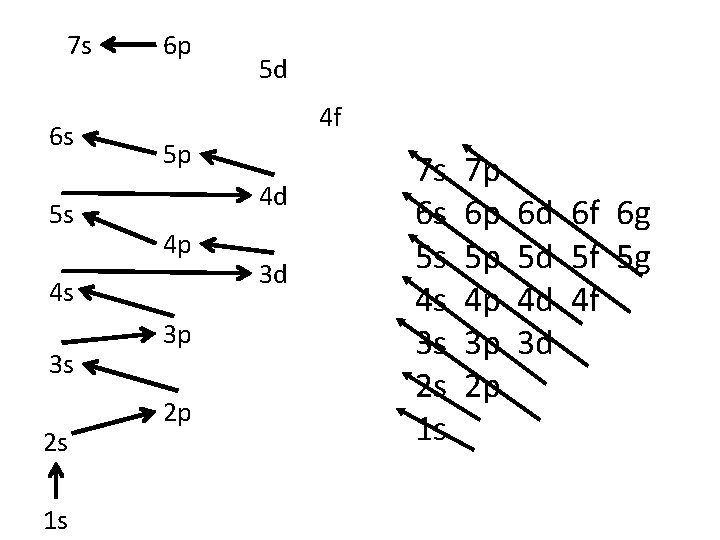
7 s 6 s 5 s 6 p 4 f 5 p 4 d 4 p 4 s 3 s 2 s 1 s 5 d 3 p 2 p 3 d 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 2 p 6 d 6 f 6 g 5 d 5 f 5 g 4 d 4 f 3 d

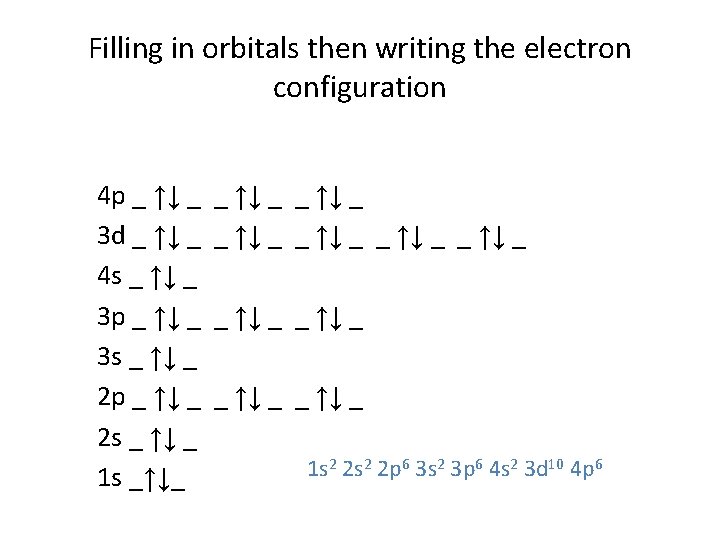
Filling in orbitals then writing the electron configuration 4 p _ ↑↓ _ 3 d _ ↑↓ _ 4 s _ ↑↓ _ 3 p _ ↑↓ _ 3 s _ ↑↓ _ 2 p _ ↑↓ _ 2 s _ ↑↓ _ 1 s _↑↓_ _ ↑↓ _ _ ↑↓ _ _ ↑↓ _ 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6

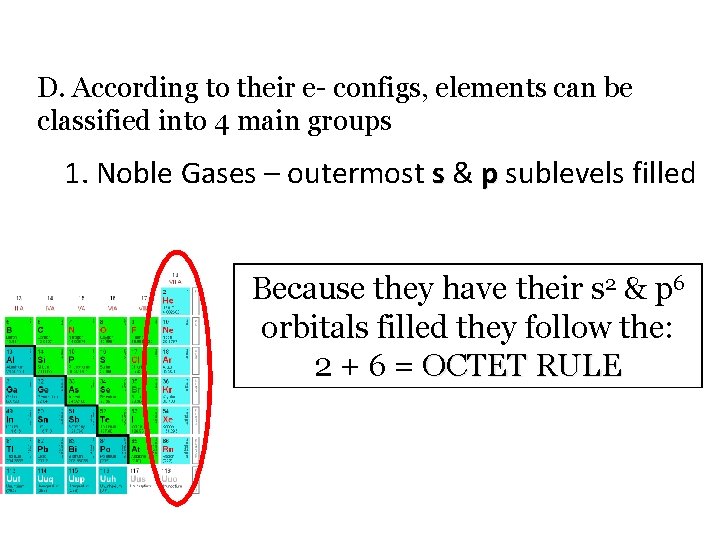
D. According to their e- configs, elements can be classified into 4 main groups 1. Noble Gases – outermost s & p sublevels filled Because they have their s 2 & p 6 orbitals filled they follow the: 2 + 6 = OCTET RULE

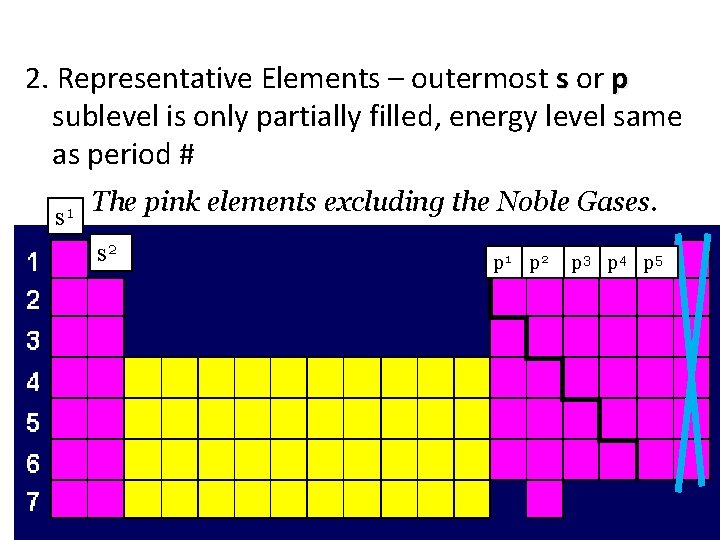
2. Representative Elements – outermost s or p sublevel is only partially filled, energy level same as period # s 1 The pink elements excluding the Noble Gases. s 2 p 1 p 2 p 3 p 4 p 5

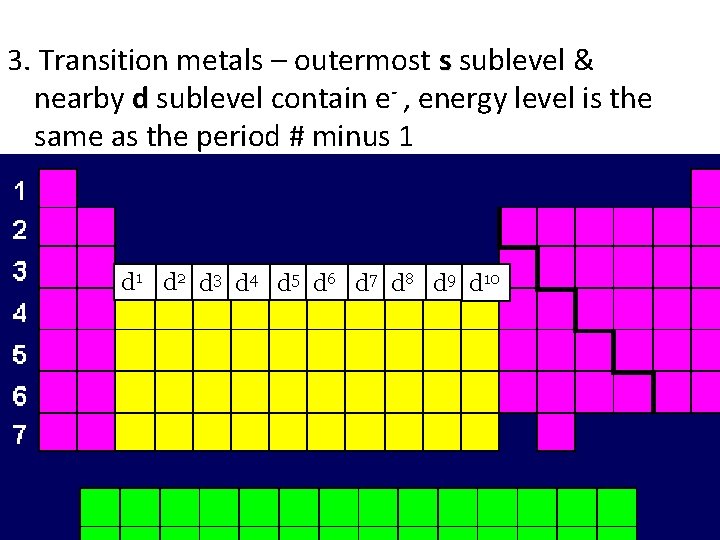
3. Transition metals – outermost s sublevel & nearby d sublevel contain e- , energy level is the same as the period # minus 1 d 2 d 3 d 4 d 5 d 6 d 7 d 8 d 9 d 10

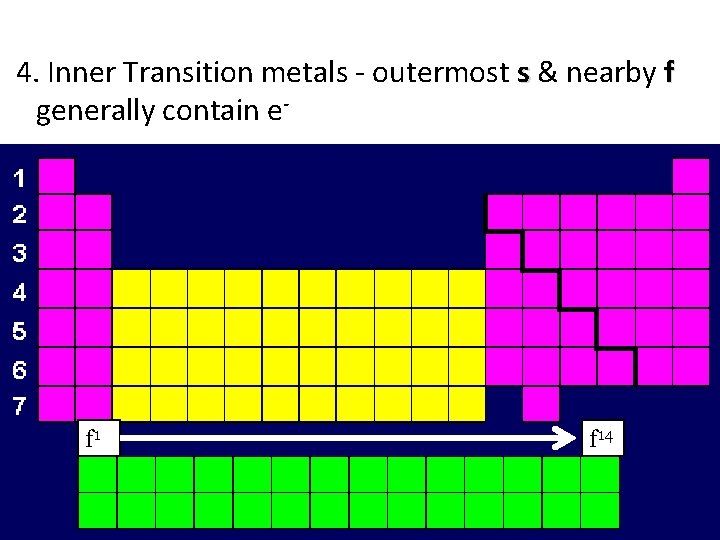
4. Inner Transition metals - outermost s & nearby f generally contain e- f 14

Your Periodic Table should look like this. s 1 s 2 p 1 p 2 p 3 p 4 p 5 s 2 p 6 d 1 d 2 d 3 d 4 d 5 d 6 d 7 d 8 d 9 d 10 f 14

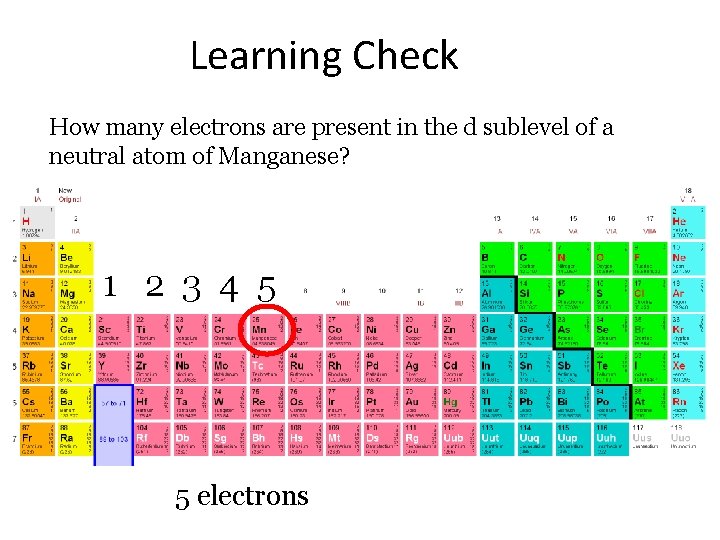
Learning Check How many electrons are present in the d sublevel of a neutral atom of Manganese? 1 2 3 4 5 5 electrons

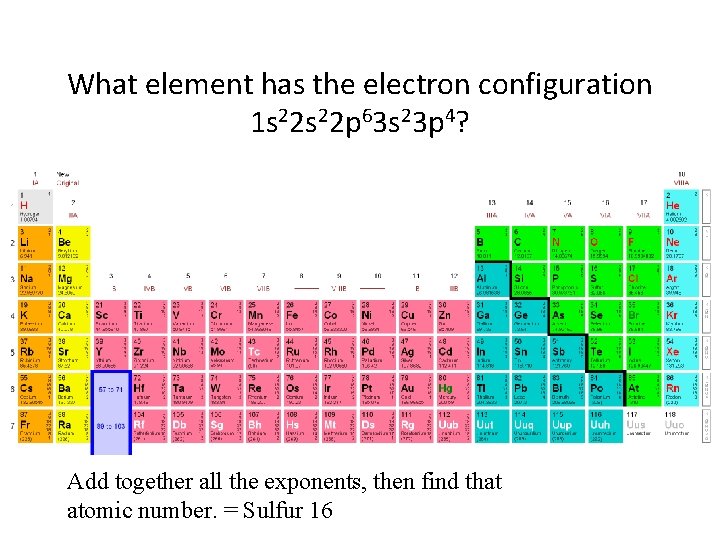
What element has the electron configuration 1 s 22 p 63 s 23 p 4? Add together all the exponents, then find that atomic number. = Sulfur 16

E. Using the Noble Gases to write Shorthand • Write the noble gas that is in the previous row. • Use the symbol of the noble gas, put it in brackets, then write the rest of the configuration. Write the e- config using Noble Gas notation for Cobalt. It would be written [Ar] 4 s 2 3 d 7 • Write the e- config for Tin (Sn). • [Kr] 5 s 2 4 d 10 5 p 2

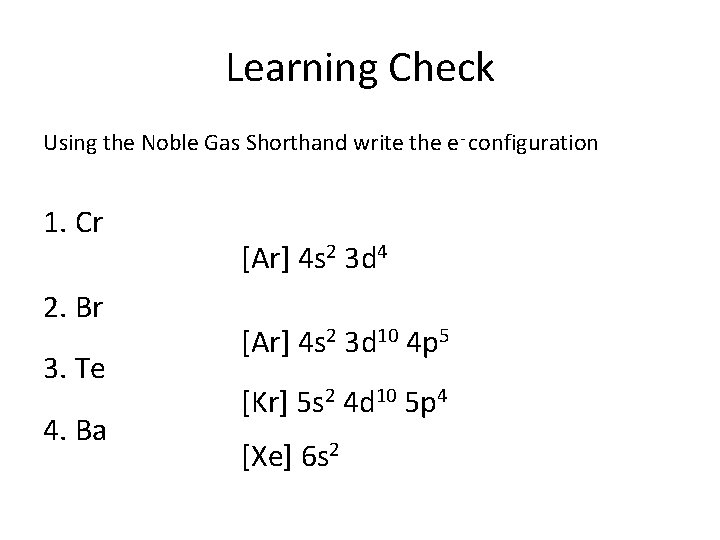
Learning Check Using the Noble Gas Shorthand write the e- configuration 1. Cr 2. Br 3. Te 4. Ba [Ar] 4 s 2 3 d 4 [Ar] 4 s 2 3 d 10 4 p 5 [Kr] 5 s 2 4 d 10 5 p 4 [Xe] 6 s 2

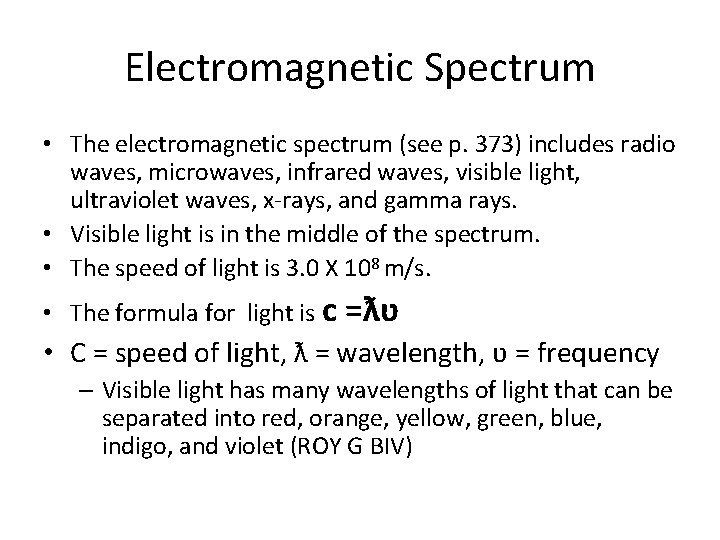
Electromagnetic Spectrum • The electromagnetic spectrum (see p. 373) includes radio waves, microwaves, infrared waves, visible light, ultraviolet waves, x-rays, and gamma rays. • Visible light is in the middle of the spectrum. • The speed of light is 3. 0 X 108 m/s. • The formula for light is c =ƛʋ • C = speed of light, ƛ = wavelength, ʋ = frequency – Visible light has many wavelengths of light that can be separated into red, orange, yellow, green, blue, indigo, and violet (ROY G BIV)

Atomic Emission Spectrum • Every element gives off light when it is excited by the passage of an electric current through its gas or vapor. • The atomic emission spectrum occurs when the light that is given off by an element in its excited state is passed through a prism. It consists of a few lines called a line spectra or discontinuous spectra. Each line on the spectra corresponds with a frequency. • See page 374. • Work problems # 11 and 12 on page 375.

Planck’s Constant • In 1900, German Physicist Max Planck used math to explain why objects, such as iron, that are heated change color. • He said energy can be quantized. The size of an emitted or absorbed quantum depends on the size of the energy change. A small energy change involves the emission or absorption of low frequency radiation. A large energy change involves the emission or absorption of high frequency radiation.

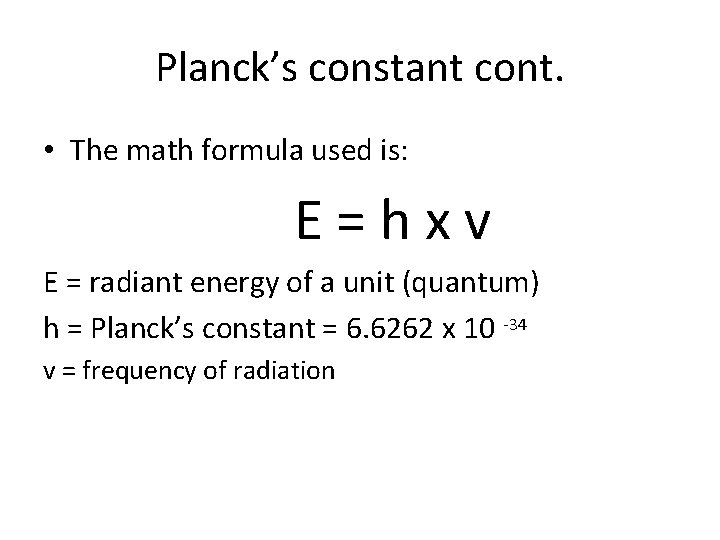
Planck’s constant cont. • The math formula used is: E=hxv E = radiant energy of a unit (quantum) h = Planck’s constant = 6. 6262 x 10 -34 v = frequency of radiation

Planck’s constant cont. • In 1905, Albert Einstein used Planck’s work to call quanta of light photons. He then used this information to explain the photoelectric effect (metals eject/emit electrons called photoelectrons when light shines on them). • Work problems 13 and 14 on p. 379.